Practice Essentials
Transthyretin (TTR) is a protein that functions as a transporter of thyroxine and retinol and is produced chiefly by the liver (> 95%), with additional production within the choroid plexus of the brain and the retinal pigment epithelium. Mutated transthyretin is associated with the formation of amyloid fibrils, leading to the development of TTR-related amyloidosis (ATTR). These fibril proteins are deposited into various organs and tissues, preferentially the nervous system and cardiac tissue, resulting in their inherent dysfunction. [1]
Signs and symptoms
The presenting signs and symptoms in patients with ATTR are fairly nonspecific and are often attributed to more common diseases affecting both the heart and the peripheral nervous system (PNS) and autonomic nervous system. There are three main types of ATTR, identified by the organ system involved: cardiac, neuropathic, and leptomeningeal.
Cardiac ATTR
Patients with cardiac deposition typically present with the following typical symptoms of chronic heart failure (CHF):
-
Symptoms suggestive of right-sided CHF (ie, dyspnea on exertion, peripheral edema, hepatomegaly, ascites, elevated jugular venous pressure), diastolic dysfunction, and/or arrhythmias (ie, palpitations, lightheadedness, syncope, ECG changes)
-
Heart failure with preserved ejection fraction (HFpEF) predominates in ATTR
-
Patients may also present with atrial arrhythmias or conduction system disease due to amyloid fibril deposition within areas responsible for electrical impulse conduction.
-
Cardiomegaly may be noted on chest imaging/echocardiogram. [1]
Neuropathic ATTR
Neuropathic involvement in patients affected by ATTR–familial amyloid polyneuropathy (FAP) is classically a symmetric, ascending length−dependent, sensorimotor, axonal polyneuropathy subtype and may include the following:
-
PNS sensimotor impairment affecting all functional classes of nerve fibers: motor, sensory and autonomic fibers (diarrhea or contipation, urinary incontinence, orthostatic hypotension, sexual impotence, glaucoma)
-
Lower-limb neuropathy (eg, in patients with the TTR V30M mutation)
-
Upper-limb neuropathy (eg, TTR I84S, TTR L58H) [1]
-
ATTR V30M variant: Lower extremity weakness, pain, and/or impaired sensation; autonomic dysfunction, often manifesting as sexual or urinary dysfunction [2]
-
Weakness and paresthesias of one or both hands, due to carpal ligament deposits (eg, in variant TTR L58H, normal-sequence TTR); symptoms of localized carpal ligament deposition sometimes precede other clinical manifestations by as long as 20 years.
Leptomeningeal involvement
Patients with rare TTR variants that cause CNS disease may present with the following features:
-
Nystagmus and pyramidal signs, with spastic paraparesis [3]
-
Seizures, subarachnoid hemorrhages, cerebrovascular attacks (ischemic strokes), dementia, in patients with leptomeningeal/cerebrovascular deposits: [3]
-
Hearing loss, cerebellar ataxia, in patients with isolated leptomeningeal disease (rare) [4]
See Presentation for more detail.
Diagnosis
Physical examination findings in patients with ATTR depend on the organ involved, which is affected by the presence and genetic identity of a TTR variant. Symptoms consistent with HFpEF, along with concurrent peripheral/autonomic neuropathy, warrant consideration of ATTR as a diagnosis. A complete family history is of great value for genetic inheritance patterns.
Biopsy
All types of amyloidosis are diagnosed definitively on the basis of demonstration of Congo red–binding material in a biopsy or autopsy specimen. Subcutaneous fat aspiration often provides sufficient tissue for diagnosing amyloid, as well as for further studies (eg, immunostaining). Biopsy of an organ with impaired function (eg, heart, GI tract) can definitively establish a cause-and-effect relationship between organ dysfunction and amyloid deposition. See the image below.
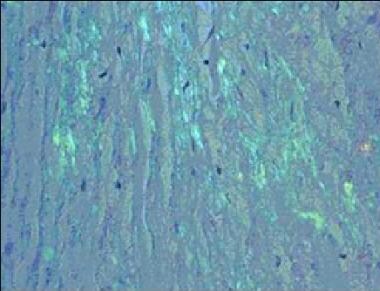 Transthyretin-related amyloidosis. Congo Red staining of a cardiac biopsy specimen containing amyloid, viewed under polarized light.
Transthyretin-related amyloidosis. Congo Red staining of a cardiac biopsy specimen containing amyloid, viewed under polarized light.
