Practice Essentials
Vulvovaginitis is a general term referring to many types of vaginal infection, although this article focuses on the following disorders, which affect the vulvar region:
-
Vulvovaginal candidiasis
-
Atrophic vaginitis
-
Vulvar vestibulitis
-
Contact dermatitis
Signs and symptoms
Acute vulvovaginal candidiasis
-
Vulvar pruritus and burning - Primary symptoms of the disease
-
Erythema and edema of the vestibule and of the labia majora and minora
-
Thrush patches - Usually found loosely adherent to the vulva
-
Thick, white, curdlike vaginal discharge
Chronic vulvovaginal candidiasis
-
Marked edema and lichenification of the vulva with poorly defined margins
-
Grayish sheen made up of epithelial cells and organism covering the area
-
Severe pruritus and burning
-
Irritation and pain
Atrophic vaginitis
-
Vaginal soreness
-
Postcoital burning
-
Dyspareunia
-
Burning leukorrhea
-
Occasional spotting
Vulvar vestibulitis
-
Primary vulvar vestibulitis (20% of cases) - Introital dyspareunia that starts from initiation of sexual activity or intolerable pain consistently present upon insertion of a tampon or vaginal speculum in women who have never been sexually active
-
Secondary vulvar vestibulitis - Introital dyspareunia that develops after a period of comfortable sexual relations, tampon use, or speculum examinations
Usual symptoms of vulvar vestibulitis include pain, soreness, burning, and a feeling of rawness that is aggravated by stress, exercise, tight clothing, coitus, and tampon use. The pain is usually not considered constant but is elicited by any attempt to enter the vagina.
Other symptoms may include the following:
-
Irritating vaginal discharge
-
Vulvar burning sensation
-
Small spots of erythema around the vestibular glands, with rare ulceration
Contact dermatitis
Pruritus is the cardinal symptom. However, an acute reaction may develop as a result of exposure to a potent irritant that involves the mucosa, leading to the following symptoms:
-
Burning, rawness, and pain
-
Red and edematous skin followed by exudation and weeping
-
Erosion, ulceration, or necrosis - If the irritant is potent enough
Contact dermatitis of long duration may include lichenification, scaling, thickening of the skin, and white plaques.
See Clinical Presentation for more detail.
Diagnosis
See the list below:
-
Vulvovaginal candidiasis - Wet-mount test or potassium hydroxide (KOH) preparation to confirm the presence of Candida; fungal culturing may be used if the diagnosis is uncertain [1]
-
Atrophic vaginitis - Vaginal pH measurement and wet-mount test (although history and physical examination generally provide sufficient diagnostic information); a wet mount often shows white blood cells and a paucity of Lactobacillus
See Workup for more detail.
Management
Vulvovaginal candidiasis
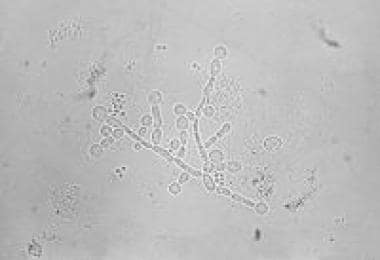
Uncomplicated sporadic vulvovaginal candidiasis usually is caused by strains of C albicans (see the image below). Most of these strains exhibit sensitivity to azole-based antifungal agents. A number of antimycotic regimens are available for the treatment of vulvovaginal candidiasis, including with oral and topical agents.
Although an optimal regimen has not yet been established for the treatment of recurrent vulvovaginal candidiasis, therapies include ketoconazole (400 mg/day), itraconazole (50-100 mg/day), fluconazole (100 mg/wk) for 6 weeks, and clotrimazole (500-mg vaginal suppositories once per wk). [2] An intravaginally administered boric acid suppository also may be used for treatment.
Atrophic vaginitis
Treatment usually entails the use of topical vaginal estrogen for 1-2 weeks to alleviate symptoms.
Vulvar vestibulitis
Pain management strategies have included the following:
-
Sex therapy
-
Behavior modification
-
Biofeedback
-
Acupuncture
-
Topical anesthetic
-
Topical corticosteroid
-
Petroleum jelly or vitamin A and D ointment - To provide a protective coating
-
Wet compresses with aluminum acetate
-
Anti-inflammatory agents
Surgical excision may be considered as a last resort in the treatment of vulvar vestibulitis. Success rates of 60-80% have been reported.
