Introduction, Definition of Terms, and Anatomy
Nonmalignant disorders account for most of the dermatologic lesions seen by urologists. For most of these conditions, the diagnosis can be ascertained through clinical history and presentation, but, on occasion, biopsy may be necessary. This review focuses on cutaneous diseases that are either specific to the male genitalia or frequently involve this body region.
Topics in this article are divided into groups based on their initial clinical presentation and most common clinical appearance. The discussion focuses on diagnosis, treatment, and clinical outcome. Attempts are made to include the most recent and relevant treatment options, emphasizing the use of pharmaceuticals and their impact. US Centers for Disease Control and Prevention (CDC) recommendations are stressed, and promising experimental treatments are noted. Although presenting a comprehensive review of each disorder is not the goal of this article, a thorough and understandable knowledge base for the treatment of patients with these disorders is provided, along with references that lead to more detailed and comprehensive studies of the subtopic.
Although rare in the United States and minimally emphasized in medical schools, some infectious diseases are showing resurgence because of increased world emigration and the spread of resistant forms of disease.
Dermatology terms
In the field of dermatology, the use of specific terms to describe clinical findings allows communication of essential information that may suggest a differential diagnoses. To a nondermatologist, these terms can be confusing. Therefore, this article begins with definitions of terms used to describe the most common dermatologic appearances.
-
Macules: These are small defined areas of color change that are not palpable (ie, flat or sometimes collapsed). Macules tend to be smaller than 1 cm.
-
Patch: This is a larger (> 1 cm) area of color change that is nonpalpable (ie, level with the skin). In essence, a patch is a large macule.
-
Papules: These are small lesions that are palpable (ie, feels like a bump). They tend to be smaller than 1 cm and can be any color or texture.
-
Plaque: This is a large (> 1 cm) raised area with palpable borders. Basically, it is a large papule with extensions along the edges. Note that the height is not increased. A plaque is large, raised, and flat. Edges can be palpated, and borders are well delineated. Like papules, they can be any color or texture. Often, this represents a confluence of papules.
-
Nodule: This is a large (> 1.5 cm) papule. Unlike plaques, an increase in the lesion's height is noted.
-
Vesicles: These are fluid-filled blisters, usually smaller than 1 cm in diameter. A distinct characteristic is that the vesicle collapses when it is incised and the fluid is removed. It can be considered a fluid-filled papule.
-
Bulla: These are fluid-filled blisters larger than 1 cm in diameter.
-
Pustules: These are vesicles filled with neutrophils.
Dermatologic anatomy of the male genitalia
The male external genitalia are composed of the penis and scrotum. The penis is divided into the more distal glans and the shaft, or body, which is anchored by its root in the perineal pouch. The prepuce (foreskin) covers the glans. It is a thin loose covering of keratinizing skin with associated underlying eccrine (sweat) and sebaceous glands and a highly vascular stroma without underlying adipose tissue. The prepuce is composed of the following 5 layers:
- External keratinizing epidermis
- Underlying dermis with eccrine and sebaceous glands
- Fine strands of dartos muscle
- Lamina propria
- Internal squamous mucosa.
The glans is an extension of the highly vascular corpus spongiosum and is covered by squamous epithelium that is keratinized in the circumcised male. The scrotum extends the keratinized squamous epithelium with underlying dartos muscle and associated external spermatic fascia. Here, the dermis contains hair follicles in association with eccrine, apocrine, and sebaceous glands. Scattered fat cells are present, although well-formed subcutaneous adipose tissue usually is not.
Most dermatologic disorders are confined to the epidermis, underlying dermis, and associated adnexal structures. These areas include the keratinized epithelium, the underlying dermis with its rich vasculature, associated smooth muscle, and associated hair follicles and sweat and sebaceous glands.
Patient education
For patient education resources, see STDs in Men (Sexually Transmitted Diseases in Men).
Trichomycosis and Folliculitis
Trichomycosis
Trichomycosis (see Trichomycosis Pubis) is a bacterial infection of the hair shaft that is found in areas bearing sweat glands, in particular the axillary area, but the pubic region may also be affected.
Pathophysiology
Three Corynebacterium species have been known to cause trichomycosis. [1] Corynebacterium tenuis is the causative organism associated with most cases. Although up to 33% of adults have colonization by this bacterium in the inguinal or axillary regions, factors such as hyperhidrosis predispose to more extensive growth and resultant clinical manifestations. [2]
Clinical presentation/diagnosis
Patients typically present with yellow, black, or red pinpoint nodules on the hair shafts in the inguinal region. [3] Nodules are often on the scrotum and occasionally on the base of the penis shaft. These lesions can be associated with erythema and itching. The nodules fluoresce under examination with a Wood lamp. Superinfection with other dermatophytes has been noted.
Differential diagnoses
Trichomycosis is clinically differentiated from nits, lice, or fungal infection with a Wood light examination.
Treatment and outcome
Treatment involves shaving and alleviating the hyperhidrosis with drying agents and antimicrobials. Topical antimicrobials such as bacitracin, clindamycin, or erythromycin are effective in most cases. Oral erythromycin may also be used. [4]
Most cases respond to therapy; however, recurrence in not uncommon.
Folliculitis
Folliculitis (see Folliculitis) is inflammation of the hair follicle that is often infectious in origin.
Pathophysiology
External factors, such as trauma from shaving or rubbing, often predisposes to bacterial colonization and overgrowth in this region, leading to folliculitis. Organisms most commonly identified include Staphylococcus, Pseudomonas, and Candida species. Less common etiological agents include herpesvirus and dermatophytes. Noninfectious folliculitis can be caused by various factors, such as irritation from shaving or the use of topical irritants. Novel therapeutics, such as tumor necrosis factor (TNF)–alpha inhibitors and epidermal growth factor receptor inhibitors, are associated with pustular folliculitis eruptions. [5, 6]
Clinical presentation/diagnosis
Patients present with small red papules or pustules centered around the follicular orifice (see the image below). Lesions often involve the base of the penile shaft or the scrotum and may be mildly painful or pruritic.
Differential diagnoses
When the lesions are follicular based and inflamed, the diagnosis of folliculitis is fairly straightforward. However, determining the etiology requires bacterial culture, viral (Tzanck) preparation, or fungal (potassium hydroxide [KOH]) preparation. Biopsy is not usually necessary.
Treatment and outcome
Treatment is directed at the causative organism and includes topical or oral antimicrobials, as indicated. Improved hygiene and antimicrobial cleansers may also be helpful. Patients should be advised to obtain new hygiene products (eg, razors). [4]
Lesions generally resolve with therapy, and serious complications are very rare. Treatment failures may result from lack of identification of the etiologic organism or the presence of resistant bacterial strains, such as methicillin-resistant Staphylococcus aureus.
Balanoposthitis, Balanitis, and Candidiasis
Balanoposthitis
Balanoposthitis is defined as the inflammation of the foreskin and the glans penis in uncircumcised males (see Balanoposthitis). Multiple bacterial and fungal etiologic agents are associated with the condition. Complex infections have also been well documented.
Pathophysiology
Balanoposthitis is commonly identified in young boys (< 5 y). Predisposing factors include limited retraction of the foreskin and poor hygiene in the area. The lack of hygiene leads to bacterial infection. [7, 8] Anaerobic organisms are the most common bacteria isolated from lesions. [9] Candida infection appears to also be a common cause of disease. In older males, the condition has other etiologies, including the following [10, 11] :
-
Intertrigo
-
Irritant dermatitides
-
Infection with Candida or other fungi
-
Viral infections
Clinical presentation/diagnosis
Patients present with erythema, swelling, and pain in the foreskin area. In adults, clinical history and diagnostic tests, including KOH and Tzanck preparations, can help determine the correct diagnosis. Biopsy is generally not necessary, although a lack of response to treatment warrants a biopsy to exclude premalignant or malignant lesions.
Differential diagnoses
The differential diagnoses include intertrigo (see Intertrigo); irritant dermatitis; and candidal (see Candidiasis, Cutaneous), viral, or fungal infections. [7, 8] As emphasized above, the clinical history, in association with KOH and Tzanck preparations, often helps to determine the correct diagnosis. In adult patients, erythroplasia of Queyrat is a diagnostic consideration, and biopsy should be performed upon clinical suspicion of a neoplastic process. Rare causes include amebiasis (see Amebiasis), usually in men who have sex with men. [12] This etiology should be suspected in patients who have a poor response to antibiotic therapy. Examination for trophozoites is necessary for diagnosis.
Treatment and outcome
Treatment includes topical antibiotics and antifungals. Topical corticosteroids are used to reduce inflammation. Additional treatment includes proper hygiene, with washing and drying of the prepuce. Circumcision is usually effective and may be indicated if medical therapy fails. [13, 10]
The outcome in treated patients is favorable, and serious complications are rare. Uncommonly, untreated cases can result in cellulitis and gangrene. Treatment failures should prompt further clinical examination and consideration of other etiologies. Failure of response in the setting of appropriate treatment suggests a premalignant or malignant lesion. Biopsy is necessary to rule out primary and secondary malignancies that involve the penis. One case report describes the presentation of acute promyelocytic leukemia as an ulcerating balanoposthitis. [14]
Plasma cell balanitis
Plasma cell balanitis, also known as Zoon balanitis and balanitis circumscripta plasmacellularis (see Balanitis Circumscripta Plasmacellularis), was first described by Zoon in 1952. This variant of balanitis almost exclusively affects uncircumcised males and is characterized microscopically by a plasma cell–rich inflammatory infiltrate. [15] Although far less common, an equivalent condition, plasma cell vulvitis, is seen in the vulvar region of females. [16]
Pathophysiology
The etiology of this condition is unknown. Some reports have suggested the lesions represent a plasma cell–rich variant of lichen planus.
In one study, immunoglobulin (Ig) E and IgG were determined to be major immunoglobulin classes in plasma cellular infiltrate; thus, the disorder may have more in common with a hypersensitivity reaction than an infectious process. Immunoperoxidase studies revealed low IgM levels and a mixed kappa-to-lambda ratio, which is consistent with a polyclonal B-cell reaction. [17] Another premise explains the disorder as a disturbed preputial ecology that results in the nonspecific balanitis. Progression to a malignant B-cell proliferation has not been reported, although a report of carcinoma of the penis postdating Zoon balanitis has been published. No association with human papillomavirus (HPV) and Zoon balanitis has been identified.
Clinical presentation/diagnosis
Although the mean age at presentation is 53 years, patients aged 18-88 years are described in two series, and presentation in children as young as 12 years has been described. The men are uncircumcised in most series.
Patients present with a solitary, glistening, sharply demarcated, large (2-3 cm), erythematous, speckled patch on the glans or inner prepuce. Rarely, multiple patches can erode and ulcerate. Clinical involvement is typically (85%) on both the glans and prepuce or prepuce only. Presentation on the glans alone is less common. Occasionally, discharge is the presenting symptom. The clinical course is typically chronic, and the initial presentation is delayed an average of 12 months.
