Overview
Noninvasive ventilation (NIV) refers to the administration of ventilatory support without using an invasive artificial airway (endotracheal tube or tracheostomy tube). The use of noninvasive ventilation (see the video below) has markedly increased over the past two decades, and noninvasive ventilation has now become an integral tool in the management of both acute and chronic respiratory failure, in both the home setting and in the critical care unit. Noninvasive ventilation has been used as a replacement for invasive ventilation, and its flexibility also allows it to be a valuable complement in patient management. Its use in acute respiratory failure is well accepted and widespread. It is the focus of this review. The role of noninvasive ventilation in those with chronic respiratory failure is not as clear and remains to be defined.
Historical background
An interest in the methods of artificial respiration has long persisted, stimulated by attempts at resuscitation of drowning victims. Reports dating from the mid 1700s document a bellows-type device being the most commonly used form of respiratory assistance. Negative-pressure tank-type ventilators came into use in the next century, with a prototype developed by Dalziel in 1832. This spawned a variety of cuirass and tank negative-pressure ventilators, with the general principle of enclosing the thorax, creating negative pressure to passively expand the chest wall and lungs. This led to the Drinker-Shaw iron lung in 1928, which was the first widely used negative-pressure ventilator. In 1931, Emerson modified these large devices, and the Emerson tank ventilator became the standard for ventilatory support. The Emerson tank ventilator was especially crucial in the treatment of poliomyelitis victims.
Rudimentary devices that provided continuous positive airway pressure were described in the 1930s, but the negative-pressure ventilators were the predominant method of ventilatory support until the polio epidemics overwhelmed their capacity in the 1950s. Development of positive-pressure valves delivered through tracheostomy tubes permitted the delivery of intermittent positive pressure during inspiration. This quickly replaced the negative-pressure ventilators, further supported by the development of the cuffed endotracheal tube and bedside ventilators. However, positive-pressure ventilation delivered through either a translaryngeal endotracheal tube or a tracheostomy tube was also associated with a host of complications, specifically injury to the larynx and trachea, as well as other issues involving the timing of extubation, preservation of speech, and the ability to continue swallowing.
In the 1980s, increasing experience with positive-pressure ventilation delivered through a mask in patients with obstructive sleep apnea led to this type of ventilatory support, initially in patients with neuromuscular respiratory failure. Success led to its adoption in other conditions, and noninvasive ventilation became especially promising in the treatment of patients with decompensated chronic obstructive pulmonary disease. In the ensuing 20 years, noninvasive positive-pressure ventilation delivered via a mask has been widely adopted, to the point where it is a first-line therapy in some medical centers. The conditions and patients best suited for noninvasive ventilation are discussed.
Note the images below.
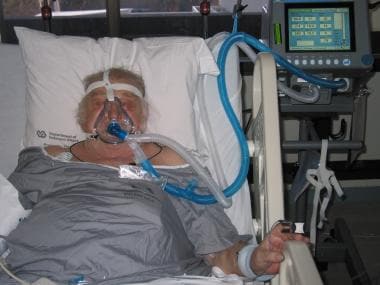
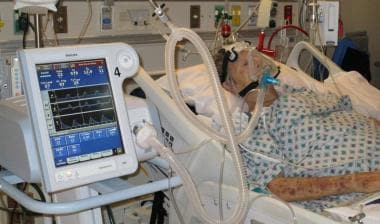 Patient with decompensated congestive heart failure undergoing noninvasive ventilatory support with BiPAP ventilation and an orofacial mask.
Patient with decompensated congestive heart failure undergoing noninvasive ventilatory support with BiPAP ventilation and an orofacial mask.
Methods of Delivery
Noninvasive positive-pressure ventilation
Positive-pressure ventilation delivered through a mask has become the predominant method of providing noninvasive ventilatory support and is the focus of this and subsequent sections. Early bedside physiologic studies in healthy patients and in patients with respiratory conditions document successful ventilatory support (ie, reduction in respiratory rate, increase in tidal volume, decrease in dyspnea) with reduction in diaphragmatic electromyography (EMG), transdiaphragmatic pressures, work of breathing and improvement in oxygenation with a reduction in hypercapnia.
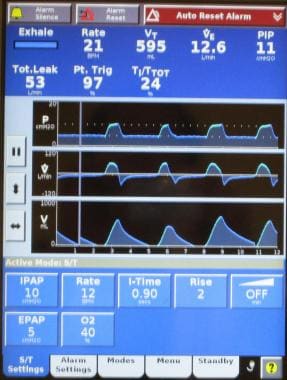
Ventilatory support can be achieved through a variety of interfaces (mouth piece or nasal, face, or helmet mask), using a variety of ventilatory modes (eg, volume ventilation, pressure support, bilevel positive airway pressure [BiPAP; see the image below], proportional-assist ventilation [PAV], continuous positive airway pressure [CPAP]) with either ventilators dedicated to noninvasive ventilation (NIV) or those capable of providing support through an endotracheal tube or mask. Older models of noninvasive ventilators required oxygen to be bled into the system, but current models incorporate oxygen blenders for precise delivery of the fraction of inspired oxygen (FIO2).
Noninvasive negative-pressure ventilation
Negative-pressure ventilators provide ventilatory support using a device that encases the thoracic cage starting from the neck, and devices range from a whole-body tank to a cuirass shell. The general principal is the same with a vacuum device, which lowers the pressure surrounding the thorax, creating subatmospheric pressure and thereby passively expanding the chest wall with diaphragmatic descent, all leading to lung inflation. Exhalation occurs with passive recoil of the chest wall.
This was the predominant technology during the polio epidemics, but these devices were bulky and cumbersome to use. Upper airway obstruction was also a problem. These ventilators have been largely supplanted by the more widespread positive-pressure noninvasive ventilators; however, some patients continue to be treated with this modality. While the bulk of the experience lies in patients with chronic respiratory failure, specifically neuromuscular respiratory failure, reports described successful application in patients with acute respiratory failure.
With respect to the two modes, positive-pressure ventilation has supplanted negative-pressure ventilation as the dominant mode of delivery of noninvasive ventilation. Positive-pressure ventilation is more effective than negative-pressure ventilation in unloading the respiratory muscles, at least under investigational conditions. The primary focus of this article is positive-pressure noninvasive ventilation, and the mention of "noninvasive ventilation" will refer to positive-pressure delivery. That being stated, the reader should be aware that in certain patients and under certain circumstances, negative-pressure ventilatory support may also be acceptable. [1]
High-flow nasal oxygen
As a result of prospective, randomized clinical trials, another option has emerged for the patient with hypoxemic respiratory failure. Heated, humidified, high-flow nasal cannula oxygen (HFNC) has been available for over a decade, but refinements and increasing clinical experience have made it a solid alternative for management that exists in the spectrum of options before noninvasive and invasive mechanical ventilation. This modality was initially developed for neonatal patients, and refinements have permitted its use in adults. Conventional oxygen therapy is not well tolerated at high flow rates because of problems with unheated and nonhumidified oxygen. The high-flow nasal cannula oxygen systems are able to heat and humidify, improving patient tolerance and comfort. The high flow rates have other advantages in that high flow rates minimize room air entrainment, thereby increasing the FIO2 that can be provided to patients; are able to wash out dead space carbon dioxide, improving the efficiency of oxygen delivery; and the increased flow rate translates into positive end-expiratory pressure (PEEP). The amount of PEEP provided is a function of the flow rate but falls somewhere in the range of 0.35-0.69 cm water for each 10 L/min of increased flow rate. [2] Therefore, while high-flow nasal cannula devices technically do not provide assisted support or augment inspired tidal volume as provided by the other forms of mechanical ventilation, the small amount of positive pressure provided does help reduce the work of breathing and improve breathing patterns similarly to that achieved with CPAP. An intact respiratory drive is required with this modality, which means that it is not suited for patients with hypoventilation or a blunted respiratory drive. It is reasonable to consider this modality as a method of providing low-level positive pressure. While this is not assisted ventilation, it is at its most rudimentary level, is a form of noninvasive ventilation.
The focus of this review is on noninvasive ventilation provided through a mask-ventilator interface, but it is important to recognize that the option of high-flow nasal cannula oxygen exists and may be a viable option for some patients. It seems especially suited to the recently extubated, postoperative patient and those with mild-to-moderate hypoxemic respiratory failure as may occur in patients with decompensated heart failure. [3] There may be efficacy in other conditions. Additional comments have been included to highlight additional clinical features unique to high-flow nasal cannula oxygen.
General Considerations
The key to the successful application of noninvasive ventilation is in recognizing its capabilities and limitations. This also requires identification of the appropriate patient for the application of noninvasive ventilation (NIV). Patient selection is crucial for the successful application of noninvasive ventilation. Once patients who require immediate intubation are eliminated, a careful assessment of the patient and his or her condition determines if the patient is a candidate for noninvasive ventilation. This requires evaluation on several levels, and it may involve a trial of noninvasive ventilation. The following variables and factors help identify patients who may be candidates for noninvasive positive-pressure ventilation.