Laboratory results for different types of amyloidosis are generally nonspecific, including the following:
-
Complete blood count: Normochromic normocytic anemia
-
Chemistry panel: Electrolyte abnormalities (due to heart failure or malabsorption)
-
Kidney function tests: Evidence of varying degrees of proteinuria and diminished glomerular filtration rate in patients with renal deposition
Other tests include electrocardiography, nerve conduction studies, and genetic studies (eg, polymerase chain reaction, electrospray ionization mass spectrometry, single-strand conformation polymorphism analysis and/or direct sequencing).
Imaging studies
-
Radiolabeled P-component scanning
-
Cardiac imaging (eg, 2-dimensional echocardiography, electrocardiography, or both; CT scanning; nuclear scintigraphy, cardiac MRI)
See Workup for more detail.
Management
Patisiran, vutrisiran, inotersen, and eplontersen have been approved by the FDA for treatment of polyneuropathy caused by hereditary transthyretin-mediated amyloidosis (hATTR) in adults. Tafamidis and tafamidis meglumine have been FDA approved for treatment of transthyretin amyloid cardiomyopathy. [5] The advent of these disease-modifying agents has significantly reduced the role of liver transplantation for hATTR. [70]
Diuretics are the mainstay of therapy for amyloid-related CHF, but must be used with caution due to the restrictive physiology involved.
Surgery
Depending on the organ and/or tissue involvement, surgical intervention for patients with ATTR may involve the following:
-
Liver transplantation: An effective therapy for familial amyloid polyneuropathy, as it removes the source of mutant TTR, but increasingly obviated by successful pharmacologic therapy
-
Combination heart/liver or liver/kidney transplantation in select patients
-
Carpal tunnel release
-
Vitrectromy
See Treatment for more detail.
Background
The amyloidoses are a wide range of diseases of secondary protein structure, in which a normally soluble protein forms insoluble extracellular fibril deposits, causing organ dysfunction. All types of amyloid contain a major fibril protein that defines the type of amyloid, plus minor components. Over 20 different fibril proteins have been described in human amyloidosis, each with a different clinical picture (see Amyloidosis). One such protein that forms human amyloid fibrils is transthyretin (TTR).
TTR acts as a transport protein for thyroxine in plasma. TRR also transports retinol (vitamin A) through its association with the retinol-binding protein. It circulates as a tetramer of four identical subunits of 127 amino acids each. TTR was once called prealbumin because it migrates anodally to albumin on serum protein electrophoresis, but this name was misleading, as TTR is not a precursor of albumin. The TTR monomer contains eight antiparallel beta-pleated sheet domains.
TTR can be found in plasma and in cerebrospinal fluid and is synthesized primarily by the liver and the choroid plexus of the brain and, to a lesser degree, by the retina. Its gene is located on the long arm of chromosome 18 and contains 4 exons and 3 introns. [6]
The systemic amyloidoses are designated by a capital A (for amyloid) followed by the abbreviation for the chemical identity of the fibril protein. Thus, TTR amyloidosis is abbreviated ATTR.
Pathophysiology
Normal-sequence transthyretin-related amyloidosis
In contrast to variant ATTR, normal-sequence cardiac ATTR is associated with aging, usually developing in the seventh and eighth decades of life. This disorder is commonly of little or no clinical significance and only noted on autopsies in studies aiming at estimating its prevalence in an otherwise asymptomatic aging population. In one autopsy study of people > 85 years of age, ATTR was present in 25%. [7] The fraction of autopsied patients with clinically significant symptoms is not known.
The stimuli that lead to normal-sequence ATTR are not understood. Normal-sequence TTR forms cardiac amyloidosis predominantly in men above 60 years of age, a disorder termed senile cardiac amyloidosis (SCA). When it was recognized that SCA is often accompanied by microscopic deposits in many other organs, the alternative name senile systemic amyloidosis (SSA) was proposed. Both terms are now used. [6] The clinical manifestations of severe SCA are similar to those observed in familial ATTR and in cardiac amyloidosis of the immunoglobulin light chain type (AL).
