Background
Conization of the cervix is defined as excision of a cone-shaped or cylindrical wedge from the cervix uteri that includes the transformation zone and all or a portion of the endocervical canal. It is used for the definitive diagnosis of squamous or glandular intraepithelial lesions, for excluding microinvasive carcinomas, and for conservative treatment of cervical intraepithelial neoplasia (CIN).
While no recent changes have occurred in the technique of conization, a quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine (Gardasil) was introduced in 2006. Its widespread use is expected to reduce the number of cervical neoplasias, and, consequently the need for surgical interventions.
Conization can be performed with a scalpel (cold-knife conization), laser, or electrosurgical loop. The latter is called the loop electrosurgical excision procedure (LEEP) or large loop excision of the transformation zone (LLETZ). Combined conization usually refers to a procedure started with a laser and completed with a cold-knife technique. Laser conization can be excisional or destructive (by vaporization). Techniques for diagnostic and therapeutic conization are virtually identical. The extent of excision must be adjusted according to individual needs (see image below).
Each of these approaches has distinct benefits and disadvantages. Cold-knife conization provides the cleanest specimen margins for further histologic study, but it is typically associated with more bleeding than laser or LEEP, and it requires general anesthesia in most cases. Laser procedures are of longer duration and, especially if low-power density is used, may "burn" the margins, thus interfering with histologic diagnosis. The main advantage with this procedure is that dots produced by the laser energy can be used to accurately outline the exocervical margins. However, overall, the benefit of using laser for conization may not justify the high cost of the procedure.
LEEP procedures have several advantages, including rapidity, preservation of the margins for histologic evaluation, and virtual bloodlessness. Moreover, one can perform LEEP procedures in the office or in other outpatient settings.
Procedures that do not yield tissue for pathologic studies, such as electrocoagulation or cryosurgery, are not discussed in this article.
History of the Procedure
Procedures similar to conization were used in the early 19th century in an attempt to excise gross cervical tumors per vaginam. During the second half of the 20th century, conization evolved as an important tool for diagnosing the cause of positive cervical cytology in women without visible lesions and, later, as treatment of CIN. The diagnostic application of cold-knife conization was reduced following the widespread use of colposcopically directed cervical biopsies combined with endocervical curettage. However, conization remains an important diagnostic tool in selected situations. Therapeutic conization for CIN became an accepted modality in the management of CIN following publication of rigorous studies by Scandinavian and Austrian researchers. [1, 2, 3, 4] The precise origin of cold-knife conization is historically uncertain.
Problem
The incidence and mortality of carcinoma of the cervix have declined about 300% since the 1930s in most of North America and in Europe. The sharpest decline began in the 1950s, following the introduction of cytologic screening. Since cytology rarely provides precise diagnosis, conization of the cervix became an important tool for the determination of the accurate diagnosis of abnormal, cytologic, clinical, or colposcopic lesions. Additionally, it is a major method for the treatment of intraepithelial cervical lesions.
Epidemiology
The frequency with which conization procedures are performed depends on the number of suggested or detected cases of CIN and can only be estimated. Approximately 10-20 million cases of human papillomavirus (HPV) infection may be responsible for causing CIN or cervical carcinoma. Although a large proportion of these (an estimated 80%) regress spontaneously, for a definitive diagnosis or treatment, detected cases require colposcopy and, at times, conization.
In the United States, the American Cancer Society predicted 13,820 new cases of invasive cervical cancer and 4360 deaths due to cervical cancer in 2024. [5] The average age at diagnosis is between ages 35 and 44 years; it is rarely diagnosed in woman younger than 20 years. The mortality rate in Black women and Native American women is approximately 65% higher than in White women.
Worldwide, cervical cancer is the second leading cause of cancer death in women. It remains a major cause of mortality in regions without effective universal screening programs, particularly in developing countries.
Etiology and Pathophysiology
Etiology
Intraepithal neoplasia is induced by high-risk human papillomavirus infection. Types 16 and 18 are found in 50-80% of squamous intraepithelial lesions (SIL) and in up to 90% of invasive cancers. [6]
Pathophysiology
Human papillomavirus infection induces proliferation and atypia in the cervical epithelium. Most commonly, these changes occur in the transformation zone, or, at times, directly in the squamous or in the glandular epithelium.