Biopsy has been considered necessary for diagnosis. However, Arzberger et al report successful differentiation between balanitis and carcinoma in situ without biopsy, by in vivo use of reflectance confocal microscopy. [18]
The histopathologic features show a thinned epidermis or epithelium and a dense, superficial, bandlike, predominantly plasmacytic, inflammatory infiltrate in the dermis. The dermal infiltrate also contains lymphocytes, neutrophils, histiocytes, and eosinophils. Dilated capillaries and associated extravasated red cells and hemosiderin deposition may also develop.
Differential diagnoses
The clinical presentation of Zoon balanitis can mimic erythroplasia of Queyrat (see Erythroplasia of Queyrat [Bowen Disease of the Glans Penis]). Biopsy is necessary to differentiate these diseases. Erosive lichen planus, contact dermatitis, psoriasis, and immunobullous diseases can cause similar clinical lesions in the glans region. Balanitis xerotica obliterans (BXO; see Lichen Sclerosus et Atrophicus) can show similar microscopic features of a dense inflammatory infiltrate and a thinned epidermis; however, the infiltrate usually lacks a rich plasma cell component. Candidal balanitis should also be considered in the differential diagnoses. Although previously described as a separate entity, chronic pseudoerythroplastic balanitis now appears to be a different stage in the course of plasma cell balanitis.
Treatment and outcome
Previously, treatment focused on surgical excision or laser ablation, although the use of tretinoin (eg, Retin-A) has recently met with some success. [19, 20, 21, 22, 15] The use of topical and intralesional corticosteroids has met with mixed results, with some groups advocating use and other groups finding them (particularly topical forms) ineffective. More recently, some therapeutic success with the calcineurin inhibitors tacrolimus and pimecrolimus has been reported. [23]
Although outcome with the use of newer treatments has yielded promising results, surgical removal of the foreskin has been advocated as the standard management. [7, 13, 8] Circumcision was successful in 27 patients, with no recurrence after 3 years of follow-up. [15] Most authors report a nearly 100% curative effect with adequate circumcision.
Candidiasis
Candidal balanitis (see Candidiasis, Cutaneous) is a relatively common disorder that usually affects uncircumcised men with poor hygiene. It is common in patients with diabetes, those with HIV seropositivity, and those receiving immunosuppressive medications. Candidal balanitis has also been associated with vulvovaginitis in the sexual partner, and treatment of both partners with antifungals usually results in a greater chance of treatment success. [8]
Pathophysiology
Although common in uncircumcised men, the main association appears to be with poor hygiene and the entrapment of smegma under the prepuce. Additional factors that predispose to infection include surface maceration, diabetes mellitus, and immunosuppression. Candidal balanitis is often associated with candidal vaginal infection in the sexual partner, thus leading to a co-infection. Although many species have been isolated, Candida albicans is by far the most common. [13, 8]
Clinical presentation/diagnosis
The clinical presentation is typically that of bright-red macules or patches, often with superimposed erosions, on the foreskin and glans. (See the image below.) A whitish exudate and/or 1- to 3-mm papules or pustules (satellite lesions) may also be present. Patients also often describe an itching and burning sensation, which may be exacerbated during intercourse. Diagnosis is usually established with a KOH preparation, revealing spores and pseudohyphae. Biopsy demonstrates a neutrophilic infiltrate in the epidermis and associated lymphohistiocytic inflammation in the dermis. Diagnostic pseudohyphae are present in the stratum corneum.
Differential diagnoses
The differential diagnoses include psoriasis, reactive arthritis (Reiter syndrome), contact dermatitis, erosive lichen planus, Zoon balanitis, immunobullous disease such as pemphigus, and seborrheic dermatitis. Dermatophytes (tinea) rarely, if ever, affect the glans. In addition to these inflammatory diseases, erythroplasia of Queyrat and squamous cell carcinoma should be considered, especially in lesions unresponsive to therapy.
Treatment and outcome
Treatment is with antifungal agents, such as topical azole creams (eg, clotrimazole cream 1%, applied twice daily for 7–14 days) or nystatin. Oral antifungal agents, such as fluconazole, are also effective and may be necessary in extensive or severe cases. [10]
Lesions typically resolve within days to weeks of therapy initiation. Serious complications are uncommon. Recurrent episodes can be minimized if infected partners are also treated. In patients who fail to respond promptly, candidal superinfection of an underlying dermatosis or neoplastic process (see Differential diagnoses) should be considered.
Dermatophytosis and Scabies
Dermatophytosis
Dermatophytes are fungal organisms that colonize and infect the keratinized epidermal layer and establish an associated inflammatory host reaction. When present in the groin or male genital region, it is referred to as tinea cruris or jock itch (see Tinea Cruris).
Pathophysiology
The causative organisms for tinea cruris are present on environmental surfaces such as the floor or in communal showers. The fungi colonize and infect the superficial layers of the epidermis. The most common pathogens include the species Trichophyton rubrum, Trichophyton mentagrophytes, and Epidermophyton floccosum.
Clinical presentation/diagnosis
Patients usually present with red-brown circinate or annular patches with raised borders. The lesions are typically scaly. Sites involved include the thighs, the groin, and, rarely, the scrotum. Secondary excoriation and lichenification are common, and continued excoriation can lead to secondary bacterial infection. Diagnosis is established based on the demonstration of hyphae in KOH preparation or skin biopsy. Fungal culture may also be used.
Differential diagnoses
The differential diagnoses include psoriasis, candida, erythrasma, seborrheic dermatitis, and contact dermatitis. Involvement of the scrotum by dermatophytes is rare, and, if involved, candidal infection is more likely (see Cutaneous Candidiasis).
Treatment and outcome
Topical antifungal medications are effective. Thorough drying after cleansing to avoid maceration is helpful. In a systematic review of 129 randomized controlled trials, combinations of azoles with corticosteroids were slightly more effective than azoles alone for clinical cure, but there was no statistically significant difference with regard to mycological cure. [24]
Serious complications are rare. Recurrence is not uncommon. Bacterial superinfection can occur and requires prompt antibiotic therapy.
Scabies
The highly contagious pruritic lesions of scabies result from infestation by the mite Sarcoptes scabiei (see Scabies).
Pathophysiology
Humans are the only known reservoir for S scabiei. Scabies is typically transmitted through skin-to-skin contact with an infected individual,. [25] In addition to sexual transmission, scabies can also be transmitted by casual exposure or exposure to contaminated clothing or bedding. [26] Poor socioeconomic conditions with overcrowding can predispose to infestation.
Scabies mites create burrows up to 1 cm in length in the epidermis and lay eggs within these nests. These eggs often elicit an IgE-mediated cellular immune response and the associated pruritus. In fact, patients often have elevated serum IgE levels. Larvae emerge from their burrows in 4 days and mature in 10-14 days.
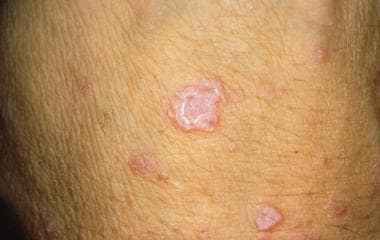
Clinical presentation/diagnosis
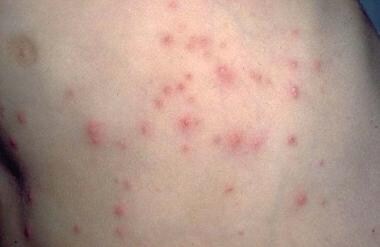
Patients often present with severely pruritic, pinpoint, red papules or vesicles (see the image below). The lesions tend to be concentrated in flexural areas, such as the genital or axillary region, or the finger web spaces. Excoriations are generally more apparent than the primary lesions. Lesions in the male genital region often appear more nodular. Diagnosis is based on demonstration of the mite, eggs, or mite feces in a scabies skin preparation, which should be obtained from several lesions because of the paucity of mites in most infestations.
Although not always necessary, biopsies reveal the female mite in the superficial epidermis, with eggs in the burrow staining positive with periodic acid-Schiff (PAS) stain. Epidermal spongiosis and an associated mixed dermal inflammatory infiltrate that contains eosinophils is usually present.
Differential diagnoses
The differential diagnoses include non-specific eczematous dermatitis, contact dermatitis, and nonscabietic arthropod bites. Nodular and treated forms (scabies incognito) may make the diagnosis more difficult. The use of topical corticosteroids may also mask a more typical presentation.
Treatment and outcome
Permethrin (5%) cream is recommended, with application to the entire skin surface below the chin. The cream should be left on for 8-14 hours (overnight) and then washed off. Second-line therapy includes the use of lindane 1% lotion/cream or oral ivermectin. Ivermectin may be repeated in 2 weeks; lower rates of treatment failure have been reported in patients treated with two doses of oral ivermectin , 95% CI 3.1-12.3) compared with those treated with one dose (7.1% vs 15.2%, respectivelyy; P = 0.021). [27]
Lindane is not recommended for pregnant women or children younger than 2 years. Use lindane as a single dose (do not repeat). [25]
Antipruritic agents may be helpful in reducing discomfort related to pruritus. [28]
All household members and close contacts must be treated simultaneously, regardless of the presence of symptoms, to remove the reservoir and to eliminate reinfection. Itching may continue for as long as one week after treatment, most likely because of the immune reaction to residual scabies material. If no response is observed, search for mites a second time before a second round of treatment is given. Clothes and bedding used in the week prior to therapy should be washed in hot water and dried in a hot dryer before subsequent use.
The overall response rate to the first treatment is approximately 90%. A particular crusted variant (hyperkeratotic or Norwegian scabies) exists, characterized by more keratotic lesions, is seen in patients with HIV or other immunocompromised states. This form is highly contagious, even to individuals who are not immunocompromised, because of the high total mite burden and may lead to localized epidemic outbreaks. Bacterial superinfection, usually by staphylococcal or streptococcal species, may occur.
Infectious Agents Affecting the Male Genitalia
Lymphogranuloma venereum
Although uncommon in the United States as a cause of genital ulcers, lymphogranuloma venereum (LGV; see Lymphogranuloma Venereum) is common in tropical and subtropical areas of the world. [29] LGV is more commonly reported in men, possibly because early manifestations of LGV are more apparent in men. [30, 31] In recent decades, outbreaks of LGV in men who have sex with men (MSM) have been reported in North America, Europe, and Australia, and the infection has become endemic in this population. [30, 32, 33, 34, 35, 36, 37, 38, 39]
Pathophysiology
Chlamydia trachomatis, an obligate intracellular bacterium that infects macrophages, is the causative agent. Serotypes L1, L2, and L3 have been associated with infection. Neither the rate of transmission nor the reservoir of C trachomatis has been clearly defined. Exposure to asymptomatic female carriers is believed to be the source of most infections. LGV infection is typically characterized by 3 stages, which are described in more detail below. After initial infection, LGV-infected macrophages migrate to the regional lymph nodes, resulting in the host inflammatory response and subsequent clinical manifestations. [40] Systemic spread may also occur.