Absolute contraindications are as follows:
-
Coma
-
Cardiac arrest
-
Respiratory arrest
-
Any condition requiring immediate intubation
Other contraindications (rare exceptions) are as follows:
-
Cardiac instability - Shock and need for pressor support, ventricular dysrhythmias, complicated acute myocardial infarction
-
GI bleeding - Intractable emesis and/or uncontrollable bleeding
-
Inability to protect airway - Impaired cough or swallowing, poor clearance of secretions, depressed sensorium and lethargy
-
Status epilepticus
-
Potential for upper airway obstruction - Extensive head and neck tumors, any other tumor with extrinsic airway compression, angioedema or anaphylaxis causing airway compromise
Other considerations that may limit application are as follows:
-
Implementation - Staff learning curve and time requirements (nursing and respiratory therapy), potential for delay in definitive therapy (limit trials of therapy)
After eliminating unsuitable candidates for noninvasive ventilation, successful application of noninvasive ventilation mandates close assessment and selection of patients and identification of conditions best suited for treatment. Not all patients with diagnoses capable of management with noninvasive ventilation (eg, chronic obstructive pulmonary disease [COPD]) are suitable candidates for treatment with noninvasive ventilation. Patients with mild disease or very severe distress may not benefit from noninvasive ventilation, which is best suited for patients with a moderate severity of illness.
Patient inclusion criteria are as follows:
-
Patient cooperation (an essential component that excludes agitated, belligerent, or comatose patients)
-
Dyspnea (moderate to severe, but short of respiratory failure)
-
Tachypnea (>24 breaths/min)
-
Increased work of breathing (accessory muscle use, pursed-lips breathing)
-
Hypercapnic respiratory acidosis (pH range 7.10-7.35)
-
Hypoxemia (PaO2/FIO2< 200 mm Hg, best in rapidly reversible causes of hypoxemia)
Not all respiratory conditions are suitable for treatment with noninvasive ventilation. Conditions that have garnered the most experience and success are generally conditions that also respond relatively quickly to treatment, for which noninvasive ventilation provides an important adjunctive support to other simultaneously administered therapeutics. These are listed below and are discussed in subsequent sections. Be aware that the list and indications continues to change as more experience is accumulated in these and newer conditions.
Suitable clinical conditions for noninvasive ventilation (most patients) are as follows:
-
Chronic obstructive pulmonary disease
-
Cardiogenic pulmonary edema
Suitable clinical conditions for noninvasive ventilation (selected patients) are as follows:
-
After discontinuation of mechanical ventilation (COPD)
-
Community-acquired pneumonia (and COPD)
-
Immunocompromised state (known cause of infiltrates)
-
Postoperative respiratory distress and respiratory failure
-
Do-not-intubate status
-
Neuromuscular respiratory failure (better in chronic than acute; avoid if upper airway issues)
-
Decompensated obstructive sleep apnea/cor pulmonale
-
Cystic fibrosis
-
Mild Pneumocystic carinii pneumonia
-
Rib fractures
Use with caution in the following clinical conditions:
-
Idiopathic pulmonary fibrosis (exacerbation)
-
Acute respiratory distress syndrome (consider helmet ventilation)
Application of Noninvasive Ventilation
Several considerations can enhance the likelihood of successful noninvasive ventilation (NIV). In addition to these factors, the experience and expertise of front-line health care providers, specifically nursing and respiratory therapy staff, cannot be underestimated. This is not a concern in hospitals where noninvasive ventilation is well established, but it is an important factor in facilities where noninvasive ventilation has been infrequently administered or not used at all.
Location of application
It can be used in the ICU, especially if there is the possibility of intubation.
It can be used in a step-down unit (lower severity of illness), as follows:
-
Moderately severe COPD (pH >7.30)
-
Do-not-intubate status
-
Intermittent or nocturnal ventilatory support
It can be used in the ward setting (not recommended if intubation is a consideration), as follows:
-
Suitable in specialized units
-
Same considerations as step-down unit
-
Emergency department - Local considerations, expertise may mirror ICU or step-down unit
Patient interfaces
In its simplest terms, noninvasive ventilation differs from invasive ventilation by the interface between the patient and the ventilator. Invasive ventilatory support is provided via either an endotracheal tube or tracheostomy tube. Noninvasive ventilatory support uses a variety of interfaces, and these have continued to evolve with modifications based on patient comfort and efficacy. Many of the interfaces or masks were initially used in patients with obstructive sleep apnea before they were adapted for use in patients to provide noninvasive ventilatory support.
Nasal masks and orofacial masks were the earliest interfaces, with subsequent development and use of full face masks, mouthpieces, nasal pillows, and helmets. Nasal masks and orofacial masks are still the most commonly used interfaces. Orofacial masks are used almost twice as frequently as nasal masks. Both have advantages and disadvantages in the application of noninvasive ventilation.
Note the images below.
Proper fitting of the mask or other interface is another key component to successful noninvasive ventilation. The mask or interface may be held in place (without straps applied) by the patient or therapist to familiarize the patient with the mask and ventilator. Typically, the smallest mask providing a proper fit is the most effective. Straps hold the mask in place, with care to minimize excess pressure on the face or nose. Leaks are the bane of all of the interfaces, but excess pressure applied with the straps increases the risk of pressure necrosis and skin breakdown. Straps should be tight enough to prevent leaks, but with enough slack to allow passage of one or two fingers between the face and the straps.
The nasal and orofacial masks may be first-line and second-line options, specifically in patients who may have prior familiarity with these interfaces or in those who may have had difficulty with orofacial or nasal masks. Clinical trials have not demonstrated the superiority of any interface, although the nasal mask may be more effective in patients with a lower severity of illness. [4] In patients with a higher severity of illness, the orofacial mask and total face mask appear to result in comparable outcomes.
The main considerations regarding the choice of an orofacial mask or nasal mask are outlined below.
Orofacial mask general advantages are as follows:
-
Best suited for less cooperative patients
-
Better in patients with a higher severity of illness
-
Better for patients with mouth-breathing or pursed-lips breathing
-
Better in edentulous patients
-
Generally more effective ventilation
Orofacial mask cautions and disadvantages are as follows:
-
Claustrophobic
-
Hinder speaking and coughing
-
Risk of aspiration with emesis
Nasal mask general advantages are as follows:
-
Best suited for more cooperative patients
-
Better in patients with a lower severity of illness
-
Not claustrophobic
-
Allows speaking, drinking, coughing, and secretion clearance
-
Less aspiration risk with emesis
-
Generally better tolerated
Nasal mask cautions and disadvantages are as follows:
-
More leaks possible (eg, mouth-breathing or edentulous patients)
-
Effectiveness limited in patients with nasal deformities or blocked nasal passages
While orofacial masks and nasal masks are the most commonly used interfaces, other patient ventilator interfaces through which noninvasive ventilation can be applied include mouthpieces, nasal pillows, total face masks, and even a helmet device, which encompasses the entire head. Experience with helmet devices is limited but increasing, and it has been successful in patients who are unable to tolerate the nasal or orofacial devices.
Ventilators
The choice of ventilators available to provide noninvasive ventilatory support has continued to expand. Early noninvasive ventilatory support was applied using either large bedside critical care volume ventilators or smaller volume or pressure specialty ventilators devoted to noninvasive ventilation. While the critical care ventilators had more options, they were also less tolerant of leaks. The specialty ventilators had fewer options and range, but they were more leak tolerant.
Many critical care ventilators currently in use also have a noninvasive ventilation option, either as part of the original device or available as an upgrade option. The ideal device is dependent on a number of factors, including familiarity by staff and available options. The differences between the bedside critical care ventilator and specialty noninvasive ventilator continue to diminish as differences related to ventilator options, range of support, and leak tolerance are corrected in both devices. The distinction in function and capability has blurred, and there are devices that are capable of providing both invasive and noninvasive ventilation with a mere switch of ventilator settings. Nevertheless, most hospitals continue to provide noninvasive support with the specialty ventilator.
Modes of ventilation
Choosing the initial mode of ventilation is based in part on past experience, in part on the capability of ventilators available to provide support, and in part on the condition being treated. Most patients who are provided noninvasive ventilation are provided support with pressure ventilation, with continuous positive airway pressure (CPAP), which is the most basic level of support. CPAP may be especially useful in patients with congestive heart failure or obstructive sleep apnea.
Bilevel positive airway pressure (BiPAP) is probably the most common mode noninvasive positive pressure ventilation and requires provisions for inspiratory positive airway pressure (IPAP) and expiratory positive airway pressure (EPAP). The difference between IPAP and EPAP is a reflection of the amount of pressure support ventilation provided to the patient, and EPAP is synonymous with positive end-expiratory pressure (PEEP). Some noninvasive ventilation is provided using proportional-assist ventilation (PAV), which provides flow and volume assistance with each breath. Clinical trials have not demonstrated a significant difference between PAV and pressure-support ventilation with BiPAP. [5, 6] However, BiPAP is the most commonly available and more frequently used modality for noninvasive ventilation. PAV remains available on many ventilator models, but use is much less common than BiPAP.
