Practice Essentials
Peyronie disease (PD) is named after French surgeon François Gigot de la Peyronie, who described the condition in 1743. [1] PD is characterized by curvature in the penile shaft (see the image below) that is often preceded by painful erections and accompanied by an area of fibrosis. The characteristic angulation is frequently associated with erectile dysfunction (ED), either as a result of buckling of the penile shaft with intromission or because of a lack of rigidity distal to the area of associated fibrosis. This lack of rigidity seems to be the result of compromise to the distal penile blood supply.
Over the years, various medical and surgical therapies have been used to treat this condition, whose etiology remains uncertain. The number and variety of these attempts at treatment stand as a testament to their relative lack of effectiveness. [2, 3]
This article defines the problem of PD, outlines a reasonable evaluation of patients with PD, reviews the medical therapies that have been used, and presents the surgical options that are currently available. The indications for treatment are discussed, as are the expected outcomes. The goal is to impart a practical approach to the diagnosis and treatment of PD in the clinical setting.
For patient education information, see Peyronie's Disease (Curvature of the Penis).
Problem
PD is a curvature of the penis that is usually associated with a palpable area of fibrosis in the tunica albuginea. The curvature is usually obvious when the penis is erect but is occasionally noticeable even when the penis is flaccid. The fibrotic area, known as a plaque, can vary in firmness and sometimes becomes calcified. The penile curvature is often preceded by painful erections and may be associated with ED.
Epidemiology
Frequency
The rate of PD is reported to be 0.39-3%. However, that is probably an underestimation because of the embarrassment most men feel about having this condition. Underreporting may also result from men whose symptoms are mild or nondebilitating not seeking medical attention. Although this condition usually affects men aged 40-70 years, several authors have reported cases in younger individuals. [4, 5, 6]
Schwarzer et al found a prevalence of 3.2% for the new appearance of a palpable plaque, in a large population-based self-report survey sent to 8000 men and answered by 4432 (55.4%). The prevalence by age was 1.5% for men aged 30-39 years, 3% for men aged 40-49 years, 3% for men aged 50-59 years, 4% for men aged 60-69 years, and 6.5% for men aged 70 or older. Associated with the plaque, 84% reported penile angulation; 47% reported painful erections; 32% noted the triad of plaque, angulation, and pain; and 41% also reported ED. [7]
Mulhall et al found the prevalence of PD to be 8.9% in 534 men who were screened for prostate cancer in the United States. These investigators also found that a significant proportion of men with PD also had hypertension and diabetes. [8]
Etiology
The etiology of PD remains enigmatic. PD has been associated with deficiency in vitamin E, ingestion of beta-blocking agents, and elevations of serotonin levels. PD is associated with Dupuytren contractures and with HLA-B7, implying a genetic link to its etiology. [9]
More recently, PD has been thought to result from vascular trauma or injury to the penis. The injury may be trivial or involve only microscopic vessels and tissues. This triggers the release of cytokines that activate fibroblast proliferation and produce collagen, the main matrix component of a Peyronie plaque, within the tunica albuginea.
Usta et al found that the presence of diabetes, hyperlipidemia, hypercholesteremia, or hypertension was not statistically related to the severity of penile curvature in men with PD. The number of comorbidities also did not affect the degree of curvature. The rates of hypertension, hypercholesterolemia, diabetes, hyperlipidemia, and heart disease were significantly higher in men with PD and ED compared with those with PD alone, but the authors believed that this relationship was more likely related to the ED rather than to the PD. [10]
To evaluate the association between PD and ED, El-Sakka studied the prevalence of PD in a population of men known to have ED. [11] In this prospective study, 1440 men with ED were assessed with the International Index of Erectile Function (IIEF) and categorized as having mild (12%), moderate (38%), or severe (50%) ED. Eight percent were found to have PD primarily based on the presence of a penile plaque. An analysis of sociodemographic factors also identified significant associations between PD and the following risk factors for ED:
-
Age
-
Obesity
-
Smoking (duration and number of cigarettes per day)
-
Longer duration and increased severity of ED
-
Diabetes mellitus
-
Dyslipidemia
-
Psychological disorders
No significant association was found between PD and hypertension or ischemic heart disease in this study. How much these risk factors contribute to the development of PD and how ED and PD influence each other remain unclear.