Clinical presentation/diagnosis
The disease affects sexually active individuals, with the acute manifestations being more common in men than in women. Following an incubation period of 3-30 days, an asymptomatic papule or pustule appears at the site of exposure. The lesion may erode, resulting in a painless discrete ulceration. Spontaneous healing occurs within one week, and this first stage often goes unnoticed by the patient. [40]
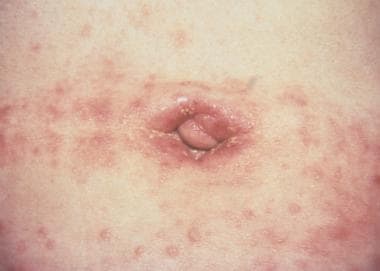
The second stage occurs 2-6 weeks later, with lymphadenitis of the ipsilateral inguinal and/or femoral nodes and associated lymphangitis. The painful lymph nodes are referred to as buboes. Buboes can become fluctuant and rupture, or they may become hard nonsuppurative nodules. In approximately one third of patients, inflammation of the nodes above and below the Poupart ligament can result in the so-called groove sign (see the image below). Constitutional symptoms such as fever, arthralgias, and malaise may develop during this second stage. [40]
The third stage of LGV infection, also called the genitoanorectal syndrome, is attributed to the host chronic inflammatory response to C trachomatis in the infected region. This stage is characterized by symptoms such as proctocolitis, perirectal fistulas, abscesses, strictures, and rectal stenosis. [40]
LGV can be diagnosed based on demonstration of the organism in culture, typically by aspiration and culture of the infected lymph node. However, culture is expensive and technically difficult, and the optimal growth medium, cycloheximide-treated McCoy cells or HeLa cells, is not readily available. Even under optimal conditions, yields of only 50% are reported. [41]
Because of the challenges associated with culture, serologic tests are typically used for diagnosis. An LGV complement fixation antibody titer is probably the most useful test and typically shows positive results within 2 weeks of infection. A titer of greater than 1:64 is highly indicative of active C trachomatis infection. [41] Caution must be used when this test is interpreted, however, as low-level positive titers may result from cross-reactivity to other chlamydial serotypes. Recently, real-time polymerase chain reaction (PCR) assays have shown success in the diagnosis of LGV. [42, 43, 44, 45]
In the United States, national reporting of LGV ended in 1995. If required by state law, these cases should be reported to the health department.
Differential diagnoses
The differential diagnoses include granuloma inguinale (see Granuloma Inguinale [Donovanosis]), chancroid, herpesvirus infection, and syphilis (see Syphilis). These conditions can often be distinguished based on clinical presentation, serology, evaluation of exudate or ulcer smears, and appropriate microbiologic culture. Anorectal involvement can mimic the clinical presentation of inflammatory bowel disease.
Treatment and outcome
The recommended treatment is doxycycline 100 mg PO bid for 21 days. Asymptomatic patients and contacts of LGV patients should receive the same treatment. [33] Oral erythromycin 500 mg qid for 21 days is also effective. Aspiration or incision and drainage of suppurative nodes may be necessary. Significant sequelae from scarring and fibrosis may require surgical intervention. [46, 40] Azithromycin 1 g weekly for 3 weeks has been proposed as an alternative treatment, but only limited data on its efficacy are available; [47] if it is used, a test of cure with a C trachomatis nucleic acid amplification test (NAAT) 4 weeks after completion of treatment can be considered. [46]
In addition, European guidelines recommend that all MSM who report receptive anal sexual practices in the previous 6 months be screened for anorectal C trachomatis infection. Those MSM found to be anorectal C trachomatis positive should then be screened for LGV proctitis. Tests for sexually transmitted infections, including HIV (if the patient is not already known to be HIV positive), hepatitis B, and hepatitis C, should be offered before starting therapy. [33] Likewise, CDC guidelines recommend testeing for other sexually transmitted infections (especially HIV, gonorrhea, and syphilis) in patients diagnosed with LGV. [46]
Outcome varies depending on the degree of inflammation, scarring, and fibrosis. If LGV is treated early, serious complications are rare. However, in late-stage disease, serious sequelae from inflammation and scarring, such as strictures, abscess or fistula formation, and lymphedema, are not uncommon. Although rare, systemic complications such as cardiac or pulmonary infection may develop if untreated.
Primary syphilis
In the United States, the incidence of syphilis had decreased to an all-time low in 2000. Since then, however, the rate has increased almost every year, and in 2022, a total of 203,500 primary and secondary syphilis cases were reported, representing a 78.9% increase since 2018. [48] Reservoirs of infection are still present in certain populations, particularly among MSM, individuals infected with HIV, prostitutes, and urban users of crack cocaine. Infection is acquired through sexual contact with an infected individual. [40, 48]
Pathophysiology
The causative organism is the spirochete Treponema pallidum (see Syphilis). The pathogenesis is not well understood because no good animal models exist for the study of syphilis. Infection occurs mainly through sexual contact with infected individuals who are in the early stages of the disease (within the first couple of years). The organism enters through the skin and/or mucous membranes, and manifestations are noted after a 2- to 4-week incubation period. [49, 50, 51]
Syphilis infection typically involves 3 distinct clinical stages. The primary lesion, known as a chancre, develops at the site of inoculation. The next stage, secondary syphilis, results in various clinical manifestations, primarily in the skin and mucous membranes. Lesions in both the first and second stages contain numerous treponemal organisms and are consequently contagious. Following the second stage is a period of latency. Finally, the third stage, known as tertiary syphilis, is characterized by cardiovascular and/or central nervous system involvement. Tertiary syphilis is rare and develops in approximately one third of untreated infected individuals. [49, 50]
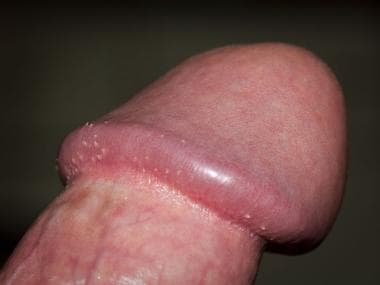
Clinical presentation/diagnosis
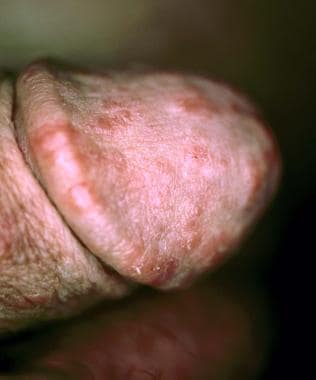
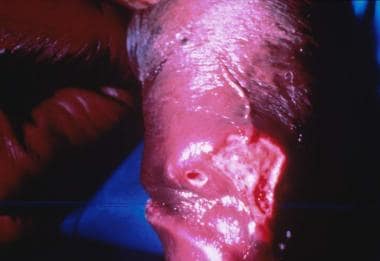
The stages of syphilis have distinct clinical manifestations. Primary syphilis presents as a discrete painless penile ulcer (chancre). The lesion is usually located on the glans, foreskin, or scrotum (see the image below). Up to 5% may present in extragenital locations. Anorectal lesions are also common in infected MSM and may present clinically as anal fissures. Nontender inguinal adenopathy may also be present. Rarely, more than one lesion is present. The chancre resolves spontaneously within 3-6 weeks. [40, 49, 50]
The second stage of syphilis, 6-8 weeks after appearance of the chancre, is characterized by skin and/or mucosal manifestations. The classic presentation is that of an asymptomatic widespread macular and papular eruption with involvement of the palms and soles. The characteristic mucous membrane lesion in this stage is called the mucous patch and typically involves the oral mucosa. The skin manifestations of secondary syphilis are protean and can range from alopecic patches in the scalp to verrucoid papulonodules known as condyloma lata. In HIV-infected patients, the primary-stage lesion may overlap secondary-stage lesions. [40, 49, 50]
The tertiary stage of syphilis is rare in the United States. Neurosyphilis, cardiovascular syphilis and gummata are characteristic of this stage. [40, 49, 50]
The diagnostic criterion standard for primary syphilis is the demonstration of the spirochete in scrapings from a syphilitic lesion with dark-field microscopy. This is technically difficult and no longer routinely performed. In clinical practice, the serologic studies are the most common method of diagnosing syphilis. Nontreponemal antibody tests, such as venereal disease research laboratory (VDRL) and rapid plasma reagin (RPR), are used as screening tests. If reactive, these are reported as an antibody titer. [40, 49]
For further diagnostic confirmation and to exclude false positivity of nontreponemal test results, specific treponemal assays are used. These include the fluorescent treponemal antibody absorption test (FTA-Abs). Once infected, the results of the nontreponemal tests and FTA-Abs remain positive, regardless of whether the patient was treated. However, the nontreponemal tests can be used to monitor response to therapy, as the titer should drop 4-fold in successfully treated patients. Occasionally, early therapeutic intervention results in conversion to a seronegative state.
Seronegative syphilis occcurs rarely in severely immunocompromised HIV-infected patients. Diagnosis in these patients is dependent on demonstration of spirochete organisms by silver stains or treponemal immunohistochemistry in paraffin-embedded lesional tissue or polymerase chain reaction (PCR) performed on lesional tissue. A rise in titer suggests reinfection or treatment failure. Microbiologic culture is not used, as the spirochete T pallidum has not been successfully cultured. [40, 49, 52, 53, 54, 55]
Differential diagnoses
The differential diagnoses include chancroid (see Chancroid), granuloma inguinale (see Granuloma Inguinale [Donovanosis]), herpesvirus infection, and LGV (see Lymphogranuloma Venereum). These conditions can often be distinguished based on clinical presentation, serology, evaluation of exudate or ulcer smears, and appropriate microbiological culture. The possibility of concomitant or superimposed infections should also be considered when the presentation is atypical or if the patient is HIV seropositive.
Treatment and outcome
Intramuscular penicillin G benzathine (2.4 million U in single dose) is still the preferred and recommended treatment for primary, secondary, and latent syphilis. A second dose is sometimes administered one week later. Doxycycline (100 mg bid for 2 wk) or tetracycline (500 mg qid for 2 wk) is an alternative in patients sensitive to penicillin. Patients should be aware that treatment might result in a transient acute febrile reaction with accompanying headache and myalgias (Jarisch-Herxheimer reaction) that may occur within 24 hours after initiation of treatment, especially in early cases.
Treatment failures occur in all groups; thus, serology must be obtained at 3, 6, and 12 months to ensure a decrease in antibody titers and a lack of reinfection.
Successful treatment in the primary and secondary stages results in complete resolution of the disease. If left untreated, approximately 50% of patients progress to secondary syphilis and the remainder enter a latency period. Following the latent period, tertiary syphilis develops in approximately 30% of untreated patients. Antimicrobial therapy is not effective in eradicating the tertiary stage of the disease. [40] Syphilis is often seen in association with HIV infection; therefore, evaluation of HIV status is recommended in patients with syphilis. The clinical presentation of syphilis in patients infected with HIV may be atypical and the course can be accelerated.