While volume ventilators can be used to provide noninvasive ventilatory support, the previously described modes are preferred because they provide better patient comfort and synchrony and are more tolerant of the leaks that accompany all noninvasive ventilatory interfaces.
Initial ventilator settings and adjustments
Adequate ventilation and oxygenation, correction of respiratory failure, and adequate patient tolerance and comfort are the primary goals of noninvasive ventilation, and adjustments are made to achieve these endpoints. Initial settings focus on achieving adequate tidal volumes, usually in the range of 5-7 mL/kg. Additional support is provided to reduce the respiratory rate to less than 25 breaths/minute. Oxygen is adjusted to achieve adequate oxygenation, with a pulse oximetry goal of greater than 90%. Serial arterial blood gas measurements are essential to monitor the response to therapy and to guide further adjustments in the ventilator. The following provides some guidance on titration of ventilator settings in patients with respiratory distress and who have never been placed on noninvasive ventilation. In those patient who may have chronic noninvasive support, the initial values should be based on prior support levels. The listed levels may be inadequate and would thus increase the likelihood of intolerance or failure. If there is uncertainty, it is important to perform a bedside titration with increasing levels based on patient comfort or exhaled tidal volumes. These adjustments can be made within minutes and can be done without obtaining blood gases.
Initial IPAP/EPAP settings are as follows:
-
Start at 10 cm water/5 cm water
-
Pressures less than 8 cm water/4 cm water not advised as this may be inadequate
-
Initial adjustments to achieve tidal volume of 5-7 mL/kg (IPAP and/or EPAP)
Subsequent adjustments based on arterial blood gas values are as follows:
-
Increase IPAP by 2 cm water if persistent hypercapnia
-
Increase IPAP and EPAP by 2 cm water if persistent hypoxemia
-
Maximal IPAP limited to 20-25 cm water (avoids gastric distension, improves patient comfort)
-
Maximal EPAP limited to 10-15 cm water
-
FIO2 at 1.0 and adjust to lowest level with an acceptable pulse oximetry value
-
Back up respiratory rate 12-16 breaths/minute
Pressure control (PC) and average volume assured pressure support (AVAPS) ventilation
The above considerations and approach to adjustment are best suited for those with COPD or chronic heart failure as the primary cause of their hypercapnia or hypoxemic respiratory distress and failure. Patients with neuromuscular disorders (amyotrophic lateral sclerosis, postpolio syndrome, muscular dystrophy) or thoracic cage disorders (severe kyphoscoliosis) may fare better with other ventilatory modalities. The most current noninvasive ventilators have PC or average AVAPS options. In PC ventilation, both the inspiratory pressure and the inspiratory time are set and fixed. This differs from BiPAP in which the patient controls the inspiratory time. This modality may be useful in the neuromuscular disease patient who does not have the respiratory muscle strength to generate an adequate inspiratory time. Setting an increased inspiratory time may increase the tidal volume provided, but it may also increase patient-ventilator dyssynchrony if the set inspiratory time is longer than the patient's desired inspiratory time.
AVAPS is another option in these neuromuscular disease patients and has also been used in those with severe obesity-hypoventilation syndrome. It should be noted that AVAPS is not generally used for those patients with acute respiratory distress and is better suited for management as they recover or have recovered from their acute decompensated state. Although most experience with AVAPS in COPD is with chronic respiratory failure, some investigators have noted favorable outcomes when used in acutely decompensated COPD patients. [7]
As with any pressure-cycled mode, the dependent variable is volume and it may vary widely if there is patient dyssynchrony, changes in lung compliance, or changes in resistance that can occur with changes in body position that occurs in the very morbidly obese. [8] A fixed pressure support setting will not compensate for these changes, and, as a result, delivered tidal volume will fall. AVAPS allows a target tidal volume to be identified with a range of pressure support settings that fluctuate to meet the target tidal volume. AVAPS uses an internal algorithm to make changes in the pressure support supplied to achieve the target volume, but these changes are small and occur over minutes (typically 1-2.5 cm water per minute). That is why rapidly changing, acute respiratory conditions are not suited for AVAPS as the ventilator adjustments may not be timely enough to meet the patient's requirements. Typically, the pressure support required to produce the target volume during bedside titration is used to identify the minimal pressure with the set minimal pressure (min P), typically 2-3 cm water lower to allow flexibility for adjustment in the AVAPS mode. The maximal pressure (max P) is typically set in the 20-25 cm water range as higher pressures are not well tolerated. The min P is at least 8 cm water and usually higher. Additional parameters that are part of AVAPS setting are the target tidal volume, respiratory rate, EPAP, and inspiratory time.
Predictors of successful noninvasive ventilation
Importantly, recognize that certain parameters may predict successful noninvasive ventilation or failure of noninvasive ventilation, so that patients are not subjected to continued treatment when optimal treatment requires intubation and mechanical ventilation. This includes changes during a trial of noninvasive ventilation. The changes, in turn, are a reflection of the patient's ability to cooperate with noninvasive ventilation, patient-ventilatory synchrony, and noninvasive ventilation effectiveness. Trials of noninvasive ventilation are usually 1-2 hours in length and are useful to determine if a patient can be treated with noninvasive ventilation. Extended trials without significant improvement are not recommended because this only delays intubation and mechanical ventilation (unless patients are do-not-intubate status).
Predictors of success, with a response to a trial of NIV (1-2 h), are as follows:
-
Decrease in PaCO2 greater than 8 mm Hg
-
Improvement in pH greater than 0.06
-
Correction of respiratory acidosis
Predictors of failure are as follows:
-
Severity of illness - Acidosis (pH < 7.25), hypercapnia (>80 and pH < 7.25), Acute Physiology and Chronic Health Evaluation II (APACHE II) score higher than 20
-
Level of consciousness - Neurologic score (>4 = stuporous, arousal only after vigorous stimulation; inconsistently follows commands), encephalopathy score (>3 = major confusion, daytime sleepiness or agitation), Glasgow Coma Scale score lower than 8
-
Failure of improvement with 12-24 hours of noninvasive ventilation
Late admission predictors of failure (>48 h after initiation of noninvasive ventilation) are as follows:
-
Lower functional status (Activity score < 2 = dyspnea light activity)
-
Initial acidosis (pH ≤7.22)
-
Hospital complications (pneumonia, shock, coma)
Certain patients may benefit from a trial of therapy; however, limiting trials is important to avoid delays in definitive therapy. Trials may be as short as a few minutes, in patients with immediate failure, and probably should not exceed 2 hours if patients fail to improve.
Objective criteria for discontinuation are important to limit trials in patients in whom noninvasive ventilation ultimately fails. This specifically refers to intubation criteria, which carry a subjective element but have been defined in the literature in investigational studies. All these criteria are subject to some degree of interpretation in the context of the patient's clinical status. Importantly, recognize the following as guidelines to assist with the decision to intubate a patient. Most patients who meet these criteria are candidates for intubation, but a few may be able to be managed with continued noninvasive ventilation.
Prediction of failure to high-flow nasal cannula oxygen
Although the focus has been on noninvasive ventilation (NIV), there has been increasing use of high-flow nasal cannula (HFNC) oxygen in clinical situations where NIV had previously been used. This also warrants objective measures that may identify patients in whom HFNC oxygen support fails and who require intubation. This is important, as delayed intubation of patients with progressive hypoxemic respiratory failure has been associated with increased mortality. This led to the identification of the ROX index, defined as the ratio of pulse oximetry oxygen saturation and fraction of inspired oxygen to respiratory rate [(SpO2/FIO2)/RR]. In an inception and validation cohort of patients with pneumonia and on HFNC oxygen, values of the ROX index greater than 4.88 measured at 2, 6, and 12 hours, 18 and 24 hours after initiation of HFNC were found to identify those patients who would not need intubation. [9] Lower ROX index scores not only identified those requiring intubation, but was also associated with poor outcomes, specifically mortality. The authors identified the hours between the 12th and 24th hours as the most vulnerable in their cohort, with an increased risk of failure in those with ROX index scores lower than 3.85.
Intubation criteria
Major criteria (any one of the following) are as follows [10, 11] :
-
Respiratory arrest
-
Loss of consciousness with respiratory pauses
-
Gasping for air
-
Psychomotor agitation requiring sedation
-
Heart rate less than 50 bpm with loss of alertness
-
Hemodynamic instability with systolic blood pressure less than 70 mm Hg
Minor criteria (two of the following) are as follows:
-
Respiratory rate greater than 35 breaths/minute
-
pH less than 7.25 and decreased from onset
-
PaO2 less than 45 mm Hg despite oxygen
-
Increase in encephalopathy or decreased level of consciousness
Intubation guidelines
Any one of the following [12] :
-
pH less than 7.20
-
pH 7.20–7.25 on 2 occasions 1 hour apart
-
Hypercapnic coma (Glasgow Coma Scale score < 8 and PaCO2 >60 mm Hg)
-
PaO2 less than 45 mm Hg
-
Cardiopulmonary arrest
Two or more of the following in the context of respiratory distress:
-
Respiratory rate greater than 35 breaths/minute or less than 6 breaths/minute
-
Tidal volume less than 5 mL/kg
-
Blood pressure changes, with systolic less than 90 mm Hg
-
Oxygen desaturation to less than 90% despite adequate supplemental oxygen
-
Hypercapnia (PaCO2 >10 mm increase) or acidosis (pH decline >0.08) from baseline
-
Obtundation
-
Diaphoresis
-
Abdominal paradox
Noninvasive Ventilation in COPD
Patients with underlying chronic obstructive pulmonary disease (COPD) who present with an exacerbation of their COPD and hypercapnic respiratory distress or respiratory failure are the group most likely to be successfully treated with noninvasive ventilation (NIV). Exacerbations increase the work of breathing in these patients and may exceed the patient's ability to adequately ventilate through a variety of mechanisms, including increasing hyperinflation with decreased diaphragmatic excursion and strength, increasing intrinsic positive end-expiratory pressure (PEEP), ineffective or inadequate tidal volume generation, respiratory patterns, and increased respiratory frequency. Noninvasive ventilation effectively unloads the respiratory muscles, increasing tidal volume, decreasing the respiratory rate, and decreasing the diaphragmatic work of breathing, which translates to an improvement in oxygenation, a reduction in hypercapnia, and an improvement in dyspnea.