Bjekic et al examined the risk factors for PD in a case-controlled study of 82 men with PD matched against 264 men who had neither a history nor signs of PD. [12] The study identified the following as risk factors: (1) genetic predisposition in association with a family history of Dupuytren contracture; (2) minor vascular penile trauma, either accidental or iatrogenic from cystoscopy or transurethral resection of the prostate (TURP); (3) systemic vascular diseases, including diabetes mellitus, hypertension, and hyperlipidemia; and (4) smoking and alcohol consumption. The use of propranolol and a history of nongonococcal urethritis were also found to be risk factors for PD. How these risk factors contribute to the initiation of PD remains elusive.
Agrawal et al reported that patients with PD have evidence of systemic arterial impairment that is endothelial-dependent, compared with healthy controls without PD. [13] Both groups were free of other risk factors for endothelial dysfunction and atherosclerosis. The authors suggest that these systemic abnormalities might be of clinical relevance to the development of PD. Further evaluation is necessary to confirm and interpret these interesting findings.
Casabe et al identified ED and coital trauma as the only independent risk factors for developing PD, in a study that compared 317 consecutive patients with PD and a control group of 147 consecutive patients who presented for prostate examination. Patients were queried regarding ED, coital trauma (defined as strong pain or "cracking" during sexual intercourse), diabetes mellitus, hypertension, cardiovascular disease, dyslipidemia, and lower urinary tract symptoms. The authors speculate that coital trauma may be related to suboptimal penetration capacity in men with ED, and recommend treatment to improve the quality of erections in men with ED along with the use of lubricant gel to avoid friction during intercourse and to diminish coital trauma. [14]
A literature review by Gianazza et al found a significant prevalence of diabetes mellitus in patients with PD, based on data that were somewhat heterogeneous due to population selection. While these authors admit that the role of diabetes in the development of PD is controversial, they cite impairment in wound healing, penile elasticity, nociception, and smooth muscle collagenization due to diabetes as possible contributing factors in PD. [15] These authors also note that glycemic control an ameliorate PD symptoms, and that while patients with diabetes who undergo surgical treatment are less likely to experience recurrence of curvature, they are more likely to develop de novo ED after surgery and infection after implantation of a penile prosthesis.
Pathophysiology
During normal erectile function, neural stimulation results in relaxation of the cavernosal smooth muscle tissue and the cavernosal arteries, bringing blood into the penis and the trabecular spaces within the corporeal bodies. This state initially results in both increased blood flow into the corporeal bodies and pooling of more blood within these organs. As a result, they expand and stretch the surrounding tunica albuginea. This thick expansile layer is composed of collagen and elastic fibers in an irregular framework. It normally stretches in length and width to its maximum size.
Expansion results in stretching, compression, and closure of the subtunical venules that perforate the tunica and drain blood from the corpora. Consequently, blood cannot drain easily from the penis, which results in an increase in intracorporeal hydrostatic pressure. This increased pressure allows the penis to become rigid once the tunica has reached its maximum length and width. The process is similar to overinflating a car tire: Once the maximum length of the steel belts in the tire is reached, further inflation increases the tire's rigidity only.
In PD, the initial inflammatory response is characterized by chronic lymphocytic and plasmacytic infiltration of the tunica albuginea. This may be the result of minor penile trauma, as can happen unnoticed during intercourse. Devine et al propose that such trauma can result in a dorsal and ventral delamination injury to the tunica albuginea, causing inflammation, induration, and fibrin deposition between the tunical layers. [16] If scar tissue formation and extracellular matrix deposition exceed collagen and matrix degradation, as Levine et al postulate, then increased collagen is deposited in the tunica albuginea, resulting in fibrosis and plaque formation.
Luangkhot et al reported that men with PD have more type III collagen than type I collagen in their tunica albuginea. [17] Chiang et al also found increased type III collagen in the adjacent penile tissue in men with PD and in the tunica in men with corporeal veno-occlusive dysfunction (venous leak syndrome). [18] This may indicate that a more generalized penile abnormality is involved in PD.
Transforming growth factor–beta (TGF-beta) is a cytokine involved in tissue repair and scar formation. El-Sakka et al showed that its production is up-regulated in men with PD and that it may be responsible for the increase in collagen deposition associated with this condition. [19]
Gonzalez-Cadavid and Rajfer used two rat models to explore the relationship of TGF-beta1 and fibrin in PD. [20] These models consisted of injection of either TGF-beta1 or fibrin into the tunica of the rat. The authors were able to derive and confirm several conclusions as to the role of microtrauma, cytokines, myofibroblasts, and oxidative stress in the initiation and progression of plaque. Among the several other findings of the study, they also helped to characterize the antifibrotic effects of long-term phosphodiesterase type 5 (PDE5) inhibitor use in these patients.