Granuloma inguinale
Granuloma inguinale (donovanosis; see Granuloma Inguinale [Donovanosis]) is a sexually transmitted disease that is most commonly seen in developing countries. It is extremely rare in the United States. Occurring predominantly in tropical areas, including Southeast Asia, India, the Caribbean, and aboriginal Australia, possible outbreaks may be related to travel from endemic regions. [40, 56]
Pathophysiology
Granuloma inguinale is caused by Calymmatobacterium granulomatis, a gram-negative, pleomorphic, obligate intracellular bacillus. C granulomatis is present within histiocytes in the papules or ulcerated lesions. The actual rate of infectivity is low, ranging from 0.4-0.5%, and incubation periods vary from 1 day to 1 year, with an average of 17 days.
Clinical presentation/diagnosis
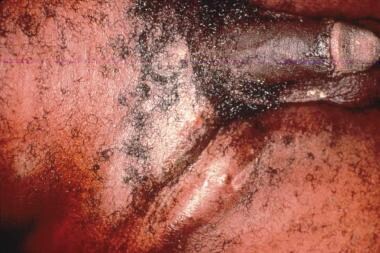
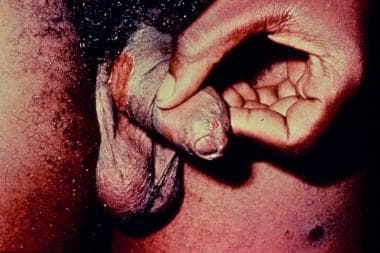
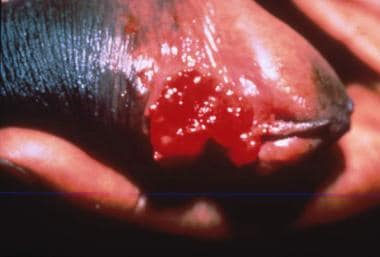
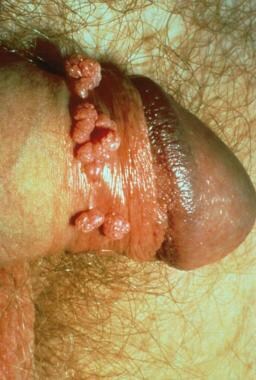
The disease is characterized clinically by multiple indurated papules or nodules that coalesce to form large ulcers. The lesions have a beefy red appearance and bleed easily. In males, the glans, prepuce, coronal sulcus, and shaft of the penis are most commonly affected. (See the images below.) Perianal and inguinal involvement may also occur. Autoinfection is common, with the formation of so-called "kissing" lesions. Primary lymph node infection does not occur, but regional lymphadenitis may develop.
The classic presentation is generally recognizable with careful clinical examination. Granuloma inguinale can take on various phenotypes, with more severely ulcerative lesions, large dry vegetative masses, and painful, foul-smelling necrotic variants. When an atypical presentation occurs, the presence of a superimposed infection must also be considered. Diagnosis depends on the demonstration of macrophages with intracellular bacteria. This is accomplished through a scraping of an ulcer or a biopsy of lesional skin. [40, 50]
The histologic appearance of the prototypical ulcer reveals acantholytic epidermal borders with pseudoepitheliomatous hyperplasia. The ulcer bed contains prominent granulation tissue and vascular ectasia. Pathognomonic macrophages with cytoplasmic vacuoles contain bipolar staining bacilli (Donovan bodies). These are best demonstrated in Giemsa or Warthin-Starry stained sections. [40, 41]
Reliable culture is not available, with the last documented successful culture in 1962. Recent attempts at growth using coculture with monocytes have been reported. [57] Testing through indirect immunofluorescence methods has been under investigation. PCR based assays have also been used for the diagnosis of granuloma inguinale. [58, 59]
Differential diagnoses
The differential diagnoses include syphilis (see Syphilis), chancroid (see Chancroid), herpesvirus infection, and LGV (see Lymphogranuloma Venereum). These conditions can often be distinguished based on clinical presentation, serology, evaluation of exudate or ulcer smears, and appropriate microbiological culture. The possibility of concomitant or superimposed infections should also be considered when the presentation is atypical or if the patient is HIV seropositive.
Although the typical presentation is rather pathognomonic, variant presentations may pose a diagnostic challenge. The verrucous form may simulate condyloma (see Condyloma Acuminatum) or carcinoma, and the necrotic variant may lead to consideration of chancroid (see Chancroid). Smear cytology with stains should support the correct diagnosis.
Treatment and outcome
First-line treatment for granuloma inguinale is with doxycycline (100 mg bid) or trimethoprim-sulfamethazine (TMP-SMZ) (double-strength bid) for at least 3 weeks or until all lesions have cleared. The CDC recommends ciprofloxacin (750 mg bid) or erythromycin (500 mg qid) as second-line treatments for refractory disease. Tetracycline resistance has been reported. [40]
Treatment is usually effective, but relapses can occur within 6-18 months. Close clinical monitoring of patients is recommended. Although granuloma inguinale does not tend to produce generalized symptoms, systemic dissemination, if left untreated, can lead to death. Lymphatic obstruction can lead to lymphedema and scrotal elephantiasis. Scarring and fibrosis are also well-documented sequelae of untreated locally aggressive disease and may require surgical intervention for repair. Extragenital lesions are a consequence of autoinoculation or dissemination.
Chancroid
Chancroid (see Chancroid) is a sexually transmitted disease found primarily in Africa, the Caribbean basin, and Southeast Asia. The disease is rare in the United States. During 2014–2018, the number of reported cases has fluctuated, ranging from 11 in 2015 to three in 2018, with cases located in California, Texas, and South Carolina. [48]
Pathophysiology
The causative organism is Haemophilus ducreyi, a facultative, gram-negative coccobacillus. It adheres to the surface of epithelial cells and produces cytotoxins that are associated with cellular damage and ulcer formation. [60] Inhibition of phagocytosis is another mechanism of pathogenesis that has recently been reported. [61]
Clinical presentation/diagnosis
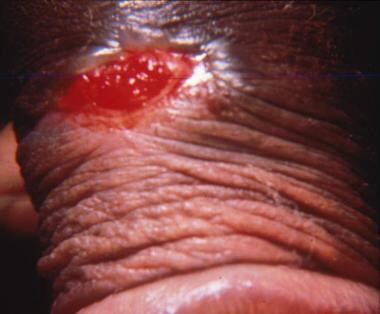
Initial lesions occurs in 3 days to 2 weeks after exposure. Tender erythematous papules develop into painful ulcers 0.3-2 cm in size (see the image below). Lesions may coalesce to form large ulcers. In half of cases, chancroid is associated with painful lymphadenopathy. Approximately 25% of these cases progress to a suppurative bubo that ulcerates. [40, 50]
Diagnosis depends on the demonstration of the organisms in tissue sections, in culture, or, more recently, in PCR-based assays. [59] In biopsy specimens, the microscopic features include the identification of 3 zones: a surface fibrinoid exudate with neutrophils and debris, an intermediate zone of granulation tissue, and a deep zone of a mixed inflammatory infiltrate with numerous plasma cells.
H ducreyi bacteria is occasionally identified in Giemsa-stained sections but are generally better seen in smears. [41] Although not sensitive or specific, a Gram-stained smear of the ulcer base can show gram-negative coccobacilli in chains and clusters (railroad-track/school-of-fish pattern). Cultures are diagnostically definitive, but growth of the organism requires special media, and isolation rates are variable. [62, 41]
Atypical presentations are common, often because patients have more than one disease. Because of the difficulty in diagnosis, the CDC recommends establishing a provisional diagnosis based on the following: one or more painful genital ulcers, clinical presentation and lymphadenopathy suggestive of chancroid, and negative laboratory results for T pallidum and herpes simplex virus (HSV).
Differential diagnoses
The differential diagnoses include syphilis (see Syphilis), granuloma inguinale (see Granuloma Inguinale [Donovanosis]), herpesvirus infection, and LGV (see Lymphogranuloma Venereum). These conditions can often be distinguished based on clinical presentation, serology, evaluation of exudate or ulcer smears, and appropriate microbiological culture. The possibility of concomitant or superimposed infections should also be considered when the presentation is atypical or if the patient is HIV seropositive.
Treatment and outcome
Chancroid can be successfully treated with antibiotics. Differences in local sensitivity have been noted, and H ducreyi can acquire both gram-negative and gram-positive resistance factors. Current antibiotic regimens include the following [46, 40] :
-
Azithromycin 1 g PO single dose
-
Ceftriaxone 250 mg IM single dose
-
Ciprofloxacin 500 mg PO bid for 3 days
-
Erythromycin base 500 mg POP tid for 7 days
Ceftriaxone was effective in the United States but has been associated with treatment failures in Kenya. Fleroxacin has been effective in Kenya. Trimethoprim-sulfamethoxazole has been ineffective. Because of the nature of the disease, sexual partners should also be treated. Vaccines for chancroid are currently in development. [63]
Treatment with antibiotic therapy resolves the clinical lesions. The population at risk for chancroid is also at risk for HIV infection. Patients with HIV infection may not respond as well to the outlined antibiotic regimens.
Inflammatory Disorders of the Male Genitalia
Balanitis xerotica obliterans
Balanitis xerotica obliterans (BXO), also known as lichen sclerosus, is an inflammatory disorder that usually affects the anogenital skin. Lichen sclerosus is a benign disorder of unknown etiology and is far more prevalent in women than men. In men, it is commonly referred to as BXO, as it usually involves the glans penis and the foreskin. Uncircumcised males are usually affected, and BXO is an established cause of phimosis and meatal stenosis in this population. Among those with BXO, complications often arise as a consequence of the sclerosis and scarring typical of this disorder.
Pathophysiology
The exact pathogenesis of BXO is unknown. Both an infectious and an autoimmune etiology have been proposed. An association with Borrelia burgdorferi has been reported in some series; however, this has not been substantiated in other reports. Although some patients with BXO are more likely to have an accompanying autoimmune disorder, pathogenic antibodies have not been consistently demonstrated in the affected skin or sera of those with this condition. BXO/lichen sclerosus may be a superficial variant of morphea (localized scleroderma) because they share similar histopathologic features, and the two conditions may coexist in the same patient.
Clinical presentation/diagnosis
BXO occurs in a wide age range, including young prepubescent boys and older men. Males with BXO typically present with phimosis and/or recurrent balanitis. In addition, pain during urination or erection may also be a presenting symptom. BXO usually involves the glans, prepuce, urethral meatus, and, occasionally, the shaft. [64, 65] The clinical presentation is that of well-defined, atrophic pale-gray to ivory-white macules or patches that resemble cigarette paper. Punctate areas of hemorrhage, fissures, and erosions are commonly apparent because of the pruritic nature of the lesions. Focal areas of hyperkeratosis may also develop.