Noninvasive ventilation is an important adjunct to other conventional therapy (eg, bronchodilators, corticosteroids, antibiotics). COPD is an ideal condition for noninvasive ventilation, given the rapid reversibility with treatment and added support that can be provided by noninvasive ventilation. Most experience with noninvasive ventilation has accrued with either bilevel positive airway pressure (BiPAP) or pressure support ventilation, less so with volume ventilation and continuous positive airway pressure (CPAP), which is infrequently used as a mode of ventilatory support in these patients.
While prospective randomized trials have involved relatively small numbers of patients (< 1000 total), a consistent treatment benefit has been demonstrated, and noninvasive ventilation has been recommended as first-line therapy in the management in COPD patients with hypercapnic respiratory failure. Systematic reviews and meta-analyses have all come to the same conclusion. Noninvasive ventilation reduces the need for intubation, mortality, complications, and length of stay in patients with COPD.
However, the magnitude of the benefit of noninvasive ventilation differs given some inconsistencies in the included studies. The largest review concluded that noninvasive ventilation decreased the intubation rate by 28% (95% confidence interval [CI], 15-40%), in-hospital mortality rate by 10% (95% CI, 5-15%), and absolute reduction in length of stay by 4.57 days (95% CI, 2.30-6.38 d). [13] The benefit was most pronounced in patients with more severe COPD exacerbations, defined by an initial pH of less than 7.30. The magnitude of effect was even more pronounced in this group, with intubation rates decreased by 34% (95% CI, 22-46%), mortality reduction of 12% (95% CI, 6-18%), and absolute reduction in the length of stay by 5.59 days (95% CI, 3.66-7.52 d). Investigations with less severely affected patients did not demonstrate any benefit in any of these outcomes.
In another review, greater improvement in respiratory acidosis, hypercapnia, and tachypnea was noted after 1 hour on noninvasive ventilation, along with fewer complications related to intubation. [14]
Additional experience with noninvasive ventilation in hypercapnic COPD has helped to identify possible thresholds for its application. Severely hypercapnic patients with severe respiratory acidosis and lethargy or even frank coma related to the hypercapnia were often excluded from trials of noninvasive ventilation because of concerns for progressive respiratory failure and an inability to cooperate with noninvasive ventilation as a result of their carbon dioxide narcosis.
In some centers, patients with an initial pH of less than 7.25 and a Glasgow Coma Scale score of less than 11 had noninvasive ventilation failure rates of 70% or greater. [15] Some report successful application of noninvasive ventilation in patients with a Glasgow Coma Scale score less than 8 and an average pH of 7.13 ± 0.06 (mean ± standard deviation), with 76 (80%) of 95 patients responding to treatment with noninvasive ventilation. [16] Others have had less success, grading sensorium using a Kelly score of 4 or more defined as a stuporous patient, only intermittently able to follow commands, and an average pH of 7.22, where the success rate was 55% in a group of 20 patients. [17] However, in all of these series, improvement after 1-2 hours noninvasive ventilation was predictive of success.
Local experience and expertise also play significant roles in determining the successful limits of noninvasive ventilation in COPD patients. Patients who are not cooperative and have a pH that approaches 7.20 must be evaluated with caution because they have a higher risk of failure with noninvasive ventilation and would therefore benefit from earlier intubation (if an option), especially if they do not respond to a short trial of noninvasive ventilation.
Another benefit with noninvasive ventilation may be a reduction in nosocomial infections associated with its application. This was a finding suggested by earlier investigations, because averting endotracheal intubation also avoids a major risk factor for ventilator-associated pneumonia (ie, the endotracheal tube). Experience in a case-control study suggests a reduction in nosocomial pneumonia from 22% to 8%, with fewer days in the ICU and lower mortality (26% down to >4%) in those treated with noninvasive ventilation as opposed to those who received endotracheal intubation. [18, 19]
The case for first-line use of noninvasive ventilation in the management of acute exacerbations of COPD has been further supported by review of large administrative databases. [20, 21] Noninvasive ventilation has better outcomes than invasive mechanical ventilation when used as the initial treatment in those with acute exacerbations of COPD on several levels, including length of stay, hospital-acquired pneumonia, and mortality. However, those in whom noninvasive ventilation fails and who require intubation have a worse outcome, about three times higher than those initially treated with noninvasive ventilation. However, successfully treated patients do not have the same severity of illness as those who are initially subject to intubation and mechanical ventilation, and those in whom noninvasive ventilation fails likely represent a group whose worse outcomes are further testaments to their borderline status. [22] This further emphasizes the importance of proper patient selection for noninvasive ventilation, with the best-suited patients identified from the clinical trials summarized below.
Summary
COPD is the most suitable condition for noninvasive ventilation.
Noninvasive ventilation is most effective in patients with moderate-to-severe disease
Hypercapnic respiratory acidosis may define the best responders (pH 7.20-7.30). Noninvasive ventilation is also effective in patients with a pH of 7.35-7.30, but no added benefit is appreciated if the pH is greater than 7.35. The lowest threshold of effectiveness is unknown, but success has been achieved with pH values as low as 7.10.
Obtunded COPD patients can be treated, but the success rate is lower.
Improvement after a 1- to 2-hour trial may predict success.
Also see the clinical guidelines summary, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. [23]
Noninvasive Ventilation in Cardiogenic Pulmonary Edema
Respiratory insufficiency due to cardiogenic pulmonary edema or congestive heart failure (CHF) is another condition that is effectively treated with noninvasive ventilation (NIV). Respiratory failure due to heart failure is potentially a rapidly reversible condition, similar in its reversibility to decompensated chronic obstructive pulmonary disease (COPD), and noninvasive ventilation is an ideal adjunct to the other treatments used in the management of CHF.
The pathophysiology of respiratory failure in CHF is related to a combination of pulmonary vascular congestion, interstitial edema, and alveolar fluid accumulation. This leads initially to hypoxemic respiratory failure, and patients with CHF who further deteriorate manifest hypercapnic respiratory failure. Positive-pressure ventilation is beneficial because it recruits alveoli, increases functional residual capacity, and allows breathing on the more compliant portion of the lung's pressure-volume curve, thereby decreasing the work of breathing, improving ventilation-perfusion relationships, and eventually correcting hypoxemia and hypercapnia. Positive intrathoracic pressure also decreases preload and left ventricular afterload, both beneficial effects in patients with intravascular volume overload.
These beneficial effects can be achieved with continuous positive airway pressure (CPAP), which has been recommended as a first-line therapy in CHF patients. The other ventilator modalities, such as bilevel positive airway pressure (BiPAP), pressure support ventilation, or volume ventilation, have also been used and some controversy exists regarding their efficacy when compared with CPAP.
Note that CPAP has long been recognized as effective in the management of CHF, with initial reports dating from as early as 1938 using very simple pressure devices. Randomized prospective trials comparing its efficacy with oxygen were not conducted for almost 50 years, and small trials also confirmed its effectiveness in correcting gas exchange abnormalities, even in patients with profound respiratory acidosis, with a general benefit of both a reduction in intubation rates and mortality rates.
The experience with noninvasive ventilation provided using BiPAP or pressure support modalities, however, has been mixed. Some investigators found no benefit with their applied noninvasive ventilation, and some noted more complications, specifically higher rates of myocardial infarction. Other investigators found greater benefit in symptom relief and oxygenation but no differences in intubation rates or mortality rates or benefits in a post hoc analysis involving hypercapnic patients. Meta-analyses do suggest a benefit with CPAP, with a risk reduction in intubation of 60% (RR, 0.40; 95% CI, 0.27-0.58) and a decrease in mortality rate of 47% (RR, 0.53; 95% CI, 0.35-0.81). Noninvasive ventilation has also demonstrated a risk reduction in intubation rates of 52% (RR, 0.48; 95% CI, 0.34-0.76), but not for mortality rates. No differences were noted when comparing CPAP and noninvasive ventilation. [24, 25]
The largest randomized trial comparing oxygen, CPAP, and noninvasive ventilation as adjunctive treatments to conventional therapy in patients with CHF did not identify any intubation or mortality benefit. [26] Patients with CPAP and noninvasive ventilation (BiPAP) did have more rapid resolution of symptoms and correction of gas exchange abnormalities and pH compared with the oxygen group. No difference was noted between CPAP and noninvasive ventilation (BiPAP).