Mutant transthyretin-related amyloidosis
TTR mutations accelerate the process of TTR amyloid formation and are the most important risk factor for the development of clinically significant ATTR. More than 100 amyloidogenic TTR variants cause systemic familial amyloidosis. The age at symptom onset, pattern of organ involvement, and disease course vary, but most mutations are associated with cardiac and/or nerve involvement. The gastrointestinal tract, vitreous, lungs, and carpal ligament are also frequently affected. [6]
In a retrospective cross-sectional study of 284 ATTR and non-ATTR patients, the most common ATTR mutations were as follows [8] :
-
Thr60Ala (24%)
-
Val30Met (15%)
-
Val122Ile (10%)
-
Ser77Tyr (5%)
ATTR is caused by a single-point mutation, of which more than 100 have been described, that promotes destabilization of the native quarternary structure into a beta-pleated sheet–predominant, insoluble and inactive form. This conformational change hypothesis has been researched in vitro with a key finding that tetramer dissociation is a required and generally rate-limiting step in amyloid fibril formation.
Energetic studies have suggested that amyloidogenic mutations destabilize the native quaternary and tertiary structures of TTR, thereby inducing conformational changes that lead to dissociation of the tetramers into partially unfolded species, which can subsequently self-assemble into amyloid fibrils. However, the wild-type (wt) TTR form can also result in amyloid deposits found in peripheral nerves and cardiac tissue in patients affected by the disease, usually in older patients. It is expected that the process of amyloid aggregation will be further elucidated in the future to address this and other concerns. [9]
When the peripheral nerves are prominently affected, the disease is termed familial amyloidotic polyneuropathy (FAP). When the heart is involved heavily but the nerves are not, the disease is called familial amyloid cardiomyopathy (FAC).
Epidemiology
United States
The most common amyloidosis-associated TTR variants in the United States are as follows:
-
TTR V30M - Also the most widespread variant worldwide and most common cause of FAP
-
TTR T60A - Most common in an area centered in West Virginia
-
TTR L58H - Most commonly seen in Maryland but also throughout the United States
-
TTR S77Y - Also found in Europe
-
TTR I84S - Found in an area centered in Indiana
Cardiac ATTR amyloidosis has a progressive increase in prevalence in people older than 80 years and is seen in about 15% of autopsies, with one study finding a prevalence of about 25%. In this setting, the deposited TTR is usually of normal sequence (wt-ATTR).
International
A few amyloidosis-associated TTR variants are common in certain populations, although few data indicate population frequencies. The most common TTR variants include the following:
-
TTR V30M is found throughout Europe, in North and South America, and Japan. It is most common in some areas of northern Sweden (where it is carried by more than 1% of the population), northern Portugal, and certain areas in Japan. [10]
-
TTR V122I originated in West Africa. It is carried by 3.9% of African Americans and 5% or more of the population in some areas of West Africa. [11]
Familial TTR variants
Most variants that cause familial ATTR are rare, but a few are common in certain populations. TTR variants are written, according to convention, by the normal amino acid found at a position in the mature protein, followed by the number of the amino acid from the amino terminal end, and the variant amino acid found, using either the three-letter or single-letter amino acid code. The most widely recognized TTR variants are described below.
TTR V30M
This was the first TTR variant discovered. The role of TTR in amyloidosis was first established when TTR was found in the fibrils in several kindreds with autosomal dominant amyloidosis affecting the peripheral nerves, heart, and other organs.
This syndrome was first described in Portugal in the 1950s and later in Japan and Sweden. [12] The fibrils in patients in all 3 endemic areas were found to contain TTR that carried a substitution of methionine for valine at position 30, arising from a point mutation.
TTR V30M has now been found worldwide. It is the most widely studied TTR variant, and has served as a prototype for variant-sequence ATTR. The disease in the TTR V30M kindreds was termed FAP because early symptoms arose from peripheral neuropathy, but these patients actually have systemic amyloidosis, with widespread deposits often involving the heart, gastrointestinal tract, eye, and other organs. [10]
TTR V122I
This variant, carried by 3.9% of African Americans and over 5% of the population in some areas of West Africa, increases the risk of late-onset (after age 60 years) cardiac amyloidosis. It appears to be the most common amyloid-associated TTR variant worldwide. Affected patients usually do not have peripheral neuropathy. [11]
-
TTR T60A: This variant causes late-onset systemic amyloidosis with cardiac, and sometimes neuropathic, involvement. This variant originated in northwest Ireland and is found in Irish and Irish American patients. [13]
-
TTR L58H: Typically affecting the carpal ligament and nerves of the upper extremities, this variant originated in Germany. It has spread throughout the United States but is most common in the mid-Atlantic region. [13]
-
TTR G6S: This is the most common TTR variant, but it appears to be a neutral polymorphism not associated with amyloidosis. It is carried by about 10% of people of white European descent. [13]
Currently, about 100 TTR variants are known, with varying geographic distributions, degrees of amyloidogenicity, and organ predisposition. Currently known TTR variants are listed in the table below. [6] For organ involvement, the following abbreviations are used: PN = peripheral nerves, AN = autonomic nervous system, H = heart, L = liver, LM = leptomeninges, K = kidney, S = skin, E = eye, GI = gastrointestinal tract, CL = carpal ligament, and CNS = central nervous system.