The diagnosis can be established clinically; however, biopsy is often necessary for confirmation. The characteristic histopathologic findings include epidermal orthokeratosis and atrophy with focal vacuolar change of the basal layer and hyalinization (sclerosis) of the papillary and superficial reticular dermis. [66, 67, 68] A superficial perivascular lymphocytic inflammatory infiltrate is frequently present.
Differential diagnoses
The differential diagnoses include vitiligo (see Vitiligo), scar, and atrophic lichen planus. Symptoms are usually absent in patients with vitiligo. Patients with scars may have a history of prior trauma or inflammation. Patients with lichen planus frequently have characteristic lesions in other sites. Biopsy is often necessary to distinguish these disorders, as well as to exclude the possibility of early squamous cell carcinoma. [65]
Treatment and outcome
For early-stage disease, topical therapies may be effective. However, late-stage disease with extensive sclerosis is unlikely to respond well to medical therapy, and surgical intervention is often required. Circumcision is the therapy of choice for lesions that involve the foreskin. Topical agents, such as testosterone creams, petrolatum, and antifungals, are generally ineffective. Although high-dose topical corticosteroids have been used with success in women (in some cases even reversing the course of disease), they have not been studied in detail in men. Based on limited studies in men, topical corticosteroids may be effective, particularly in early disease. [67] However, long-term corticosteroid treatment may cause epidermal atrophy or lead to reactivation of latent infections such as HPV.
Other treatment modalities involving surgery, laser therapy, and retinoids produce limited success. [69, 70, 71] Topical tacrolimus is often effective. [72] If urethral involvement and stenosis are present, meatotomy or dilation may be necessary.
The outcome of BXO is variable and depends on the degree of sclerosis and scarring. Patients may require surgical intervention for structural or functional impairment. Meatal stenosis is a complication of urethral meatal involvement that may require surgical management.
BXO is associated with penile squamous cell carcinoma; however, the actual incidence of squamous cell carcinoma in patients with BXO is unclear. [73] One study showed that squamous cell carcinoma developed in 5.8% of patients with BXO. [74] Some studies have suggested that HPV may be a cofactor in the development of penile squamous cell carcinoma in patients with BXO. [75] However, other studies have shown non-HPV–associated penile squamous cell carcinoma arising in patients with BXO. In females, lichen sclerosus is also associated with vulvar squamous cell carcinoma in a minority of cases.
Lichen planus
Lichen planus is a pruritic papulosquamous skin disorder of unclear etiology. The skin, mucosal surfaces, and/or nails may be affected. Patients may have genital involvement along with widespread cutaneous disease, or the disease may be restricted to the genital and/or mucosal regions.
Pathophysiology
Although several hypotheses have been proposed, the specific etiology of lichen planus is unknown. Cell-mediated immune response and alteration of epidermal antigenicity appear to be important in the pathogenesis. Genetic factors play a role, as lichen planus is significantly associated with HLA-DR1 and HLA-DQ1. In recent years, an epidemiological association has been established between hepatitis C viral infection and lichen planus.
Clinical presentation/diagnosis
Usually, the onset of genital lichen planus occurs in the fourth to sixth decade of life. A possible delayed hypersensitivity reaction to an unknown epidermal antigen is the current theory. The cellular immune response to basal epithelial cells causes initial destruction of the basal cell layer by T cells. Familial cases and cases associated with liver cell abnormalities, including chronic active hepatitis and primary biliary cirrhosis, have been reported. HLA-DR1 is associated with lichen planus.
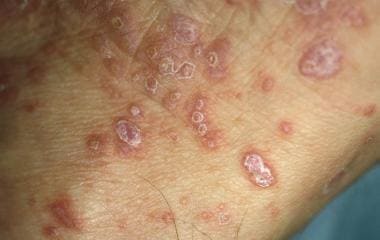
The classic presentation is that of widespread discrete, flat-topped, polygonal papules that have a predilection for the flexural surfaces. The individual papules are violaceous in color and often have overlying white lines known as Wickham striae. (See the images below.) The lesions tend to be extremely pruritic, and, as they heal, considerable dyspigmentation is notable at sites of prior involvement. Nails and the oral mucosa are frequently involved. Genital involvement is common in males, affecting up to 25% of patients with widespread disease.
Occasionally, penile involvement may occur in the absence of other cutaneous lesions. In the genital region, the lesions often appear annular, eroded, or ulcerated. Patients may present with itching, pain, or dysuria. In the absence of characteristic cutaneous lesions, biopsy is necessary to establish the diagnosis. The histopathology shows epidermal hyperkeratosis, acanthosis, wedge-shaped hypergranulosis, and interface alteration of the basal keratinocytes. The dermis displays a superficial, dense bandlike, predominantly lymphocytic inflammatory infiltrate. Apoptotic keratinocytes, known as Civatte or cytoid bodies, are typically present.
Differential diagnoses
Complete evaluation of the oral mucosa, skin, and nails is often helpful in establishing the diagnosis of lichen planus. The differential diagnoses include psoriasis, candidiasis, fixed drug eruption, early erythema multiforme, herpesvirus infection, syphilis, Zoon balanitis, BXO, erythroplasia of Queyrat, and squamous cell carcinoma. Most of these conditions can be differentiated on clinical grounds; however, microbiologic culture, skin biopsy, and serologies may be necessary for diagnosis in some cases.
Treatment and outcome
The standard therapy for genital lichen planus is topical corticosteroids. Symptoms and clinical response generally improve within several weeks. When severe, systemic corticosteroids (ie, prednisone) in doses ranging from 30-40 mg daily, tapered over 6-8 weeks, can be helpful. In steroid-resistant cases, therapy with systemic retinoids or topical immune modulators such as tacrolimus, pimecrolimus, [76] or cyclosporin may be helpful.
In most cases, lichen planus resolves spontaneously within 1-2 years. Recurrence and remissions separated by years are not uncommon. Scarring in the form of dyschromia—both hyperpigmentation and hypopigmentation—is a characteristic sequela of this disease. Phimosis due to lichen planus has been reported and may require circumcision if severe or unresponsive to medical therapy. Penile squamous cell carcinoma arising in lesions of lichen planus has been very rarely reported.
Fixed drug eruption
Fixed drug eruptions are erythematous lesions that develop within hours after a drug is taken and recur at the same site upon subsequent exposure to the same drug (see Drug Eruptions). [77] The lesions may be solitary or multiple and show a predilection for the lips, extremities, and genitalia. A large number of drugs have been reported to elicit fixed drug eruptions. The most common drugs implicated include sulfonamides, tetracyclines, trimethoprim-sulfamethoxazole (TMP-SMZ), ibuprofen, phenolphthalein, barbiturates, acetyl-salicylic acid, and quinine. An increased susceptibility is associated with HLA-B22. [78]
Pathophysiology
The specific pathogenic mechanism underlying the recurrent lesions in fixed drug eruptions is not fully understood. Current concepts of pathogenesis have focused on cell-mediated responses that involve effector CD8+ T cells. In addition, the recurrence at a fixed site appears to be related to memory T cells at the lesional site. Recent evidence has shown that apoptosis of keratinocytes by CD8+ T cells may play an important role in the pathogenesis of fixed drug eruptions.
Clinical presentation/diagnosis
Fixed drug eruptions present as erythematous to violaceous plaques that develop 30 minutes to several hours after a drug is taken. Some lesions show vesiculation. Rare cases of widespread bullous fixed drug eruption, clinically resembling toxic epidermal necrolysis (TEN), have been reported. Lesions typically involve the lips, hands, feet, or genitalia. The glans is the typical site involved in penile lesions. Pruritus and burning may be associated symptoms. The lesions resolve in days and leave a hyperpigmented brown macule that eventually fades. Re-exposure to the offending drug elicits lesions at the same site. Additional sites may become involved over time. The clinical history and characteristic presentation are often sufficient for diagnosis; however, diagnosis is often confirmed with biopsy.
The histopathologic features of fixed drug eruption show interface alteration of the basal epidermis with focal necrotic keratinocytes, a superficial dermal mixed inflammatory infiltrate with scattered eosinophils, and pigment incontinence in the papillary dermis.
Differential diagnoses
The clinical differential diagnoses of penile fixed drug eruption include erythema multiforme, lichen planus, and immunobullous diseases. In the widespread bullous variant of fixed drug eruption, TEN is in the differential diagnoses.
Treatment and outcome
Cessation of the associated drug induces resolution of lesions. Topical corticosteroids can be used to treat symptomatic lesions.
Recurrence is common if the patient is re-exposed to the offending drug. Generally, the outcome is excellent with no significant long-term complications. Postinflammatory hyperpigmentation may present a cosmetic concern in some patients.
Psoriasis
Psoriasis is a chronic skin disorder that affects approximately 2% of the population. The most common form of psoriasis is plaque-type psoriasis, which is characterized by the presence of well-demarcated, silvery-scaled, erythematous plaques. A significant percentage (5-42%) of patients with psoriasis may also have arthritis, which may be debilitating if it is severe.
Pathophysiology
The specific pathogenic mechanisms underlying psoriasis are not fully understood, but several factors, including genetic and environmental factors, play a role. A polygenic inheritance pattern has been proposed, as a positive family history is detected in up to one third of patients. HLA antigens, specifically HLA-Cw6 and HLA-DR7, confer a greater relative risk of developing psoriasis. Infections (streptococcal pharyngitis in particular), psychogenic stress, medications, and cutaneous injury have all been identified as triggers of psoriasis, particularly in genetically predisposed individuals.
Experimental evidence suggests that psoriasis is a T-lymphocyte–mediated skin disease. In addition, several reports have demonstrated an altered expression of cytokines, particularly interleukin (IL)–10, tumor necrosis factor (TNF)–a, and IL-8, in psoriatic skin lesions. The imbalance of cytokines likely plays a role in the acceleration of the keratinocyte turnover time in the epidermis of psoriatic lesions. Polymorphonuclear cells are also thought to be involved in the pathogenesis of psoriasis, although their exact role is unclear.
Clinical presentation/diagnosis
Psoriasis may present in individuals of any age, but most patients present before the fifth decade of life. Several variants of psoriasis exist, and any area of the skin may be affected. The most common form of psoriasis is chronic plaque-type psoriasis. Sites of predilection include the knees, elbows, and scalp; however, the trunk, nails, intergluteal fold, and genitals may also be involved. The classic psoriatic lesion is that of a sharply marginated, erythematous plaque with silvery scale.
In the pustular form of psoriasis, lesions are characterized by erythematous macules or patches with overlying pinpoint sterile pustules. This and other forms of psoriasis (erythrodermic, guttate, inverse) do not exclusively affect the genitals. Nail involvement may appear as nail plate thickening, yellow-brown discoloration ("oil staining"), and/or separation of the nail plate from the nail bed (onycholysis). In a significant number of males with plaque-type psoriasis, genital involvement may occur, but the genital region is rarely the exclusive site of involvement. Any portion of the scrotum or penis may be affected. In the genital region, rather than appearing as distinctly elevated, silvery-scaled plaques, the lesions often have less scale and are barely raised (or nonraised) erythematous lesions.