Contact dermatitis
Treatments include the following:
-
Removal of the inciting agent
-
Triamcinolone ointment (0.1%) - Applied twice daily for irritant contact dermatitis
-
Wet compresses of aluminum acetate - For severe lesions
-
Hydrocortisone (0.5-1%) and fluorinated corticosteroids in lotions or creams
See Treatment and Medication for more detail.
Background
Vulvovaginitis, a general term referring to many types of vaginal infection, is the most common gynecologic condition seen by practitioners rendering primary care to women. Discharge, burning, and pruritus are the most common symptoms, accompanied by signs of vulvar irritation, such as erythema and excoriation of the vulvar skin. (See Presentation.)
Traditionally, the 3 classic entities of vaginitis include bacterial vaginosis, Trichomonas infection, and candidiasis. This article, however, focuses on disorders that affect the vulvar region, including the following (see the image below):
-
Vulvovaginal candidiasis
-
Atrophic vaginitis
-
Vulvar vestibulitis
The differential diagnosis for women with symptoms of vulvovaginitis is complex. Discharge, burning, and pruritus usually are the presenting symptoms, with signs of vulvar irritation that may include erythema and excoriation of the vulvar skin. Primary or secondary infections, skin irritants, or contact dermatitis may produce vulvar irritation. Irritation from bodily fluids such as urine and normal vaginal secretions may cause symptoms when the environment is kept moist, as with tight-fitting or occlusive clothing. (See Pathophysiology and Etiology, Presentation, and Workup.)
Because each disorder produces a similar clinical presentation, a careful history must be taken, an examination must be performed, and the vaginal discharge should be examined. Along with medical treatment, the patient must be encouraged to avoid etiologic agents and to make necessary changes in her habits. (See Treatment and Medication.)
Patient education
For patient education information, see the Pregnancy Center and the Women's Health Center, as well as Vaginal Infections (Vaginitis), Candidiasis (Yeast Infection), Vaginal Yeast Infection Treatment, Female Sexual Problems, and Trichomoniasis.
Anatomy
The vulva, the external genitalia of the female, includes the labia majora and minora, the clitoris, and the vestibule of the vagina. The skin of the vulva is sensitive to the vaginal environment and hormonal, metabolic, and allergic influences. It is composed of stratified squamous epithelium that contains hair follicles, sebaceous sweat glands, and apocrine glands.
During the reproductive years of a healthy woman's life, the vagina maintains a moist environment that is in constant fluctuation. The secretion of an alkaline transudate from the vaginal epithelium and cervical glands maintains this moist environment with a pH ranging from 3.8-4.5. In addition, the vagina and its microflora form a unique, balanced environment that can change under pressure from external stimuli but returns to normal with removal of the stimuli. It can vary in degree during sexual activity, pregnancy, and the menstrual cycle.
The vaginal epithelium consists of 3 cell layers; ie, superficial, intermediate, and basal. The cells in these layers are capable of storing glycogen under the influence of estrogen. Glycogen is available in the fully mature cells in the superficial layer of the epithelium. With elevated levels of either exogenous or endogenous estrogen, all levels of the epithelium thicken as a result of glycogen storage. With diminishing levels of estrogen, the layers become thin and atrophic.
Pathophysiology and Etiology
In an adult woman's reproductive years, the bacterial flora of the healthy vagina contains numerous microorganisms, including aerobic and anaerobic gram-positive and gram-negative bacteria. Lactobacillus and Corynebacterium predominate over other bacteria such as Streptococcus, Bacteroides, Staphylococcus, and Peptostreptococcus.
Lactobacillus and Corynebacterium produce lactic and acetic acid from glycogen, thus maintaining the low vaginal pH. Additional bacteria are kept in check by the acid-producing bacteria and are rarely pathogenic, but they may become pathogenic if the environmental balance is affected.
Vaginal pH may increase with age, menstrual cycle phase, sexual activity, contraceptive choice, pregnancy, the presence of necrotic tissue or foreign bodies, or the use of hygienic products or antibiotics. [3]
Vulvovaginal candidiasis
Vulvovaginal candidiasis can be an acute, chronic, recurrent, or persistent condition that can involve the vulva, vagina, and adjacent crural areas. The specific causative agent belongs to the genus Candida. These organisms are found in almost all humans and many animals. An estimated 10-50% of reproductive-aged American women are considered opportunistic carriers.
The species C albicans is identified approximately 85-90% of the time. However, an increased frequency of other Candida species, such as C glabrata, C tropicalis, and C krusei, has been reported. The emergence of these other Candida species may possibly be due to widespread use of over-the-counter drugs, long-term use of suppressive azoles, and the use of frequent short courses of antifungal drugs.