Presentation
The natural history of PD is variable. Progression can occur over several years. If the fibrosis becomes calcified, the angulation becomes quite stable. Earlier studies described PD as being a self-limited condition, with spontaneous resolution in most cases. However, this does not appear to be accurate. In 1990, Gelbard et al found that the plaque completely resolved without treatment in only 13% of men, while 40% described their condition as progressive and 47% noted no change. [4] Because spontaneous resolution is unlikely in most men, most investigators recommend intervention if and when the condition impacts significantly upon sexual function. Devine et al proposed that the earlier that medical intervention is initiated, the more likely it is to be successful. [21]
Men with PD may present with any combination of the following:
-
Penile pain, which is more pronounced during erections
-
Penile angulation, which may be apparent only with an erection or may be noted in a flaccid penis
-
A plaque that is usually palpable at the site of and on the acute side of the angulation
-
An indentation in the shaft, typically at the site of the plaque (causing an hour-glass deformity in the shaft)
-
Decreased erectile function, either from loss of rigidity or from penile buckling caused by the angulation
The signs and symptoms often follow the usual progression of PD. Progressive angulation can approach a maximum angle of 90°, thus complicating intromission.
Penile pain per se almost always resolves without therapy, and this has led to controversy in assessing therapeutic outcomes. Alternatively, some patients note pain in the penis that may subside before the appearance of a palpable plaque or of penile angulation. A less common manifestation is a palpable plaque with no associated pain or angulation.
For practical purposes, PD can be divided into acute and chronic phases. The acute phase usually lasts for the first 18-24 months and is characterized by a changing inflammatory pattern that may include penile pain, some curvature, and a penile nodule. The chronic phase is characterized by a stable plaque, often with calcification, and penile angulation. Loss of erectile ability is associated more often with the chronic phase.
Indications
If Peyronie disease (PD) is diagnosed early in the disease course (ie, within the first 6 mo of the onset of any symptoms), attempt nonsurgical therapy. If it is not initially successful, maintain such therapy for a reasonable time (≥6 mo) before considering more invasive measures.
Waiting for a sufficient duration after the inception of symptoms is necessary to ensure that the condition is stable and to be certain that spontaneous resolution will not occur. This duration is governed by the severity and debilitating effects of the symptoms. It should be in the range of 1-2 years. During this time, nonsurgical treatments can be applied. A significant endpoint in the observation period is the presence of calcification in the plaque. This usually indicates a stable plaque, and no further angulation or resolution can be anticipated at this point. If the plaque and/or penile angulation have remained unchanged for 6 months, the condition can be assumed to be stable and surgical intervention can be contemplated.
Surgery should not be undertaken unless the condition is debilitating, preventing satisfactory intercourse. Erectile dysfunction (ED) that is not responsive to pharmacologic therapy is an indication to use surgical approaches that address both the curvature and the ED. The authors do not believe that operating to correct small curvatures simply for cosmetic reasons is reasonable. Usually, some degree of curvature remains afterwards, either from residual disease or from resultant scar tissue, and patients are not satisfied with the results.
Relevant Anatomy
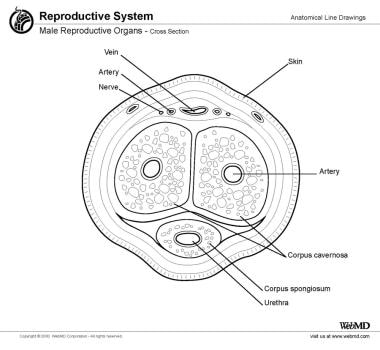
The penile shaft is made up of 3 erectile bodies: the 2 corpora cavernosa dorsally and the corpus spongiosum containing the urethra ventrally (see the image below). They are surrounded by vessels, fascia, and skin. The corpora are composed of erectile tissue that surrounds the cavernosal artery located within each corpus. This tissue is spongelike, being composed of many vascular spaces capable of expanding and filling with blood after adequate smooth muscle relaxation from proper neural stimulation.
The corpora cavernosa are on adjacent sides of the median raphe. This fenestrated midline structure acts as a buttress to the shaft, connecting to the tunica albuginea dorsally and ventrally. It is composed of the same elastic fibers as the tunica albuginea and it allows free communication between the 2 corpora cavernosa.
The tunica albuginea is a tough fibrous layer that surrounds the corpora cavernosa. It is composed of 2 layers of elastic tissue and collagen. This layer gives the penis rigidity as the intracorporeal pressure increases. The plaque of Peyronie disease (PD) develops in this layer, although the plaque can also extend into the underlying corporeal tissue.