Do note, however, that this trial randomized and treated patients in an emergency department setting, and treatment with noninvasive ventilation averaged approximately 2 hours. Admission to a critical care unit was an outcome measure, and the overall intubation rate for all of the groups was less than 3%. This is distinctly different from other trials, in which the intubation rates for the control groups were approximately 31%. This raises some question about the comparability of these three groups of patients with patients reported in other trials, which included patients requiring ICU-level care.
Noninvasive ventilation is effective in patients with CHF. CPAP is probably the most effective mode, achieving a reduction in intubation rates and mortality rates, with a little less effectiveness noted with noninvasive ventilation (BiPAP). Subsequent experience with BiPAP has not identified an increased risk with therapy, specifically no increased risk of myocardial infarction; therefore, the choice of ventilatory support may be a local or patient-based decision. Subsequent trials comparing BiPAP or its equivalent with CPAP have failed to demonstrate the superiority in patient outcomes of one mode to the other. [27] Patients with CHF who can be treated in an emergency department setting may only realize a symptom or dyspnea benefit from noninvasive ventilation (whether CPAP or BiPAP) as opposed to oxygen alone.
Summary
Noninvasive ventilation is well suited for patients with cardiogenic pulmonary edema.
CPAP and BiPAP modalities both are effective, with CPAP possibly being more effective.
The greatest benefits are realized in relief of symptoms and dyspnea.
A decrease in intubation and mortality rates is not a universal experience.
Patients with hypercapnic respiratory acidosis may derive the greatest benefit from noninvasive ventilation.
Importantly, adjust to standard therapy, including diuresis.
Benefit may be seen with as few as 2 hours of support.
Noninvasive Ventilation and High-Flow Nasal Cannula Oxygen After Extubation
Interest in the use of noninvasive ventilation (NIV) after discontinuation of mechanical ventilation is considerable. Postextubation respiratory insufficiency requiring reintubation can occur in more than 20% of patients. Many of the pathophysiologic derangements discussed in earlier sections also occur in the postextubation period, including increased respiratory load, hyperinflation, diaphragmatic dysfunction, and increases in preload and afterload, all of which can contribute singly or in unison to hypercapnia, hypoxemia, and eventual respiratory failure. In addition, patients may have incurred some upper airway trauma with intubation or may have developed upper airway edema, which, in turn, can contribute to partial upper airway obstruction, which is another factor contributing to an increased respiratory workload.
Noninvasive ventilation can ameliorate some of the pathophysiologic derangements that occur following extubation and has been used in 2 primary postextubation scenarios. Patients in whom weaning trials have failed or those who do not meet extubation criteria have been extubated to noninvasive ventilation support as part of an early extubation approach or as an adjunct to weaning. Early extubation with noninvasive ventilation support may be able to prevent some of the complications associated with endotracheal intubation, specifically nosocomial pneumonia. In addition, noninvasive ventilation allows for speech with preservation of oropharyngeal function. Noninvasive ventilation has also been applied to patients who were identified as candidates for extubation based on weaning and/or extubation criteria but then developed postextubation respiratory distress.
While mixed groups of patients are encountered, the vast majority of patients managed with postextubation noninvasive ventilation support have had underlying chronic obstructive pulmonary disease (COPD), and this is the population that seems especially suited to noninvasive ventilation in general and, specifically, to noninvasive ventilation–supported weaning.
A systematic review and meta-analyses of noninvasive ventilation and weaning in slightly more than 500 patients (mostly COPD patients) found that the use of noninvasive ventilation reduced mortality rates by 45% (RR, 0.55; 95% CI, 0.38-0.79), ventilator-associated pneumonia rates by 71% (RR, 0.29; 95% CI, 0.19-0.45), weighted duration of ICU stay by 6.27 days (95% CI, 8.77-3.78 d), and hospital days by 7.19 days (10.8-3.58 d) compared with a conventional weaning approach. The duration of endotracheal intubation was reduced by 7.81 days (95% CI, 11.3-4.31 d), as was the need for tracheostomy. However, reintubation rates were not decreased. [28] A more recent report suggests that hypercapnic patients may fare better if treated with noninvasive ventilation following extubation. [29]
These results conflict with reports of increased adverse outcomes (reintubation, mortality) when noninvasive ventilation is applied later in the course of patients on mechanical ventilation, after they have fulfilled criteria for extubation, are extubated, and develop respiratory distress. Noteworthy is that only approximately 10% of the patients in these trials had COPD, and this is the group that seemed to benefit the most from noninvasive ventilation. [30] On the other hand, in a smaller prospective randomized trial of 97 high-risk patients with mixed causes of respiratory failure including COPD, ARDS, and pneumonia, application of NIV 1 hour after extubation was associated with a lower reintubation rate (8.3 % vs 24.5%, P = .027). [31]
High-flow nasal cannula oxygen (HFNC) may have a role in this setting, probably better than face mask oxygen. [32] Its potential benefit may lie in its ability to provide higher levels of oxygen and a small amount of positive pressure compared with standard oxygen therapy. Its performance and role compared with NIV has undergone additional investigation. A randomized, multicenter trial involving 830 patients following cardiothoracic surgery found comparable failure rates (reintubation) between HFNC (21%) compared with NIV provided as BiPAP (21.9%). BiPAP-randomized patients had a higher PaO2/FIO2 than HFNC patients, but no differences were noted in a host of secondary outcome measures, including PaCO2, pH, respiratory rate, dyspnea score, comfort score, nosocomial pneumonia, pneumothorax, and length of stay in the ICU or hospital. [33]
A prospective randomized trial involving 604 patients with a mix of initial of both medical and surgical diagnoses, including ARDS, decompensated heart failure, and COPD exacerbations, and postoperative patients, with the majority either undergoing abdominal or neurosurgery, compared 24 hours of postextubation support with either HFNC or BiPAP. After 24 hours, patients were switched to standard oxygen therapy. No differences in reintubation were noted between HFNC and BiPAP in the first 24 hours, but all-cause reintubation was a little lower with BiPAP (19.1%) versus HFNC (22.8%), as was respiratory related-reintubation (BiPAP 15.9% vs HFNC 16.9%), but none of these outcomes was statistically significant and this supports the conclusion of noninferiority of HFNC versus BiPAP for mixed causes of intubation. [34]
This experience led to a comparison of HFNC alone with HFNC and NIV postextubation in patients at high risk for extubation failure, defined as those older than 65 years and with underlying chronic cardiac or pulmonary disease. [35] These entities included those with left ventricular dysfunction defined by a left ventricular ejection fraction of less than or equal to 45% and prior cardiogenic pulmonary edema, ischemic heart disease, chronic atrial fibrillation, COPD, obesity-hypoventilation syndrome, or restrictive lung disease. Patients were also stratified on the presence or absence of hypercapnia (PaCO2 >45 mm Hg). Randomization to HFNC postextubation represented 48 hours of continuous HFNC oxygen, whereas the HFNC and NIV group were first treated with NIV for at least 4 hours postextubation and for 12 hours daily with HFNC during the period when off NIV in the 48 hours after extubation. If those in either group were stable after 48 hours, they were switched to conventional oxygen. The primary outcome was reintubation within 7 days of extubation ,and the investigators had preestablished reintubation criteria. Of 641 patients who were randomized, the NIV and HFNC group had lower reintubation rates at 48 hours, 72 hours, ICU discharge, and 7 days (11.8% vs 18.2%, P = .02) than the HFNC group. It should be noted that NIV was continued beyond the initial postextubation window of 48 hours in 25% and HFNC oxygen was continued in that group in 35% of patients. However, mortality was not different between the two groups, whether assessed during the ICU stay, hospital stay, or at 90 days. Of those in whom HFNC oxygen failed, 20 patients were then treated with NIV and half of those patient eventually needed reintubation. This report supports the combined use of NIV and HFNC oxygen as a successful postextubation strategy in those patients at high risk for respiratory failure.
In a meta-analysis of 18 trials involving 3881 patients, HFNC was associated with lower rates of intubation compared with conventional oxygen therapy in those with acute respiratory failure. However, no differences in intubation rates, ICU mortality, or ICU length of stay was found between HFNC and NIV in patients with acute respiratory failure. [36] This suggests that HFNC oxygen is not inferior to NIV in the management of these patients and can be an alternative method of support if there is concern for the use of NIV.
Overall, experience to date suggests that NIV can help facilitate weaning and discontinuation of mechanical ventilation in selected patients. Patients with underlying COPD are the best candidates, but other groups may also benefit from an early-extubation approach followed by NIV support. COPD patients who develop respiratory distress after meeting criteria for extubation are most likely to benefit from NIV, but this is not established and use of NIV in these patients (as well as any other patient who develops postextubation respiratory distress) should be done with caution. There are better outcomes with NIV provided as prophylactic or preemptive ventilatory support after extubation, but before the development of respiratory distress. For those who may not be able to tolerate NIV interfaces, HFNC oxygen is an acceptable alternative and in those patients with underlying cardiac and/or respiratory disease, the combination of NIV and HFNC oxygen reduces the incidence of postextubation respiratory failure and need for reintubation.