Pruritus is frequently present but is typically not severe. Genital psoriasis can usually be diagnosed on clinical grounds. Because most patients also have nongenital involvement, thorough skin examination, particularly of the scalp, elbows, knees, nails, umbilicus, and intergluteal fold, is necessary. If characteristic psoriatic lesions are lacking, a skin biopsy may be necessary for confirming the diagnosis.
The histopathologic features of a well-developed psoriasis lesion show epidermal hyperparakeratosis, hypogranulosis, acanthosis, and regular psoriasiform hyperplasia with thinning of the suprapapillary plate. Intracorneal and intraepidermal microabscesses are focally present. The dermis displays dilated vascular channels in the papillary dermis and a superficial, mild-to-moderate, mixed inflammatory infiltrate. Lesions of pustular psoriasis typically exhibit more extensive intraepidermal microabscesses and less pronounced acanthosis and hyperplasia of the epidermis.
Differential diagnoses
The differential diagnoses of psoriasis involving the male genitalia include candida, tinea, seborrheic dermatitis, lichen planus, syphilis, Zoon balanitis, and early pemphigus. If the patient has systemic symptoms, reactive arthritis should be considered. If a solitary lesion is present, skin biopsy may be necessary to exclude the possibility of erythroplasia of Queyrat, Bowen disease, or squamous cell carcinoma.
Treatment and outcome
Low- to mid-potency topical corticosteroids are the mainstay of therapy for genital psoriasis. With long-term topical corticosteroid application, atrophy can occur and may limit its use in this particular body region. Topical therapy with calcipotriol (see calcipotriene), a vitamin D analogue, or calcineurin inhibitors such as tacrolimus or pimecrolimus are also potential options. These latter therapies can be used alone or in combination with topical corticosteroids to minimize adverse effects. Other topically applied medications include the keratolytic agent, salicylic acid, and the retinoid tazarotene. The irritant effect of the latter often limits its use in the genital area.
In most cases, topical medications are effective in reducing the signs and symptoms of genital psoriasis. Systemic agents such as methotrexate, cyclosporine, anti–TNF-a agents (infliximab and etanercept), adalimumab, apremilast, and secukinumab are generally reserved for patients with severe, widespread, or refractory psoriatic disease.
The outcome for most patients with psoriasis is generally good. With or without treatment, the disease has a chronic course, often with periods of exacerbation and remission. Although rare, bacterial or fungal superinfection of genital lesions may occur. The psychosocial impact of psoriasis can be severe and is often underestimated by treating physicians.
Reactive arthritis
Reactive arthritis (formerly known as Reiter syndrome) is characterized by urethritis, arthritis, and ophthalmologic abnormalities that occur simultaneously or sequentially. Oral ulcers and psoriasiform skin lesions that affect the palms, soles, and genitalia are also common manifestations. The disease usually presents in men aged 20-40 years, and those with histocompatibility antigen HLA-B27 have a strong genetic susceptibility. [79]
Pathophysiology
The disease occurs primarily in genetically predisposed individuals, particularly those with HLA-B27, and is most commonly triggered by bacterial infectious agents. C trachomatis is responsible for triggering most cases. Enteric infection with Yersinia, Salmonella, Shigella, and Campylobacter species are also common infectious triggers for reactive arthritis. The exact pathogenetic mechanisms involved in induction of this condition are unknown.
Clinical presentation/diagnosis
Patients with reactive arthritis present with musculoskeletal, genitourinary, ocular, or mucocutaneous manifestations. These may occur simultaneously or sequentially. Symptoms of arthritis predominate in most patients. The arthritis often affects more than one joint and most commonly involves the spine (ankylosing spondylitis), knees, ankles, feet, and wrists. Ocular inflammation may be bilateral and frequently manifests as mucopurulent conjunctivitis, iritis, or iridocyclitis. Cutaneous involvement classically occurs on acral body surfaces, specifically the palms, soles, and penis. Palmoplantar lesions, often referred to as keratoderma blennorrhagica, are typically erythematous macules or patches with overlying thick, waxy, keratotic scale. Sterile exudate may be evident under these hyperkeratotic plaques.
On the penis, the lesions are referred to as balanitis circinata. These initially manifest as isolated or coalescing red erosions with a slightly edematous margin on the glans and meatus. Urethritis frequently manifests as pain, dysuria, and exudative discharge. Reiter syndrome is diagnosed based on the presence of a constellation of findings, and no specific test is diagnostic. Clinical correlation with laboratory, radiographic, and histological studies is necessary. Individuals infected with HIV may be at higher risk for developing reactive arthritis.
The histopathologic features of reactive arthritis are essentially identical to pustular psoriasis. The lesions show lesion show epidermal hyperparakeratosis, hypogranulosis, mild acanthosis, and mild psoriasiform hyperplasia. Prominent intracorneal and intraepidermal microabscesses are present. The dermis displays dilated vascular channels in the papillary dermis and a superficial, mild-to-moderate, mixed inflammatory infiltrate.
Differential diagnoses
The differential diagnoses of reactive arthritis that involves the male genitalia include psoriasis, candida, seborrheic dermatitis, lichen planus, syphilis, Zoon balanitis, Behçet disease, and cutaneous immunobullous diseases such as pemphigus.
Treatment and outcome
Treatment of the urethritis with agents such as tetracyclines or macrolides is recommended. Arthritis can often be managed with nonsteroidal anti-inflammatory agents such as indomethacin. Occasionally, corticosteroid injections, methotrexate, or other systemic agents are necessary. Treatment of the skin and genital lesions is similar to that of psoriasis (see Psoriasis treatment).
Although some patients with reactive arthritis undergo spontaneous remission, many patients experience a chronic relapsing course. Serious sequelae, such as immobility from joint involvement or blindness from ocular involvement, can occur if untreated.
Viral Lesions
Molluscum contagiosum
Named in 1817 by Bateman, molluscum contagiosum is a common cutaneous viral infection that is frequently seen in children and sexually active adults. Transmission is through skin-to-skin contact. In adults, the genital region is frequently involved. Facial involvement is uncommon in adults, except in individuals with HIV seropositivity.
Pathophysiology
The causative agent is a DNA poxvirus that infects the epithelium and leads to cellular proliferation and production of viral particles. When infected cells rupture, the particles are released and lead to infection of neighboring cells. After an incubation period of 2-7 weeks, papules develop and may persist for 2-6 months. Autoinfection is common.
Clinical presentation/diagnosis
Molluscum contagiosum infection is characterized by discrete, translucent, dome-shaped, skin-colored-to-whitish papules. A central umbilication or dell is often present and is a hallmark of these virally induced lesions. The lesions range from 1-8 mm in size, but larger lesions may be seen in individuals infected with HIV. Any part of the male anogenital region may be affected, including the penis, scrotum, and surrounding skin.
Molluscum contagiosum can be diagnosed based on a molluscum crush preparation. A curette is used to remove the lesion. The firm yellow-white contents of the lesion are gently crushed between 2 glass slides and evaluated microscopically. The characteristic ovoid molluscum bodies can be demonstrated.
The histopathology shows a papular morphology with central umbilication and surrounding epidermal hyperplasia. The diagnostic feature is the presence of large, eosinophilic, intracytoplasmic inclusions (Henderson-Patterson bodies) within lesional keratinocytes.
Differential diagnoses
The differential diagnoses of molluscum contagiosum in the genital region include condyloma acuminata/HPV infection, folliculitis, milia, ectopic sebaceous glands, and superficial epidermal inclusion cysts. When the host generates an immune response to the lesions, molluscum contagiosum may mimic furuncles or early impetigo. In patients infected with HIV who have low T-cell counts, disseminated infection by a systemic mycosis such as cryptococcus should be considered (see Histoplasmosis and Cryptococcosis). If molluscum contagiosum is unable to be distinguished with a clinical examination, a crush preparation or skin biopsy is diagnostic.
Treatment and outcome
Although the lesions are often self-limited, most patients prefer treatment because of the contagious nature. Therapy involves local destruction of the lesion through curettage, cryotherapy, or application of cantharidin. [80] Podophyllin (see podophyllum resin), topical and systemic cidofovir, keratolytic agents, silver nitrate, and laser to ablate the lesions have all been reported. In patients infected with HIV, immune reconstitution is essential to successfully eradicate molluscum infection. [81]
In the immunocompetent host, serious sequelae from molluscum contagiosum infection are extremely rare. Most lesions regress in 6-12 months, and involution occurs without scarring. Treatment prevents autoinfection and lowers the risk of transmission. On average, the full cycle, including reinoculation, runs a 2-year course. In immunosuppressed patients (particularly patients with untreated HIV infection), lesions frequently fail to respond to therapy, and bacterial superinfection can occur.
Herpes simplex
HSV infection is the most common cause of genital ulcers in developed countries (see Herpes Simplex). Most cases of genital herpes are due to HSV-2 (US seroprevalence of 22%). Higher seroprevalence rates are reported in inner cities and developing countries. HSV-1 is emerging as a significant cause of genital herpes and is involved in 5-30% of initial cases. Because the virus can establish a latent infection, recurrent clinical manifestations are common, but their impact can be lessened with antiviral treatment.
Pathophysiology
HSV is a lipid-enveloped DNA virus that predominantly infects mucosal or cutaneous surfaces. The virus replicates at the site of infection and then travels retrograde via sensory nerves to the dorsal root ganglion, where latency is established. Multiple mechanisms are involved in reactivation of the virus, including altered immunity, tissue damage, stress, and/or ultraviolet light exposure. If reactivation occurs, the virus begins replicating and manifests as active lesions at or around the site of initial exposure. Depending on the host immune response, dissemination to extracutaneous sites may occur.
Although infection cannot be eradicated, both cellular and humoral immunity are involved in limiting infection and preventing re-infection with exogenous HSV-2. Although overlap exists, HSV-2 has a predilection for the anogenital region, whereas HSV-1 predominantly affects orolabial skin and mucosa. This viral tropism for specific anatomical sites is not well understood.
HSV transmission occurs through viral shedding from active lesions in symptomatic individuals, as well as through viral shedding from asymptomatic individuals with a history of infection. Most patients infected with HSV-2 do not recall symptoms of primary infection. Most transmissions (up to 70%) occur during periods of asymptomatic shedding.
Clinical presentation/diagnosis
The clinical presentation of genital HSV infection can be classified into primary and recurrent. Primary infection is the patient's first exposure to genital HSV. Recurrent infection is a consequence of viral reactivation in those with prior exposure. Subclinical infection may occur in either case and accounts for a significant portion of cases. Symptomatic primary genital herpes manifests within 3 weeks of initial exposure. Males typically experience regional pain and an eruption characterized by clustered papulovesicles. Associated herpetic urethritis may manifest with dysuria and urethral discharge. Over the course of 2-3 weeks, the painful skin lesions progress to pustules, then to erosions or ulcerations, and then heal with crusting.