The deep dorsal vein lies in the dorsal sulcus between the 2 corpora. It is flanked on either side by a dorsal artery and a dorsal nerve bundle. The latter can either be in the form of a large visible nerve running lateral to each artery, or it can be fanned out into several fine nerve strands that progress distally along the dorsolateral aspect of the penile shaft. This second scenario can complicate reconstructive surgery because of the heightened chance of injuring one or more of these strands, resulting in anesthesia of some of the penile skin.
Lying in the ventral sulcus is the third erectile body, the corpus spongiosum. This envelops the urethra and is continuous distally with the glans penis. The corpus spongiosum is encased in Buck fascia, a tough elastic layer that continues dorsally to also enclose the corpora cavernosa, superficial to the tunica albuginea, the deep dorsal vein, and the dorsal arteries and nerves.
The next superficial layer is the Dartos fascia. This is composed of loose areolar tissue that allows free and easy movement of the skin over the penile shaft. Within this layer are contained the superficial veins of the penis. A prominent vein in this layer is the superficial dorsal vein, which is often visible through the penile skin. The outermost layer is composed of penile skin that folds back upon itself distally to form the prepuce.
Contraindications
In general, contraindications to treatment of Peyronie disease (PD) are based on the severity of signs and symptoms. If little or no loss of penile rigidity is present and if the curvature is minimal and does not compromise function, then intervention is contraindicated. In this setting, intervention is not likely to enhance function or appearance, although it will expose the patient to potential adverse effects, including a worsening of the symptoms.
Topical and/or oral treatments are contraindicated in any person who has a known hypersensitivity to any specific agent. Injectable agents are generally tolerated well and appear to have little if any systemic effects because of the small amounts injected. As with topical and oral agents, they should not be used in any person who has demonstrated an allergic reaction to the particular medication contemplated.
Surgical treatment is contraindicated in men who have minimal symptoms. In addition, surgery should not be used when the disease is not yet stable. If plaque excision is performed before the process of fibrosis is complete, the procedure is doomed to fail because further plaque and subsequent curvature will develop.
Implantation of a penile prosthesis is contraindicated in any person with an ongoing infection anywhere in the body. In addition to the contraindications mentioned above, an inflatable prosthesis is contraindicated in a patient who lacks the manual dexterity to operate the prosthesis.
-
Penile angulation indicative of Peyronie disease. Courtesy of Allen Seftel, MD.
-
MRI of the penis in the axial plane (T1-weighted image). The penis is in the erect position with the corpus spongiosum located ventrally (upper part of the frame). The tunica albuginea can be seen as the dark band outlining the corpora cavernosa. The tunica albuginea appears irregular and heterogeneous dorsally, which is consistent with the presence of a fibrous plaque. The image on the left is without annotation. The image on the right is identical but with annotation. Courtesy of Evan H. Dillon, MD
-
Precontrast MRI of the penis in the axial plane (T1-weighted image with fat saturation). Preaxial image demonstrates lack of definition of the tunica albuginea in the dorsal aspect of the penis. The image on the left is without annotation. The image on the right is identical but with annotation. Courtesy of Evan H. Dillon, MD.
-
Postcontrast MRI of the penis in the axial plane (T1-weighted image with fat saturation). Image obtained after the injection of gadolinium demonstrates enhancement of the tunica albuginea in the dorsal aspect of the penis. Enhancement reflects the presence of active inflammation in the region of the plaque. The image on the left is without annotation. The image on the right is identical but with annotation. Courtesy of Evan H. Dillon, MD.
-
Skin is incised through previous circumcision and retracted. Artificial erection is achieved with injection of normal saline via butterfly with tourniquet at base of penis. This demonstrates 45° curvature of the penile shaft to patient's left.
-
Buck's fascia has been incised along the convex, right side of the penile shaft to expose the tunica albuginea. The right dorsal neurovascular bundle has been dissected from the tunica albuginea (left side of figure). Parallel incisions have been made through the tunica albuginea to allow plication of the convex side of the shaft.
-
The isthmus of tunical tissue has been buried by suturing the proximal side of the proximal incision to the distal side of the distal incision. Another set of incisions were placed distal to the original set for further correction of the curvature. These were then sutured in a similar fashion. The second suture line can be seen closer to the corona.
-
Buck fascia and the skin layer have each been reapproximated. A repeat artificial erection demonstrates correction of the curvature. The penis is then dressed with Xeroform gauze and Coban dressing.
-
Cross-section view of the penis is shown.