Summary
Noninvasive ventilation is effective as a bridge support after early extubation.
Noninvasive ventilation is an adjunct to weaning (substitutes noninvasive support for invasive support).
Patients with underlying COPD are most likely to benefit from noninvasive ventilation after early extubation.
Noninvasive ventilation is not as effective in patients with postextubation respiratory distress.
COPD patients are a subgroup who may benefit in that situation.
Preemptive or near-immediate use (as opposed to waiting for respiratory distress) of NIV following extubation may be more effective in the transition of patients off invasive ventilatory support.
HFNC oxygen is an acceptable option for the transition off ventilatory support following extubation.
While postoperative patients may be especially suited to HFNC, it has been successful with other causes of respiratory failure.
The combination of NIV and HFNC is more effective than HFNC alone following extubation in patients with cardiac and respiratory disease.
Noninvasive Ventilation in COVID-19
There are considerable differences in opinion and controversy on the use of noninvasive ventilatory devices in patients with novel coronavirus disease 2019 (COVID-19). Much of this stems not from issues with efficacy, but from questions and concerns about aerosolization of potentially infectious exhaled gas, which may increase the possibility of spreading this infection to other healthcare workers or patients. Increased exhaled air dispersion has been demonstrated in simulation studies with mannequins with the amount of noted dispersion increasing with higher applied pressures (IPAP 18 cm water). [37] However, this does not seem to have translated to an increase in infections when reviewing the experience with severe acute respiratory syndrome (SARS), although in a small number of patients (20). [38] The authors did note endotracheal intubation was avoided in 70% of the patients with shorter ICU lengths of stay (3.1 vs 21.3 days, P< .001), with no nosocomial SARS infections noted in 105 healthcare workers involved with these patients. In addition to good infection control practices, they also mitigated the potential production of droplets with use of a facial mask (as opposed to a nasal mask), exhalation device with reduced jet-expired air, and a viral bacterial filter placed before the exhalation device. On the other hand, in a case control study to identify risk factors for a super-spreading nosocomial event defined as clusters of more than 3 cases, the use of high-flow oxygen and BiPAP were risk factors, although BIPAP did not achieve statistical significance in local-site multivariate models but only in pooled analysis. [39] In addition, the use of a dual-limb circuit noninvasive ventilator as opposed to one with a single-limb circuit, which typically has a circuit leak or leak valve to prevent carbon dioxide rebreathing, may also provide additional control of environmental aerosol generation.
In a retrospective cohort study of 26 healthcare workers who developed SARS, 38% had exposure to noninvasive ventilation (NIV), whereas 8% were exposed to high-flow oxygen, but neither modality was identified with increased transmission of SARS in a logistic regression model. [40] In a systematic review of the literature and risk of infections to healthcare workers with aerosol-generating procedures, NIV generated an odds ratio (OR) of 3.1 (95% confidence interval [CI], 1.4-6.8), but this only involved two small cohort studies. No association was noted with high-flow nasal cannula (HFNC) oxygen, but only one cohort was included in the analysis. [41] In comparison, higher ORs were identified with tracheal intubation (OR, 6.6; 95% CI, 2.3-18.9) and manual ventilation before intubation also had an increased OR (2.6; 95% CI, 1.3-6.4). This summarizes the experience and solutions applied during the SARS epidemic, which was also due to a coronavirus.
Because of the potential risk for nosocomial aerosol transmission, the role of NIV in the management of hypoxemic respiratory failure due to COVID-19 pneumonia, remains unsettled. While NIV and HFNC oxygen are options for the management of hypoxemic respiratory failure, some medical centers have raised concern for their use given the potential risk for nosocomial transmission of coronavirus. On the other hand, these options have been demonstrated to avoid intubation, which is a crucial aspect of management as patient demand has exceeded the inventory of ventilators at some sites, leading to difficult management and possible ethical conflicts in patient care.
The use of HFNC and NIV in the treatment of COVID-19 pneumonia and respiratory failure has varied greatly in series reported from worldwide sites. This may be in part a reflection of local practices, but it may also be a reflection of the severity of respiratory failure, as HFNC and NIV are not typically used in patients with severe hypoxemic respiratory failure, defined as low PaO2/FIO2 less than 200. There are also risks for aerosolization with both NIV and HFNC, but different practices may mitigate that risk as outlined above for NIV with the use of lower flows, a surgical mask on the patient, and close attention to minimizing condensation of tubing. These reports have focused on the overall experience in managing respiratory failure, and there has not been specific attention to noninvasive therapies except for one report from China. They retrospectively reviewed their experience and reported on a small group of 17 patients treated with HFNC in 7 (41%) of their patients, all with PaO2/FIO2 less than 200, but those failures also went on to be treated with NIV and only 2 (11.7% of those treated with HFNC and NIV) eventually required intubation. [42]
In three series from China, in a group of 138 patients with an overall death rate of 4.3%, 36 of their patients were treated in the ICU, with 11% receiving HFNC oxygen and 42% NIV, but it is not entirely clear in how many subjects these modalities had failed. [43] In another cohort of 201 patient with ARDS and overall death rate of 21.9%, HFNC (includes mask oxygen) use rates were 48% and NIV 30%. [44] They reported 42% of their cohort had ARDS and 33% were treated with mechanical ventilation, but they provided no information on the number in who whom HFNC oxygen and NIV failed. In a report that focused on 168 deaths, 34% received HFNC oxygen and 43% NIV, but only 20% were intubated. [45]
In the largest reported series from Italy, their cohort consisted of 1591 patients with a 26% mortality rate at the time of their report. The vast majority of patients were treated with invasive mechanical ventilation (88%) and 11% were treated with NIV. [46] In four US studies, two from Washington state, with 24 and 21 patients, respectively, with mortality rates of 50% and 67%, use of HFNC oxygen was 42% and 5%, respectively, NIV was 0% and 19%. [47, 48] In the largest case series from New York of 5700 patients, 2634 who had been discharged or died, the mortality rate was 21%, with 320 (12%) intubated. Of the intubated patients, the mortality rate was 88%. No information was provided on HFNC or NIV use. [49] In a cohort from Boston of 66 intubated patients with 16.7% mortality at the time of the report, only one patient was treated with HFNC or NIV (2%) and that patient was eventually intubated. [50]
It is evident that use of HFNC oxygen and NIV is much different in China than Italy and the United States. Until there is further analysis, it will be difficult to ascertain the true impact and/or advantage of HFNC oxygen and NIV in COVID-19 pneumonia. However, these modalities can be used, and based on the experiences with SARS, with minimal impact for healthcare workers provided proper infection control practices are in place. It should also be noted that the vast majority of the SARS experience occurred in the context of negative-pressure rooms, and this may be an important factor for the use of these modalities in COVID-19, as this has been largely unreported in these case series. One of the advantages in past use of HFNC or NIV in patients with hypoxemic respiratory failure was the ability to avoid intubation. While this may still be the case with COVID-19, the magnitude of this effect remains to be determined.
The following ventilation clinical practice guidelines in adults with COVID-19 were released by the European Society of Intensive Care Medicine and the Society of Critical Care Medicine [51] :
-
It is suggested to start supplemental oxygen if the peripheral oxygen saturation (SPO 2) is less than 92%. It is recommended to start supplemental oxygen if the SPO 2 is less than 90%.
-
In the event of acute hypoxemic respiratory failure on oxygen, it is recommended that the SPO 2 be maintained at no higher than 96%.
-
In patients with acute hypoxemic respiratory failure despite conventional oxygen therapy, it is suggested that a high-flow nasal cannula be used rather than conventional oxygen therapy.
-
In patients with acute hypoxemic respiratory failure, it is also suggested that a high-flow nasal cannula be used over noninvasive positive-pressure ventilation.
-
In these patients with acute hypoxemic respiratory failure, in the event a high-flow nasal cannula is not available and the patient has no urgent indication for endotracheal intubation, it is suggested that a trial of noninvasive positive-pressure ventilation be conducted, with close monitoring and short-interval assessment for worsening of respiratory failure.
-
While considered an option, no recommendation was made regarding helmet noninvasive positive-pressure ventilation versus mask noninvasive positive-pressure ventilation.
-
In patients receiving either noninvasive positive-pressure ventilation or high-flow nasal cannula, it is recommended they be closely monitored for worsening respiratory status; early intubation in a controlled setting is recommended if worsening occurs.
-
In patients with acute respiratory distress syndrome (ARDS) who are on mechanical ventilation, it is recommended to use low-tidal-volume ventilation (4-8 mL/kg of predicted body weight) versus higher tidal volumes (>8 mL/kg).
-
In patients with ARDS who are on mechanical ventilation, it is recommended to target plateau pressures at less than 30 cm water.