Known TTR Variants (adapted from Benson [14] and Connors et al [6] ) (Open Table in a new window)
Variant |
Geographic Focus (Ethnic Origin) |
Organs Involved |
Gly6Ser |
Caucasian |
None |
Cys10Arg |
United States (Hungarian) |
H, PN, AN, E |
Leu12Pro |
United Kingdom |
CNS, AN, L, LM |
Asp18Gly |
United States (Hungarian) |
CNS, LM |
Met13Ile |
Germany |
None |
Asp18Asn |
United States |
H |
Asp18Glu |
South America |
AN, PN |
| Asp18Gly | Hungary | LM |
Val20Ile |
United States, Germany |
H, CL |
Ser23Asn |
United States (Portuguese) |
H, E, PN |
Pro24Ser |
United States |
PN, H, CL |
Ala25Ser |
United States |
PN, H, CL |
Ala25Thr |
Japan |
CNS, PN |
Val28Met |
Portugal |
AN, PN |
Val30Met |
Argentina, Brazil, China, Finland, France, Germany, Greece, Italy, Japan, Portugal, Sweden, Turkey, United States |
PN, AN, E, LM |
Val30Ala |
United States (German) |
AN, H |
Val30Leu |
Japan, United States |
PN, AN, H, K |
Val30Gly |
United States |
E, CNS, LM |
Phe33Cys |
United States |
CL, E, K, H |
Phe33Ile |
Israel (Polish, Ashkenazi Jewish) |
PN, E |
Phe33Leu |
United States (Polish, Lithuanian) |
PN, AN, H |
| Phe33Val | United Kingdom, Japan, China | PN |
Arg34Thr |
Italy |
PN, H |
Lys35Asn |
France |
PN, H, AN |
Ala36Pro |
Greece, Italy, United States (Jewish) |
PN, E, CNS, CL |
Asp38Ala |
Japan |
H, PN, AN |
Trp41Leu |
United States (Russian) |
E, PN |
Glu42Gly |
Japan, Russia, United States |
PN, AN, H |
Glu42Asp |
France |
H |
Phe44Ser |
United States, Japan |
PN, H, AN, E |
Ala45Thr |
Italy, Ireland, United States |
H |
Ala45Asp |
United States , Ireland, Italy |
PN, H |
Ala45Ser |
Sweden |
H |
Gly47Ala |
Italy, Germany, France |
PN, H, AN |
Gly47Arg |
Japan |
PN, AN |
Gly47Val |
Sri Lanka |
H, AN, PN, CL |
Gly47Glu |
Germany, Italy, Turkey, United States |
H, K, PN, AN |
Thr49Ala |
France, Italy (Sicily) |
PN, CL, H |
Thr49Ile |
Japan |
PN, H |
Thr49Pro |
United States |
H |
Ser50Arg |
Japan, France, Italy |
PN, H, AN |
Ser50Ile |
Japan |
PN, H, AN |
Glu51Gly |
United States |
H |
Ser52Pro |
United Kingdom |
PN, AN, H, K |
Gly53Glu |
Basque |
CNS, LM, PN, H |
Glu54Gly |
United Kingdom |
PN, E, AN |
Glu54Lys |
Japan |
PN, AN, H |
Leu55Pro |
United States (Dutch, German), Taiwan |
PN, E, H, AN |
Leu55Arg |
Germany |
PN, LM |
Leu55Gln |
United States (Spanish) |
AN, E, PN |
Leu58His |
United States, Germany |
H, CL |
His56Arg |
United States |
H |
Leu58Arg |
Japan |
AN, E, CL, H |
Thr59Lys |
Italy, United States (Chinese) |
H, PN, AN |
Thr60Ala |
Ireland, United States (Appalachian), Australia, Germany, United Kingdom, Japan |
H, PN, GI, CL |
Glu61Lys |
Japan |
PN |
Phe64Leu |
Italy, United States |
PN, H, CL |
Phe64Ser |
Canada (Italian), United Kingdom |
CNS, PN, E, LM |
Ile68Leu |
Germany, United States |
H |
Tyr69His |
United States, Scotland, Canada |
E, LM |
Tyr69Ile |
Japan |
CL, H, AN |
Lys70Asn |
United States, Germany |
CL, E, PN |
Val71Ala |
France, Spain |
PN, E , CL |
Ile73Val |
Bangladesh |
PN, AN |
Asp74His |
Germany |
None |
Ser77Tyr |
Germany, France, United Kingdom |
PN, H, K |
Ser77Phe |