Pregnancy
Any host factor that affects the vaginal environment or vaginal secretions can play a role in the initiation of Candida vulvovaginitis. Pregnancy is one of the most common predisposing factors. Studies have demonstrated that up to one third of pregnant women worldwide on any day can be affected. The high levels of reproductive hormones and an increase in the vaginal environment’s glycogen content create a favorable environment for Candida species, providing an abundant source of carbon for candidal growth, germination, and adherence.
Furthermore, the acidity of the pregnant vaginal flora can suppress the growth of other microorganisms that are naturally inhibitory to Candida. Although the initial attachment of the organism occurs more readily at high pH values (6-7), the germ tube formation and the development of mycelia are favored by a low vaginal pH (< 5).
Contraception
Older studies of women using high-dose estrogens in oral contraceptives found an increase in vaginal colonization by Candida. The mechanism is believed to be similar to that found in pregnancy. However, newer oral contraceptives with a lower estrogen dose do not seem to predispose the patient to vulvovaginal candidiasis.
Other causes
Disorders associated with an altered immune response, such as acquired immunodeficiency syndrome (AIDS) and diabetes mellitus, also predispose women to Candida vulvovaginitis.
Antimicrobials are thought to predispose a patient to Candida by reducing the number of protective resident vaginal bacteria. The most common offenders are broad-spectrum agents such as tetracycline, cephalosporins, and ampicillin-like agents. Tight-fitting undergarments are another potential factor in the development of vulvovaginal candidiasis.
A study by Horowitz et al demonstrated Candida species in ejaculate fluid of partners of patients with recurrent Candida infections, but they suggested that the carrier rate may be low. [4] Traditionally, vulvovaginal candidiasis is not considered a sexually transmitted disease, because it occurs in celibate women, and Candida itself is considered part of the normal vaginal flora.
Recurrent vulvovaginal candidiasis
Although most women with vulvovaginal candidiasis respond quickly to treatment, the recurrent form of the disease, defined as 4 or more episodes of infection per year, may occur (albeit in less than 5% of healthy women). Predisposing factors for recurrent infection are apparent in only a minority of women; they include poorly controlled diabetes and immunosuppressive therapy.
Other factors that may predispose to recurrent infection include abnormalities in local vaginal mucosal immunity and genetic susceptibility. Studies have found that women with recurrent infections have a higher frequency of certain Lewis blood group antigens and specific gene polymorphisms compared with controls.
Recurrent vulvovaginal candidiasis has also been associated with a decreased in vivo concentration of mannose binding lectin (MBL) and an increased concentration of interleukin-4 (IL-4). Studies have shown that the prevalence of a variant MLB gene is higher in women with recurrent vulvovaginal candidiasis than in controls without candidiasis. Furthermore, IL-4 blocks the anti-Candida response mediated by macrophages; thus, elevation of IL-4 levels results in the inhibition of local defense mechanisms. [5, 6, 7, 8]
The role of sexual transmission in recurrent infection remains unresolved. Although controversial, most studies do not support treatment of sexual partners. [9] Horowitz et al reported on 54 women with recurrent Candida vaginitis and found no significant difference in the rate of relapse between women with untreated or treated partners. [4]
Recurrences may be caused by other species of Candida that are not equally susceptible to the usual first-line treatments. In vitro studies have shown that imidazole antifungal agents, such as miconazole and clotrimazole, are not as effective against non– C albicans fungi. C tropicalis and C glabrata are 10 times less sensitive to miconazole than is C albicans. Appropriate fungal cultures may be taken to identify the species. Treatment entails longer courses of antimycotic therapy (10-14 days), regardless of the route of administration.
Atrophic vaginitis
Extremely low estrogen production, as found after menopause or bilateral oophorectomy, can lead to atrophy of the vaginal and vulvar epithelium. Vulvovaginal atrophy is considered a natural process after estrogen withdrawal. Although menopause is the leading cause of decreased levels of circulating estrogen, atrophy of the vagina can occur in nonmenopausal women due to diminished ovarian estrogen production, as can result from cancer treatments, such as radiation therapy and chemotherapy, and immunologic disorders.
Furthermore, in postpartum women, the decline in estrogen levels in conjunction with the loss of placental estrogen and the antagonistic action of prolactin on estrogen production during lactation can lead to atrophy.