-
In patients with moderate-to-severe ARDS who are on mechanical ventilation, it is suggested to use a higher positive end-expiratory pressure (PEEP) strategy versus a lower PEEP strategy. When using a higher PEEP strategy (ie, PEEP >10 cm water), monitor patients for barotrauma.
-
In patients with ARDS who are on mechanical ventilation, it is suggested to use a conservative fluid strategy versus a liberal fluid strategy.
-
In patients with moderate-to-severe ARDS who are on mechanical ventilation, it is suggested to use prone ventilation for 12-16 hours versus no prone ventilation.
-
In patients with moderate-to-severe ARDS who are on mechanical ventilation, it is suggested to use, as needed, intermittent boluses of neuromuscular blocking agents versus a continuous infusion, to facilitate protective lung ventilation.
-
Use of a continuous infusion of neuromuscular blocking agents is suggested in the event of persistent ventilator dyssynchrony, a need for ongoing deep sedation, prone ventilation, or persistently high plateau pressures.
-
In patients with ARDS who are on mechanical ventilation, routine use of inhaled nitric oxide is not recommended.
-
In mechanically ventilated patients with severe ARDS and hypoxemia despite optimization of ventilation and other rescue strategies, a trial of inhaled pulmonary vasodilator is suggested as rescue therapy; if rapid improvement in oxygenation is not observed, taper off treatment.
-
In mechanically ventilated patients with severe ARDS and hypoxemia despite optimization of ventilation, use of recruitment maneuvers is suggested over not using recruitment maneuvers. If recruitment maneuvers are used, staircase (incremental PEEP) recruitment maneuvers are not recommended.
-
In those patients on mechanical ventilation who have refractory hypoxemia despite optimization of ventilation and who have undergone rescue therapies and proning, it is suggested to use venovenous extracorporeal membrane oxygenation (EMCO) if available; alternatively, refer the patient to center that has ECMO. However, because EMCO is resource-intensive and it requires experienced centers/healthcare workers and infrastructure, it should only be considered in carefully selected patients with severe ARDS.
Summary
HFNC and NIV have been effective in the treatment of hypoxemic respiratory failure in SARS and COVID-19.
The severity of hypoxemic respiratory failure defined as PaO2/FIO2 less than 200 may identify patients better suited for treatment with invasive ventilation.
The risk of transmission of potentially infectious aerosols may be mitigated by the use of filters, personal protective equipment, and negative-pressure rooms.
Local experience and expertise are important factors in the use of HFNC oxygen and NIV.
HFNC and NIV may avoid intubation in some patients with COVID-19 pneumonia and respiratory failure, but this remains to be determined and the use of these modalities should be made on a case-by-case basis.
Noninvasive Ventilation in Other Conditions
Noninvasive ventilation (NIV) has been used in a number of clinical situations, but it seems to be most effective in patients with acute respiratory failure due to underlying chronic obstructive pulmonary disease (COPD) or congestive heart failure (CHF). These represent the hypercapnic and hypoxemic conditions best suited for noninvasive ventilation, but, obviously, other conditions can also be treated with noninvasive ventilation.
Other diagnoses are regularly added to the list as experience accumulates. The common theme that suggests successful application noninvasive ventilation for all of these other conditions is a reasonably rapidly reversible condition with noninvasive ventilation as an adjunct to therapy. Other parameters, as outlined in General Considerations, are also important for the successful application of noninvasive ventilation. Noninvasive ventilation is likely to be successful in selected patients with these diagnoses, but the evidence to date does not support universal application of noninvasive ventilation in these patients. The following highlights the main considerations in each condition. [52, 53, 54, 55]
In community-acquired pneumonia, note the following:
-
Noninvasive ventilation not established to be beneficial
-
Secretions may be limiting factor
-
Improvement with noninvasive ventilation best achieved in patients also with COPD
-
Hypercapnic respiratory acidosis may define group likely to respond
-
Decrease in intubation rate and mortality may be limited to those also with COPD
In immunocompromised patients and hypoxemic respiratory failure, note the following:
-
Solid organ transplantation [57] - Single-center trial, approximately 50 patients; subgroup with cardiogenic pulmonary edema fared best
-
Febrile neutropenic patients - Single-center trial, approximately 50 patients; mostly hematologic malignancies or bone marrow transplantations; benefit of noninvasive ventilation in those with an identified cause of pneumonia; severity of illness relatively modest
In asthma [58] patients, note the following:
-
Similar pathophysiology to COPD; limited reported experience with noninvasive ventilation
-
Mostly case series with reported benefit
-
Prospective, randomized studies based on emergency department settings
-
Improvement in spirometry main outcome measure
-
Fewer admissions with noninvasive ventilation; intubation not an outcome measure
-
Hypercapnic asthma patients not represented in randomized trials
-
Noninvasive ventilation probably beneficial, but experience limited
In postoperative patients, [59] note the following:
-
Postoperative hypoxemia related to atelectasis or pulmonary edema
-
Randomized trials with postoperative continuous positive airway pressure (CPAP) demonstrate benefit
-
Applied as prophylactic support or with development of hypoxemia
-
Benefit noted with level CPAP levels in 7.5- to 10-cm water range
-
Lower intubation rates, days in ICU, and pneumonia
-
High-flow nasal cannula oxygen option (50 L/min and FiO2 = 0.50) may be comparable to noninvasive ventilation [33]
With rib fractures (traumatic, with nonpenetrating chest injuries), note the following:
-
Older single report using low-level CPAP (5 cm water)
-
Fewer episodes of pneumonia, duration of hospitalization
-
No mortality benefit: Hernandez et al found that in patients with hypoxemia related to severe thoracic trauma, noninvasive mechanical ventilation reduced intubation rates. In a randomized clinical trial, patients with PaO2/FiO2 ratio of less than 200 for more than 8 hours while receiving oxygen by high-flow mask within the first 48 hours after thoracic trauma were randomized to remain on high-flow oxygen mask or to receive noninvasive mechanical ventilation. The trial was halted after 25 patients were enrolled in each group because the intubation rate was much higher in controls than in noninvasive mechanical ventilation patients (40% vs 12%, P = .02). In addition, length of hospital stay was shorter in noninvasive mechanical ventilation patients (14 vs 21 d, P = .001); however, no difference in survival was observed. [63]
For do-not-intubate status (advanced disease or terminal malignancy), [64] note the following:
-
Numerous case series
-
COPD patients comprise most patients
-
Most with hypercapnic respiratory failure
-
Report of 60% success rate, but discharge home rate of 40-50%
-
Median survival following treatment 179 days in one series
-
One-year survival rate of 30%
-
Some with more distress from the mask and noninvasive ventilation than benefit
-
Issues with resource utilization and prolonging the inevitable
-
Better outcomes in CHF, awake patients, and those with strong cough (mobilized secretions)
-
Benefit in patients with malignancy if treating reversible condition
-
Benefit in dyspnea relief for patients with terminal malignancy [65]
For acute respiratory distress syndrome, [66, 67] note the following:
-
Not recommended as first-line therapy in management
-
Limited experience, but may benefit those who do not require immediate intubation
-
Noninvasive ventilation provided via mask or helmet; able to avoid intubation in approximately half - Ventilator settings in successful noninvasive ventilation are pressure support ventilation of 14 cm water; positive end-expiratory (PEEP) of 7 cm water; successfully treated patients found to have lower severity of illness (Simplified Acute Physiology Score II < 34 or improvement of PaO2/FiO2 ratio >175 after 1 h)
-
High-flow nasal cannula oxygen in a trial compared with high-flow nasal cannula plus noninvasive face mask ventilation with better mortality outcomes than noninvasive ventilation, although no statistical difference in primary outcome of intubation [68]
-
Single-center trial of selected patients with acute respiratory distress syndrome who did not improve with noninvasive ventilation demonstrated benefit with helmet ventilation with decreased intubation rate, more ventilator-free days, and decreased mortality [69] ; higher PEEP delivered and high flow rates (100-200 L/min) may explain benefit of helmet noninvasive ventilation
For severe acute respiratory distress syndrome, [70, 38] note the following:
-
Successful treatment with noninvasive ventilation during severe acute respiratory distress syndrome (SARS) outbreak
-
Noninvasive ventilation able to avoid intubation in 70%
-
Patients hypoxemic with also relatively low severity of illness (Acute Physiology and Chronic Health Evaluation II [APACHE II] score < 6)
The remaining conditions have had reports of successful noninvasive ventilation in both acute and chronic respiratory failure. Most of these processes represent patients with either chronic hypercapnia or relatively mild hypoxemia. Patients with the following diagnoses who may be candidates for noninvasive ventilation should be evaluated on a case-by-case basis:
-
Cystic fibrosis - May be useful as a bridge to lung transplantation and as an adjunct to oxygen therapy alone during sleep to improve gas exchange [71]
-
Neuromuscular respiratory disease [72, 73] - Nocturnal use may be especially effective for daytime hypercapnia; avoid in bulbar dysfunction or excess secretions; effective in patients with muscular dystrophy, kyphoscoliosis, and postpolio syndrome; some may be able to be treated with negative-pressure ventilators
-
Obesity-hypoventilation (or decompensated obstructive sleep apnea) - Corrects hypercapnia, facilitates diuresis, and provides opportunity for restorative sleep [70]
-
Upper airway obstruction (partial) - Caution if potential for complete obstruction
-
Mild Pneumocystis carinii pneumonia - Case series; may avoid intubation in selected patients
-
Idiopathic pulmonary fibrosis - Generally poor response to invasive ventilation, much less noninvasive ventilation; Successfully treated patients with a rapidly reversible cause of respiratory failure
Complications of Noninvasive Ventilation
Noninvasive ventilation (NIV) has a very different profile of complications compared with complications associated with endotracheal intubation and mechanical ventilation. This is directly attributable to the elimination of the endotracheal tube in patient management. Complications associated with its placement, the duration of placement, and removal of the tube all are averted. In addition, patients are usually not sedated as they would be if they were intubated, which further reduces complications related to sedation. Even complications common to both noninvasive ventilation and invasive ventilation occur less frequently in patients undergoing noninvasive ventilation.