France |
PN, AN, H |
Tyr78Phe |
France (Italian) |
PN, CL, S |
Ala81Thr |
United States |
H |
Ile84Ser |
United States (Swiss), Hungary |
H, CL, E, LM |
Ile84Asn |
Italy, United States |
E, H, CL |
Ile84Thr |
Germany, United Kingdom |
PN, AN, H |
Glu89Gln |
Italy/Sicily |
PN, H, CL |
Glu89Lys |
United States |
PN, H, AN |
His90Asn |
Portugal, Germany |
None |
Ala91Ser |
France |
PN, H, CL, AN |
| Gln92Lys | Japan | H |
| Ala97Gly | Japan | H, PN |
| Ala97Ser | China, France, Taiwan | PN, H |
| Gly101Ser | Japan | None |
Arg103Ser |
United States |
H |
Pro102Arg |
Germany |
None |
Arg104Cys |
United States |
None |
Arg104His |
Japan, United States (Chinese) |
None |
Ile107Met |
Germany |
H, PN |
Ile107Val |
United States(German), Japan |
PN, H, CL |
Ala109Val |
United States |
None |
Ala108Ala |
Portugal |
None |
Ala109Thr |
Portugal |
None |
Ala109Ser |
Japan |
PN, AN |
Leu111Met |
Denmark |
H, CL |
| Ser112Ile | Italy | PN, H |
Tyr114Cys |
Holland, Japan |
PN, E, H, LM, AN, CNS |
Tyr114His |
Japan |
CL, S |
Tyr116Ser |
France |
PN, CL, AN |
Thr119Met |
United States, Portugal |
None |
Ala120Ser |
Afro-Caribbean |
PN, H, AN |
Val122Ile |
Africa, United States, Portugal |
H |
Val122Ala |
United States (Alaska), United Kingdom |
PN, H, E |
Deletion of 122Val |
Ecuador, United States, Spain |
PN, CNS, GI, CL, H |
Pro125Ser |
Italy |
None |
Expression of transthyretin-related amyloidosis
Familial ATTR was traditionally thought of as a group of autosomal dominant diseases, but it is now known that disease expression is more complicated. The most abundant data pertain to TTR V30M; the following observations have been made:
-
Variation in age of onset: The usual age of disease onset among TTR V30M gene carriers in Portugal, Brazil, and Japan is in the third to fourth decade of life. However, there are late-onset cases (as seen in Sweden) in which disease onset is in the fifth to sixth decade of life.
-
Disease penetrance: In Portugal and Japan, more than 90% of TTR V30M gene carriers develop symptoms by middle age. However, in Sweden, disease penetrance is only 2%, and some V30M homozygous individuals remain asymptomatic. [10]
-
Some atypical Portuguese and Japanese kindred follow the late-onset, low-penetrance Swedish pattern. [12]
-
Some patients with no family history of amyloidosis and asymptomatic relatives with the variant gene carry the V30M variant.
-
Disease onset is earlier in males than in females. [15]
-
Age of symptom onset is progressively earlier in successive generations. This feature is referred to as anticipation. Anticipation in some neurologic disorders is caused by expansion of trinucleotide repeats. However, in ATTR, this mechanism seems not to apply.
The explanation for the above observations is not well understood. Other genetic and/or environmental variables are thought to be at play. Anticipation, incomplete penetrance, and clinically sporadic cases in kindreds with unaffected allele carriers also have been observed with other TTR variants. [13]
Race
TTR variants occur in all races.