Among its many effects, estrogen helps to maintain the collagen content of the epithelium and thus affects its thickness and elasticity. It also helps to maintain acid mucopolysaccharides and hyaluronic acid, which keep epithelial surfaces moist. During the reproductive years, estrogen stimulation is responsible for maintenance of a well-epithelialized vaginal vault. It causes the nonkeratinized stratified squamous epithelium of the vagina to be thick, rugated, and rich in glycogen. Glycogen is necessary for rapid multiplication and maintenance of lactobacilli.
Menopause
During the perimenopausal period, estrogen secretion, primarily estradiol, remains at approximately 120 ng/L. After menopause, it decreases to approximately 18 ng/L. The reduction of endogenous estrogen causes thinning of the epithelium and a diminished glycogen content. The lack of glycogen contributes to a reduction in lactic acid production and an increase in vaginal pH, thus leading to the overgrowth of nonacidophilic species and the disappearance of Lactobacillus. In some patients, this new flora may include bacteria that can incite a superficial infection in denuded regions and alter vaginal secretions. [10]
In addition, during estrogen withdrawal, the papillae of the vagina flatten and the rugae nearly disappear, leaving the vagina relatively smooth. The mucosa becomes progressively thinner and eventually may become only a few cell layers thick. A moderately thick layer of intermediate cells may be present in some areas, with only a row of basal cells in others. Eventually, the vagina becomes denuded of epithelium.
Vulvar vestibulitis
The classic definition of vulvar vestibulitis, according to Freidrich's criteria, includes the following signs and symptoms confined to the vulvar vestibule:
-
Severe pain upon touching the vestibule or attempted vaginal entry
-
Tenderness to pressure localized within the vulvar vestibule
-
Physical findings confined to vestibular erythema of various degrees
The vestibule consists of nonpigmented and nonkeratinized squamous epithelium devoid of skin. It contains mucus-secreting minor vestibular glands, ductal orifices of the Bartholin glands, Skene glands, and the urethral meatus. It is within this region that the inflammatory entity vulvar vestibulitis arises. While many theories have been proposed, the etiology of this condition remains unknown.
Histology
Histopathologic studies have not been helpful in determining etiology, demonstrating a nonspecific inflammation of the vestibular region affecting mostly the superficial stroma and occasionally the epithelium. In 1988, Pyka et al studied surgically excised specimens of the vulvar vestibule from 41 patients with vulvar vestibulitis. Pyka identified it in 66% of the specimens' minor vestibular glands. All of these glands demonstrated some degree of squamous metaplasia forming vestibular clefts. [11]
Infectious etiologies
Vulvar vestibulitis was not widely recognized until Woodruff and Parmley reported on it in the 1980s. [12] They thought that the etiology was an infection of the vestibular glands that was best treated by perineoplasty.
Marinoff and Pyka proposed that Candida may be a causative organism in vulvar vestibulitis; however, the presence of yeast in patients with the condition has not been confirmed by other reports.
More recent studies have investigated the role of human papillomavirus (HPV) infection; however, the evidence has been controversial. [13] Turner and Marinoff [14] reported a 100% rate of HPV positivity in vulvar biopsies in 7 patients with vestibulitis, while Bergeron reported negative viral findings in all 11 of his biopsies. Further studies are needed to elucidate the relationship, if any, between HPV and vulvar vestibulitis.
Noninfectious etiologies
Possible noninfectious causes of vulvar vestibulitis include the following:
-
Vulvovaginal candidiasis therapy - Some authors believe that the disease may result from allergic sensitization within the vulvar vestibule to several types of topical medication for vulvovaginal candidiasis
-
HPV therapy - Treatments for clinical and subclinical HPV, [15] such as cryosurgery, trichloroacetic acid, podophyllin, and laser treatment, have been implicated in the development of vulvar vestibulitis
-
5-fluorouracil cream - Several cases of vulvar vestibulitis have been reported after the use of this agent, which is administered for the treatment of actinic keratoses and superficial basal cell carcinoma
-
Chemical irritants - An association between such agents, including those found in feminine hygiene products, and vulvar vestibulitis has been investigated
-
Alkaline vaginal pH - This has been demonstrated to cause irritation to the vestibule; agents that alter the vaginal pH can lead to the overgrowth of anaerobic and/or the disappearance of normal flora (ie, Lactobacillus); hypotheses suggest that the constant bathing of the vestibule by an alkaline vaginal discharge may lead to chronic irritation and inflammation
-
Other - Some authors have associated vulvar vestibulitis with a history of sexual abuse, elective abortions, severe marital conflicts, depression, and anxiety
Neurologic pathophysiology
Studies have begun to focus on specific pain receptors found in the vulvar tissue. As a brief overview, the sensory innervation of the inferior portion of the vulva is primarily from the branches of the pudendal nerve. The ilioinguinal and branches of the genitofemoral nerve innervate the superior portion of the vulva. These nerve fibers are of 2 types: (1) those responsible for touch and (2) those responsible for nociception (perception of noxious stimuli).