Regional lymphadenopathy and systemic constitutional symptoms may be present. Recurrent genital HSV infection is more commonly encountered in clinical practice and is characterized by a unilateral outbreak of clustered papulovesicles that rapidly ulcerate. The most common location in males is the penis, the glans, or the shaft; however, any part of the anogenital region may be affected. Regional nongenital skin, such as that of the thigh, buttocks, or pubic area, is also a common site of recurrent genital HSV. Many individuals experience a neuropathic prodrome, such as itching, tingling, or burning, prior to the onset of lesions.
In immunocompromised individuals, particularly those infected with HIV, the presentation of genital HSV may be somewhat atypical. The lesions in this subpopulation may heal more slowly and manifest as a large well-demarcated ulceration rather than as a cluster of smaller punctate ulcers. Genital HSV may also appear as an exophytic, condylomalike nodule in patients with HIV seropositivity. The exophytic lesions have been recently described as vegetative HSV infections and can clinically mimic a neoplastic process. According to CDC criteria, nonhealing genital HSV ulcers of more than 1 month duration are an AIDS-defining illness in patients who are infected with HIV.
Isolation of the herpesvirus in cell culture is the most definitive diagnostic test. Culture of early lesions, such as that of a vesicle or moist ulcer, has the highest diagnostic yield. Tzanck smear may also be useful and can yield results more quickly than other tests. However, identification of the multinucleated epithelial cells with intranuclear inclusions is sometimes difficult and does not distinguish HSV infection from infection from other herpesviruses (ie, varicella-zoster virus).
Another useful and commonly used diagnostic test is the direct fluorescence analysis (DFA). A swab from the base of an early lesion is smeared on a glass slide provided in the DFA collection kit. The specimen is then tested with fluorescent-tagged antibodies specific for viral proteins of HSV-1, HSV-2, or both. Results are generally available within hours, and the yield approximates that of culture. PCR testing is available through reference laboratories; however, this may not be readily accessible.
Although serologic tests for HSV are available, they are not often type specific (HSV-1 vs HSV-2), and positive results do not necessarily indicate acute active infection. In some patients, particularly those with an atypical presentation, lesional biopsy may be necessary to establish the diagnosis of genital HSV.
The histopathologic features of HSV range from a vesicle with marked epidermal acantholysis in early lesions to a well-delineated ulcer in later lesions. Diagnostic viral cytopathic effects include multinucleation, nuclear chromatin margination, and nuclear molding. Early lesions typically display numerous diagnostic cells, while later lesions show only occasional diagnostic cells. In vegetative HSV lesions, there is extensive dermal fibrosis and a dense lymphoplasmacytic inflammatory infiltrate with numerous eosinophils. The microscopic features of HSV and herpes zoster virus cannot be distinguished based on microscopic features. Immunohistochemistry for HSV can help to confirm the diagnosis.
Differential diagnoses
The differential diagnoses for genital herpes include syphilitic chancre, chancroid (see Chancroid), LGV, granuloma inguinale (see Granuloma Inguinale [Donovanosis]), Behçet disease, erosive candidiasis, erythema multiforme, and cutaneous Crohn disease. Atypical lesions often mimic condyloma or squamous cell carcinoma.
Treatment and outcome
Eradication of the virus is not possible. Treatment may be required to minimize symptoms and infectivity. In immunocompetent hosts, initial clinical episodes should be treated with oral acyclovir (400 mg tid), famciclovir (250 mg tid), or valacyclovir (1 g bid) for 7-10 days or until the lesions are fully healed. If initiated promptly relative to the onset of symptoms, these drugs shorten lesion duration and reduce viral shedding but do not affect severity or frequency of recurrences.
Continuous or suppressive therapy with oral antivirals has been shown to reduce asymptomatic shedding and clinical outbreaks. As many as 80% of patients are symptom-free at 5 years. The decision to place a patient on suppressive antiviral therapy depends on the severity of recurrences along with patient-related psychosocial factors. CDC recommendations for suppressive therapy include acyclovir (400 mg bid), famciclovir (250 mg bid), or valacyclovir at 500-1000 mg daily. Acyclovir resistance is emerging, particularly in immunosuppressed transplant patients and those with AIDS. Resistance is most often due to mutations in the gene coding for viral thymidine kinase, although, in some cases, resistance is through mutation of genes coding for DNA polymerase.
If patients are unresponsive to the above-mentioned agents, attempts should be made to determine viral sensitivity. The administration of intravenous foscarnet may be necessary for acyclovir-resistant genital HSV. In vegetative HSV lesions, surgical excision may be necessary. [82]
Other than the psychosocial impact, the outcome for the vast majority of males with genital herpes infection is good. Recurrent episodes are common but tend to diminish in frequency with time. In a subset of patients, a hypersensitivity phenomenon known as erythema multiforme may occur with either initial or recurrent HSV infections. Erythema multiforme is characterized by severe oral and/or genital mucosal sloughing, and affected patients often require hospitalization and supportive care.
Immunosuppressed patients may develop debilitating anogenital ulcerations and often respond more slowly to antiviral therapy. In immunocompromised hosts, HSV-2 dissemination, particularly after primary infection, is a rare cause of meningitis.
Condylomata acuminata
Condyloma acuminatum is the most common sexually transmitted disease in the United States and manifests most commonly as a warty lesion on the penis or scrotum in males. The cause is HPV infection, which is estimated to be present in as many as 5% of adults aged 20-40 years (see Condyloma Acuminatum). Infection with specific types of HPV is associated with the development of anogenital squamous intraepithelial lesions and carcinoma.
Pathophysiology
The cause of condyloma acuminatum is the nonenveloped DNA virus HPV. HPV is also the cause of planar warts, plantar warts, verrucae vulgaris, and Bowenoid papulosis. More than 80 different genotypes of HPV have been identified, and different genotypes have a predilection for specific anatomical regions of the skin or mucosa. In most cases, HPV infection causes benign epithelial proliferations that may range from asymptomatic to disfiguring lesions. Some strains of HPV, however, play a significant pathogenic role in the development of carcinoma, particularly cervical carcinoma, but also anal and penile carcinoma.
HPV displays tropism for epithelial cells of the host. It is spread by skin-to-skin contact and is most readily inoculated into epithelium with impaired integrity. After attaching to host cells, the virus may spread through direct spread or autoinoculation, but it does not become blood-borne. HPV infects the basilar layer of epithelium or epidermis, where it incorporates its DNA and, after a variable incubation period of up to several months, induces proliferation. As the basilar compartment of cells proliferates and differentiates, the virus begins to replicate. Because of various viral and host-related factors, a clinically apparent lesion may develop. The host immune response to HPV infection is not well understood. However, both cellular and humoral immunity play a role in controlling or eradicating infection.
HPV types 6 and 11 are the most common cause of benign condyloma acuminatum that appears on the external genitalia. Types 16 and 18, and, less commonly, 31, 33, and 35, are high-risk HPV strains and are the types most commonly involved in the development of cervical carcinoma. The oncogenic potential of high-risk HPV types has been traced to viral proteins, specifically E6 and E7, that alter cell cycle and apoptotic responses in the host cell. High-risk types of HPV express E6, which interacts with and alters the function of tumor suppressor gene TP53, and E7 binds to retinoblastoma protein (RB), leading to a loss of cell-cycle control.
Clinical presentation/diagnosis
Condylomata on the male genitalia are typically acquired through sexual transmission. In fact, the association between men with penile condylomata and sexual partners who have cervical lesions is high (50-85% of males whose sexual partners have HPV lesions have penile lesions). The exact incubation period is unknown but averages several months. Condylomata may be located anywhere on the scrotum or penis, including on the glans or corona or adjacent to the meatus. Perineal and anal lesions are also common sites of involvement.
They typically present as asymptomatic, skin-colored-to-brown, sessile, cauliflowerlike lesions and can measure 1 mm to a few centimeters in size (see the image below). Occasionally, the lesions appear as flat-topped or barely elevated papules. Although sometimes solitary, a significant portion of those affected have more than one lesion at presentation. In most cases, the diagnosis is made based on history and physical examination. Skin biopsy can be used for diagnostic confirmation, if necessary.
The histopathologic features of condyloma acuminatum show epidermal papillomatosis with hyperkeratosis and acanthosis. Focal vacuolated keratinocytes (koilocytes) are typically present in the epidermal granular layer. HPV can be demonstrated with immunohistochemistry in most lesions. Atypia of keratinocytes is minimal to mild unless significant squamous dysplasia is present.
Differential diagnoses
The differential diagnoses include condyloma latum, seborrheic keratoses (see Seborrheic Keratosis), nevi, molluscum, and pearly penile papules (see Pearly Penile Papules). In immunocompromised patients, particularly individuals with HIV seropositivity, other sexually transmitted disease such as nodular herpesvirus infection should be considered. Malignancies such as Bowen disease and squamous cell carcinoma must also be considered, particularly in patients who present with refractory or atypical lesions.
Treatment and outcomes
A number of therapeutic options exist, but no treatment is virus specific. Most therapies involve destruction or removal of clinically apparent virally infected skin. Therapies most commonly used include cryotherapy, podophyllin, imiquimod cream, and podophyllotoxin 0.5% solution or gel. The former two are applied by the treating physician. The latter two are patient-applied therapies and are often used in conjunction with the former. Imiquimod is applied to lesional skin on alternating nights thrice weekly for several months. Podophyllotoxin is applied bid 3 consecutive days each week and repeated for up to 3-4 months.
Electrodesiccation, laser ablation, topical 5-fluorouracil, and surgical excision are other options if the above are ineffective. Because it is mutagenic, podophyllin should be used with caution in males infected with HIV and is not recommended for urethral lesions, in which case 5-fluorouracil 5% or thiotepa is preferred.
HPV vaccine (Gardasil 9) is indicated for prevention of condyloma acuminata caused by HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58 in boys, men, girls, and women aged 9-26 years. The vaccine is administered in two separate doses in patients 9 through 14 years old, and in three doses for patients who initiate the vaccination series at ages 15 through 26 years and for immunocompromised persons.
Condylomata may regress spontaneously; however, approximately half persist and require therapy. Female sexual partners are at high risk for cervical dysplasia, depending on the HPV subtype, and should be screened with routine Papanicolaou smear. Sexual abuse must be suspected and excluded in children with genital condylomata. In high-risk individuals, particularly immunocompromised patients, a high clinical suspicion for malignant transformation must be maintained given the association with HPV and carcinoma.
Miscellaneous Benign Lesions of the Male Genital Tract
Pearly penile papules
Pearly penile papules are asymptomatic, grouped, white papules that develop in approximately 8-20% of males (see Pearly Penile Papules). [83] They are benign and are not associated with an infectious etiology. The main clinical significance is that they are often misdiagnosed as condylomata or other sexually transmitted disease.