-
The most common variant worldwide, TTR V122I, apparently originated in West Africa, has spread throughout that area and the Americas, and is carried by 3.9% of African Americans. Therefore, cardiac amyloidosis is more prevalent among African Americans than among people of other races in the United States. [11]
-
Other variants are documented to have originated in people of European, Japanese, and Chinese ancestry. TTR variants have probably originated in all races. [13]
Sex
All TTR variants encoded on chromosome 18 are inherited with equal frequency in males and females. For unknown reasons, disease penetrance is greater and age of onset earlier in males than in females. Individual case reports and several small series suggest that normal-sequence cardiac ATTR is significantly more common in males than in females, although the sex ratio is unknown. [15]
Age
The age of onset varies widely, depending on the presence and identity of the TTR variant.
-
Normal-sequence cardiac ATTR presents after age 60 years and usually after age 70 years.
-
Variant-sequence ATTR presents in teenagers and people in their early 20s for the most aggressive variants and in people older than 50 years for many others.
-
The average age of onset for ATTR V30M is 32 years in Japan and Portugal and 56 years in Sweden. The reason for this difference is not known.
Mortality/Morbidity
Morbidity and mortality from ATTR depends on whether a TTR variant is present and, if so, which variant. Some variants cause clinical disease by age 40 years in all gene carriers and are always fatal within a few years of symptom onset. Other variants typically cause much milder, later onset disease, and some carriers of the variant genes remain asymptomatic until late in life. [16] Regardless, untreated varient TTR disease has a 5 year survival rate of approximately 75%. [17]
Central nervous system (CNS) complications are increasingly noted in liver-transplanted ATTR patients. Atrial fibrillation (AF) is a risk factor for ischemic CNS complications observed after liver transplantation. [18]
A study by Phull et al showed a high prevalence of coexistent monoclonal gammopathy of undetermined significance (MGUS) in patients with ATTR, with a rate higher than the general population. [19]
Morbidity depends on the organ(s) involved. Neuropathy and cardiomyopathy are most common. The most common immediate cause of death is cardiac failure or fatal arrhythmia. [20]
Prognosis
The natural course of TTR-FAP can be classified into the following three stages:
-
Stage I – Sensory polyneuropathy
-
Stage II – Progressive walking disability
-
Stage III – Wheelchair bound or bedridden
Life expectancy ranges from 7.3 to 11 years from onset. [21] Death is most often due to cardiac dysfunction, infection, or cachexia. [22]
The prognosis depends on the presence and identity of a TTR variant and the organ(s) involved. Patients with early-onset of variant-sequence TTR may die within a few years of diagnosis. Older patients with slowly progressive disease can live for decades after the onset of symptoms and may never develop life-threatening disease. [16]
Penetrance of the individual ATTR mutations vary. The penetrance of the same mutation in different geographic areas can also vary, for example, the Portuguese population showing much higher penetrance of the Val30Met mutation during middle age (80% at 50 years) compared with the French population (18% at 50 years). [21]
In contrast to light chain amyloidosis (AL), symptomatic cardiac involvement in ATTR does not necessarily portend a poor prognosis. Median survival in cardiac AL is about 6 months, but is several years in older patients with cardiac ATTR, even in those with a TTR variant.TTR-FAP usually proves fatal within 7–12 years from the onset of symptoms, most often due to cardiac dysfunction, infection, or cachexia. [22]
Within most of the regions in which it is endemic, clinical onset of TTR-FAP often occurs before age 40 years with progressive sensory-motor and autonomic neuropathy, leading to cachexia and eventually death. Length-dependent small-fiber sensory and motor polyneuropathy with life-threatening autonomic dysfunction is a distinguishing feature of TTR-FAP in these areas. In addition, cardiac, renal, and ocular involvement are also common. [21]
In nonendemic areas, and in endemic regions of Sweden, the onset of disease-related symptoms tends to be later in life, from age 50 years onward and with a male predominance for the late-onset TTR-FAP. Neuropathy tends to affect all fibers and may closely resemble chronic inflammatory demyelinating polyneuropathy (CIDP). Typically, sensory and motor neuropathy symptoms of upper and lower extremities occur, associated with mild autonomic symptoms. [21]
Patient Education
Education of patients is based on organ system involvement and expected symptoms. Genetic conditions and hereditary forms of transthyretin amyloidosis should be discussed with the patient in regard to familial screening.
-
Transthyretin-related amyloidosis. Congo Red staining of a cardiac biopsy specimen containing amyloid, viewed under polarized light.