Complications of noninvasive ventilation
For facial and nasal pressure injury and sores, note the following:
-
Result of tight mask seals used to attain adequate inspiratory volumes
-
Minimize pressure by intermittent application of noninvasive ventilation
-
Schedule breaks (30-90 min) to minimize effects of mask pressure
-
Balance strap tension to minimize mask leaks without excessive mask pressures
-
Cover vulnerable areas (erythematous points of contact) with protective dressings
For gastric distension, note the following:
-
Rarely a problem
-
Avoid by limiting peak inspiratory pressures to less than 25 cm water
-
Nasogastric tubes can be placed but can worsen leaks from the mask
-
Nasogastric tube also bypasses the lower esophageal sphincter and permits reflux
For dry mucous membranes and thick secretions, note the following:
-
Seen in patients with extended use of noninvasive ventilation
-
Provide humidification for noninvasive ventilation devices
-
Provide daily oral care
For aspiration of gastric contents, note the following:
-
Especially if emesis during noninvasive ventilation
-
Avoid noninvasive ventilation in patient with ongoing emesis or hematemesis
Complications of both noninvasive and invasive ventilation
These include the following:
-
Barotrauma (significantly less risk with noninvasive ventilation)
-
Hypotension related to positive intrathoracic pressure (support with fluids)
Complications avoided by noninvasive ventilation
These include the following:
-
Ventilator-associated pneumonia
-
Sinusitis
-
Reduction in need for sedative agents - Sedatives used in less than 15% of noninvasive ventilation patients in one survey
Other Issues
For use of noninvasive ventilation, note the following:
-
Utilization highly variable among hospitals, regions, and countries
-
Used extensively in Europe, with use increasing over time - Noninvasive ventilation (NIV) use exceeds 80% of patients in some European ICUs [75]
-
Used less in Canada and United States (20-50%; some areas 0%) [76] - Attributed to (1) unfamiliarity of physicians and other staff and (2) lack of proper equipment; inaccurate perception of increased time commitment; respiratory therapy and nursing time not increased
-
Practice guidelines increase usage of noninvasive ventilation
-
Coding and reimbursement issues additional impediments to implementation
Cost-analysis of noninvasive ventilation is as follows:
-
Demonstrated to be cost-effective in patient management - Savings of more than $3000 Canadian dollars; even greater cost savings if patients managed in a ward setting
-
Avoids costs of endotracheal intubation and mechanical ventilation
-
Shorter ICU and hospital stays
-
Eliminates costs associated with infectious complications - Episodes of ventilator-associated pneumonia reduced by half or more
Questions & Answers
Overview
What is noninvasive ventilation (NIV)?
What is the historical background of noninvasive ventilation (NIV)?
What is positive-pressure ventilation and how is it delivered?
What are the types of noninvasive ventilation (NIV)?
What is negative-pressure noninvasive ventilation (NIV) and how is it delivered?
What are the advantages of positive-pressure noninvasive ventilation (NIV)?
What is high-flow nasal cannula oxygen noninvasive ventilation (NIV)?
In which patients is high-flow nasal cannula oxygen noninvasive ventilation (NIV) indicated?
What is the basis of patient selection for noninvasive ventilation (NIV)?
What are contraindications for noninvasive ventilation (NIV)?
What other considerations may limit the use of noninvasive ventilation (NIV)?
Which patients are best suited for noninvasive ventilation (NIV)?
What are the patient inclusion criteria for noninvasive ventilation?
Which conditions are most suitable for treatment with noninvasive ventilation?
What are suitable clinical conditions for use of noninvasive ventilation (NIV)?
With which clinical conditions should noninvasive ventilation (NIV) be used with caution?
How does healthcare staff inexperience with noninvasive ventilation (NIV) affect outcomes?
Where is noninvasive ventilation (NIV) utilized for inpatient care?
How does noninvasive ventilation (NIV) differ from invasive ventilation?
How should masks be fitted to ensure successful noninvasive ventilation (NIV)?
What type of mask is most effective in noninvasive ventilation (NIV)?
What are the advantages and disadvantages of an orofacial mask for noninvasive ventilation (NIV)?
What are the advantages and disadvantages of a nasal mask for noninvasive ventilation (NIV)?
What are the modes of ventilation in noninvasive ventilation (NIV)?
What is the role of pressure control (PC) in noninvasive ventilation (NIV)?
What is the role average volume assured pressure support (AVAPS) in noninvasive ventilation (NIV)?
Why are 1-2 hour trials of noninvasive ventilation (NIV) conducted?
What are predictors of success in noninvasive ventilation (NIV)?
What are the predictors of failure in noninvasive ventilation? (NIV)
Why should trials of noninvasive ventilation (NIV) be limited?
What is included in the objective criteria for discontinuation of noninvasive ventilation (NIV)?
What are the predictors of failure in high-flow nasal cannula oxygen?
What are the major criteria for intubation of patients using noninvasive ventilation (NIV)?
What are the minor criteria for intubation of patients using noninvasive ventilation (NIV)?
What are guidelines for intubation?
What is the efficacy of noninvasive ventilation (NIV) for congestive heart failure (CHF)?
What is the role of noninvasive ventilation (NIV) after extubation?
What are the benefits of postextubation noninvasive ventilation (NIV) support?
Which patients are suitable for postextubation noninvasive ventilation (NIV) support?
What is the efficacy of postextubation noninvasive ventilation (NIV) support?
Which patients are the best candidates for postextubation noninvasive ventilation (NIV) support?
How can the role of noninvasive ventilation (NIV) after extubation be summarized?
What are ventilation-related recommendations and suggestions for adults with COVID-19?
When is noninvasive ventilation (NIV) indicated?
What is the role of noninvasive ventilation (NIV) in the treatment of immunocompromised patients?
What is the role of noninvasive ventilation (NIV) in the treatment of asthma?
What is the role of noninvasive ventilation (NIV) in the treatment of postoperative patients?
What is the role of noninvasive ventilation (NIV) in the treatment of rib fractures?
What are potential complications of noninvasive ventilation (NIV)?
How are facial and nasal pressure injury and sores caused by noninvasive ventilation (NIV) managed?
How is gastric distension caused by noninvasive ventilation (NIV) managed?
How is dry mucous membranes and thick secretions caused by noninvasive ventilation (NIV) managed?
How is aspiration of gastric contents caused by noninvasive ventilation (NIV) managed?
What are complications of both noninvasive (NIV) and invasive ventilation?
Which complications are avoided by noninvasive ventilation (NIV)?
How do the utilization rates for noninvasive ventilation (NIV) vary among developed countries?
What is the cost-analysis of noninvasive ventilation (NIV) use?
-
Nasal mask. Courtesy of Philips Healthcare (previously Respironics).
-
Full face mask. Courtesy of Philips Healthcare (previously Respironics).
-
Total face mask. Courtesy of Philips Healthcare (previously Respironics).
-
Patient with an exacerbation of chronic obstructive pulmonary disease (COPD) undergoing treatment with noninvasive ventilation using an orofacial mask.
-
Helmet mask. Courtesy of Harol S. R. L.
-
Oro-nasal mask. Courtesy of Philips Healthcare (previously Respironics).
-
Noninvasive ventilation. Courtesy of Therese Canares, MD, and Jonathan Valente, MD, Rhode Island Hospital, The Warren Alpert Medical School of Brown University.
-
Patient with decompensated congestive heart failure undergoing noninvasive ventilatory support with BiPAP ventilation and an orofacial mask.
-
Screen shot of ventilator graphics and information panel of a patient undergoing BiPAP ventilation.
Tables
What would you like to print?
- Overview
- Methods of Delivery
- General Considerations
- Application of Noninvasive Ventilation
- Noninvasive Ventilation in COPD
- Noninvasive Ventilation in Cardiogenic Pulmonary Edema
- Noninvasive Ventilation and High-Flow Nasal Cannula Oxygen After Extubation
- Noninvasive Ventilation in COVID-19
- Noninvasive Ventilation in Other Conditions
- Complications of Noninvasive Ventilation
- Other Issues
- Questions & Answers
- Show All
- Media Gallery
- References