The mechanism hypothesized is that the nociception fibers are innervated first instead of the touch fibers. This is followed by a prolonged innervational response of the nociception fibers, leading to an abnormal neurologic response from the dorsal horn of the medulla.
Westrom and Willen tested this theory by obtaining vulvar biopsies of 47 women with clinical vestibulitis. In 44 specimens, they noted that not only were regions of marked increase of vestibular nerve formation present, but a significant correlation was found between inflammation and nerve-bundle density. The authors concluded that a chronic inflammatory reaction in the vestibule might lead to proliferation of nerve fibers. Thus, treatment had entailed surgical removal of these nerve fibers. [16]
Contact dermatitis
The vulvar skin is a frequent site of contact dermatitis; the cutaneous response may be either allergic or irritant induced. An allergic reaction implies previous exposure to an allergen and sensitization. It is a cell-mediated (type IV) immunologic response that can occur in sensitized individuals.
Irritant-induced contact dermatitis can be acute or chronic. It may occur from acute exposure to a potent irritant or upon repeated exposure to a weak irritant. Irritants that can cause contact dermatitis include the following:
-
Moisture
-
Urine
-
Vaginal discharge
-
Topical medications
-
Anticandidal agents
-
Latex
-
Spermicidal agents
-
Cosmetics
-
Douching
-
Fragrances
-
Cleansing products
-
Underwear
Epidemiology
Occurrence in the United States
Vulvovaginal candidiasis
At some point in their lifetime, nearly 75% of all women experience an attack of vulvovaginal candidiasis, with approximately 50% of college-aged women having an episode. About half of the women who develop the condition have more than 1 episode, and a few have frequent relapses. [17, 18] Patients with recurrent or severe vulvovaginal candidiasis warrant a screening test for diabetes mellitus.
Atrophic candidiasis
After menopause, most women experience some vaginal atrophy as estrogen levels fall. The incidence of atrophic vaginitis depends on how it is defined. Vulvovaginitis related to infection is much less common after menopause. Desquamative inflammatory vaginitis, an exception, has an unknown etiology, but a Gram stain of culture often reveals streptococci. This is treated with intravaginal clindamycin cream or a topical or intravaginal steroid. [19]
International occurrence
An international study by Foxman et al on the rate of vulvovaginal candidiasis in Western nations found a high incidence of the disease in these countries. The investigators examined rates in the United States and 5 European nations, using surveys from about 6000 women aged 16 years and older. They determined that among the 6 countries, rates of vulvovaginal candidiasis ranged from 29-49%. It was also found that the recurrent form of the disease developed in more than one fifth of the reported cases. [20]
Age-related demographics
Candida species infections are most common during childbearing years. Atrophic vaginitis may develop several years after menopause. Most women with vaginal atrophy do not develop symptomatic atrophic vaginitis.
Prognosis
Vulvovaginal candidiasis
Most women with vulvovaginal candidiasis usually respond quickly to treatment. Despite therapy, however, recurrent vulvovaginal candidiasis, defined as 4 or more episodes of infection per year, can occur, although in less than 5% of healthy women. Predisposing factors for recurrent infection are apparent in only a minority of women, and include poorly controlled diabetes and immunosuppressive therapy. Other factors that may predispose to recurrent infection include abnormalities in local vaginal mucosal immunity and genetic susceptibility. Studies have found that women with recurrent infections have a higher frequency of certain Lewis blood group antigens and specific gene polymorphisms than do controls.
Atrophic vaginitis
Accumulating evidence indicates that the vaginal symptoms readily respond to estrogen treatment. With treatment, mucosal thickening with glandular function can be maintained well into the postmenopausal period.
Vulvar vestibulitis
Generally, no specific cure is available, but spontaneous resolution has been reported; thus, treatment should focus on alleviation of symptoms.
-
Candida albicans photomicrograph. Courtesy of Centers for Disease Control and Prevention (CDC).