Pathophysiology
The specific pathogenesis of pearly penile papules is unknown. The lesions are thought to represent a lesion in the spectrum of an angiofibroma. No association with HPV or other infectious etiology has been reported. [84]
Clinical presentation/diagnosis
The lesions present as pearly-white domed-shaped or filiform papules that are 1 to 3 mm in diameter. They are arranged in rows or groups on the coronal margin or sulcus of the glans. Lesions rarely involve the penile shaft. [85] Young males in the second and third decades are most commonly affected, but lesions can also present in older males. Rare cases in children as young as 11 years have been reported.
Although asymptomatic and not associated with an infectious etiology, they can be a source of concern for the patients who may suspect the lesions to be a sexually transmitted disease. The diagnosis is generally based on characteristic clinical findings, and biopsy is rarely performed for diagnosis.
The histopathologic features show a papular morphology with a normal or slightly thickened epidermis overlying a dense fibrous stroma with numerous ectatic vascular channels and scattered stellate fibroblast.
Differential diagnoses
The main clinical differential diagnoses include molluscum contagiosum and condyloma acuminatum (see Molluscum Contagiosum and Condyloma Acuminatum). These diagnoses can be ruled out based on clinical history and histopathologic findings.
Treatment and outcome
The lesions require no specific therapy. The patient may request treatment for cosmetic reasons. Cryotherapy and laser ablation have been reported as effective treatments in limited case series. [86, 87]
Penile Cysts
Penile cysts are generally divided into two main categories: median raphe (urethroid) cysts and epidermoid (follicular) cysts. Epidermoid cysts are the most common cystic lesions of the penis and occur primarily on the shaft. [88] Median raphe cysts are midline developmental cysts that occur at sites from the external urethral meatus to the anus, including the ventral penis. [89]
Pathophysiology
Epidermoid cysts arise from a disruption of the infundibular portion of the pilosebaceous unit. The histogenesis of epidermoid cysts at penile sites that lack hair follicles is less clearly understood. Median raphe cysts arise from defective closure of the median raphe or from outgrowth of urethral and paraurethral epithelium. [89]
Clinical presentation/diagnosis
The clinical presentation of both cysts is as an asymptomatic nodule. The cysts range in size from 0.2-2 cm in diameter. Epidermoid cysts usually affect the base of the penile shaft. Median raphe cysts occur on the ventral aspect of the penis from the external urethral meatus to the base of the shaft, with a predilection for the glans. The scrotum is uncommonly affected. Median raphe cysts generally occur in young males. Epidermoid cysts occur in a wide age range. The location of the lesion is important in the clinical diagnosis. Definitive diagnosis depends on biopsy.
The histopathology of an epidermoid cyst shows a unilocular cyst lined by stratified squamous and contains laminated keratin material in the cyst lumen. The median raphe cysts are lined by pseudostratified columnar epithelium with occasional mucous glands and rare ciliated cells.
Differential diagnoses
The differential diagnoses include other cysts, cutaneous adnexal neoplasms, and soft tissue neoplasms. Biopsy is necessary for a definitive diagnosis.
Treatment and outcome
Treatment of either an epidermoid cyst or median raphe cyst involves complete excision.
The outcome of treated penile cysts is generally excellent with few serious complications. If left untreated, cyst rupture can occur, leading to a significant inflammatory response and possible scarring. Damage to the urethra is a surgical complication in treating median raphe cysts near the urethra or urethral meatus. Although uncommon, recurrence can follow surgical excision and is more common in median raphe cysts.
Angiokeratoma
Angiokeratomas are papular vascular lesions characterized by marked ectasia of the superficial dermal blood vessels (see Angiokeratoma). Angiokeratomas occur both as solitary and multiple lesions. Some clinical variants of angiokeratoma are associated with enzymatic deficiencies, most commonly Anderson-Fabry disease. The clinical importance of recognizing angiokeratomas is the relationship to Anderson-Fabry disease, as well as to other metabolic enzyme deficiencies.
Pathophysiology
The specific pathogenesis of angiokeratoma is not understood. The lesions are not true hemangiomas but rather ectasias of the existing superficial dermal vascular channels. Some reports have speculated on the role of vascular congestion in the development of angiokeratomas. Angiokeratomas associated with Anderson-Fabry disease show electron-dense lipid bodies on electron microscopy; these bodies are not present in other types of angiokeratoma.
Clinical presentation/diagnosis
The clinical presentation and anatomic distribution of lesions is critical in the evaluation of patients with angiokeratomas. The individual lesions present as small, red, violaceous or black papules with a warty surface morphology. Five clinical variants of angiokeratoma have been reported, as follows:
-
Angiokeratoma of Mibelli presents as multiple warty papules in childhood and adolescence and involves the distal extremities. This variant is more common in females.
-
Angiokeratoma of Fordyce arises as solitary or multiple red or blue papules on the scrotum of elderly men. The penis, thighs, and abdomen can also be involved. A similar lesion occurs on the vulva of young females.
-
Angiokeratoma corporis diffusum presents as widespread clusters of red, blue, or black papules in a bathing trunk distribution. The penis is commonly involved. It is usually associated with Anderson-Fabry disease, an X-linked recessive disorder characterized by a deficiency of alpha-galactosidase A. Some affected patients have other enzyme deficiencies, including L-fucosidase, beta-mannosidase, neuramidase, beta-galactosidase, and alpha-N-acetylgalactosaminidase. In addition, identical lesions can be seen in patients without detectable enzymatic deficiencies.
-
Angiokeratoma circumscriptum is the least common variant and presents as unilateral red papules or a solitary plaque on the legs, arms, or trunk of infants and young children. This variant is more common in females.
-
Solitary and multiple angiokeratoma affect a wide age range and anatomic distribution but occur most commonly as solitary lesions on the lower extremities. Multiple lesions are less common.
Recognition of the bathing trunk distribution of lesions in angiokeratoma corporis diffusum is clinically significant because of the association with Anderson-Fabry disease. The disease is characterized by an X-linked recessive inheritance and a deficiency of the lysosomal enzyme alpha-galactosidase A. Males are almost exclusively affected, but heterozygous females who exhibit mild disease features have been reported. In males, the disease presents in childhood with episodes of pain in the distal extremities. Hypohidrosis with secondary heat intolerance, acroparesthesias, gastrointestinal symptoms, and vasomotor disturbances also occur early in the course of disease. Renal involvement initially manifests as proteinuria.
Angiokeratomas typically develop after puberty and show a widespread distribution in the waist, lower back, and genital region. The number of skin lesions varies and is not related to the severity of systemic disease. In addition to the characteristic distribution of angiokeratomas, ophthalmic examination demonstrates tortuous conjunctival and retinal blood vessels and whorled, linear, corneal opacities (verticillate cornea). Cardiac involvement in Anderson-Fabry disease includes conduction disturbances, hypertrophic cardiomyopathy, angina pectoris, and mitral valve insufficiency. Transient cerebrovascular events occur in approximately 25% of affected males. Renal failure is the most common cause of death.
Diagnosis of Anderson-Fabry disease depends on the characteristic clinical features and evaluation of alpha-galactosidase A enzyme assays. Despite a characteristic clinical presentation, definitive diagnosis of angiokeratoma usually requires biopsy.
The histopathologic features of all clinical forms of angiokeratoma are similar and show a papular morphology with epidermal hyperkeratosis, acanthosis, and hyperplasia. The superficial dermal vascular channels have marked ectasia and congestion. Lipid inclusions identified with electron microscopy are seen in angiokeratomas of patients with Anderson-Fabry disease, but not other variants.
Differential diagnoses
The main clinical differential diagnoses are the distinction of the clinical variants of angiokeratoma. Histopathologic features are essentially identical in all forms of angiokeratomas. Solitary erythematous angiokeratomas can resemble hemangiomas. Solitary black angiokeratomas can resemble a melanocytic lesion, including melanoma. Histopathologic features differentiate the latter entities from angiokeratoma.
Treatment and outcome
Angiokeratomas require no specific therapy. The patient may request treatment for cosmetic reasons. Cryotherapy and laser ablation have been reported as effective treatments in limited case series.
The prognosis of individual angiokeratoma lesions is excellent, with no significant serious complications. The prognosis of patients with Anderson-Fabry disease or other enzyme deficiencies depends on systemic manifestations of the diseases.
Idiopathic scrotal calcinosis
Idiopathic scrotal calcinosis is a nodular lesion characterized by dystrophic calcification in the dermis of the scrotum.
Pathophysiology
The lesions were initially thought to represent an idiopathic disorder, but more recent evidence suggests that the lesions arise from dystrophic calcification of epidermoid cysts or ectatic eccrine ducts in the scrotum. [90, 91]
Clinical presentation/diagnosis
Scrotal calcinosis presents a solitary or multiple, firm, dermal nodules in the scrotum of children and young adults. The scrotal skin overlying lesions can break down and discharge a white chalky material. Diagnosis is usually based on the clinical features. Biopsy is generally not necessary.
The histopathologic features show foci of dystrophic calcification in the dermis. Remnants of the existing epidermoid cyst or eccrine duct are occasionally identified at the periphery of the calcification.
Differential diagnoses
Multiple lesions usually do not present a diagnostic problem, but multiple cysts can have a similar presentation. The differential diagnoses of solitary lesions include cysts, cutaneous adnexal neoplasms, and soft tissue neoplasms.
Treatment and outcome
Surgical excision is the primary therapy. The outcome in patients with scrotal calcinosis is excellent, with no significant complications.
-
Pseudomonas folliculitis (Image courtesy of Hon Pak, MD)
-
Candidiasis (Image courtesy of Hon Pak, MD)
-
Scabies (Image courtesy of Hon Pak, MD)
-
Lymphogranuloma venereum (Image courtesy of Hon Pak, MD)
-
Primary chancre of syphilis (Image courtesy of Hon Pak, MD)
-
Granuloma inguinale (Image courtesy of Hon Pak, MD)
-
Granuloma inguinale (Image courtesy of Hon Pak, MD)
-
Chancroid (Image courtesy of Hon Pak, MD)
-
Lichen planus (Image courtesy of Hon Pak, MD)
-
Lichen planus (Image courtesy of Hon Pak, MD)
-
Condyloma acuminata (Image courtesy of Hon Pak, MD)
-
Pearly penile papules. Courtesy of Wiki Commons
Tables
What would you like to print?
- Introduction, Definition of Terms, and Anatomy
- Trichomycosis and Folliculitis
- Balanoposthitis, Balanitis, and Candidiasis
- Dermatophytosis and Scabies
- Infectious Agents Affecting the Male Genitalia
- Inflammatory Disorders of the Male Genitalia
- Viral Lesions
- Miscellaneous Benign Lesions of the Male Genital Tract
- Show All
- Media Gallery
- References