Presentation
The clinical diagnostic process usually begins by a pelvic examination and by taking a Papanicolaou smear. Suspicious lesions are biopsied, preferably under colposcopic control. Diagnostic conizations are performed if colposcopic biopsies require further evaluation. Therapeutic conizations are indicated if SILs (in particular HSIL) are detected.
Indications
Diagnostic conization
Diagnostic conization is indicated in the following situations:
-
Finding epithelial cell abnormalities, in particular high-grade squamous intraepithelial lesions (HSIL) or low-grade squamous intraepithelial lesions (LSIL) in the absence of gross or colposcopic lesions of the cervix
-
Unsatisfactory colposcopy, defined as the examiner's inability to view the entire transformation zone, including the squamocolumnar junction, in women with epithelial cell abnormalities
-
Uncertainty regarding the presence or absence of microinvasion or invasion following the diagnosis of CIN by directed biopsy
-
Finding CIN or microinvasive cancer during endocervical curettage
-
Cytologic or histologic evidence of premalignant or malignant glandular epithelium
-
Cytologic diagnosis inconsistent with histologic diagnosis based on directed biopsy findings
Therapeutic conization
Therapeutic conization is currently the preferred modality to treat CIN grades 2 and 3. All described approaches (ie, cold-knife, laser, LEEP) are equally effective, as found by Mitchell and colleagues. [7]
Historically, carcinoma in situ (CIN grade 3), the first identified intraepithelial neoplasia, was treated with hysterectomy. During the last quarter of the 20th century, several large published series proved the effectiveness of the more conservative conization procedure. In 1976, Kolstad and Klem reported on 1122 patients with carcinoma in situ treated with conization, with a recurrence rate of 2.3% and an unexpected discovery of small invasive carcinomas in 0.9%. [1] Bjerre et al reported treatment failure in 7% of their patients who received therapeutic conization. [2]
Controversies exist as to the necessity of removing the entire endocervical canal, including the internal os, in all cases. This approach, recommended by at least 2 studies, may increase the risk of cervical incompetence in women who desire posttreatment pregnancy. The author believes that determining the probability of high endocervical involvement fairly accurately is possible by performing endocervical curettage or by obtaining cytology specimens with an endocervical brush. If the results of these tests are negative for CIN or glandular atypia and if the patient wishes to preserve her childbearing potential, the author preserves the cranial extremity of the endocervical canal.
In addition to conization, CIN can also be treated by hysterectomy or by other destructive methods, such as cryotherapy, laser vaporization conization, or radical electrocoagulation. The decision to use hysterectomy or conization is usually based on the grade and extent of the disease, the patient's age, the desire for childbearing, and the history of recurrence after conservative management. Because destructive methods such as cryotherapy yield no specimen for histologic studies, their use should be limited to those women in whom an accurate preoperative diagnosis has been established by directed biopsy findings.
Relevant Anatomy
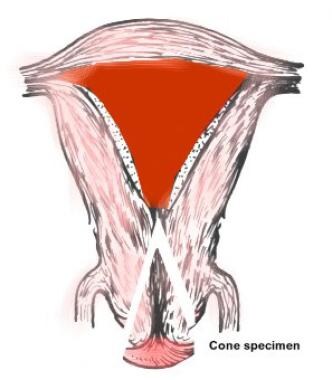
The cervix is typically 2.5 cm long. It communicates with the endometrial cavity of the corpus uteri through the internal os and with the vagina through the external os. The vaginal portion (also called exocervix or portio vaginalis) is covered by stratified squamous epithelium, and the cervical canal is covered by columnar epithelium, which also forms endocervical glands, more correctly called clefts. The 2 epithelia meet at the squamocolumnar junction. In most adult women, the squamocolumnar junction is not an abrupt meeting point, but a zone containing irregular areas of glandular and metaplastic squamous epithelium. The size of this transformation zone varies from 2-15 mm (see image below).
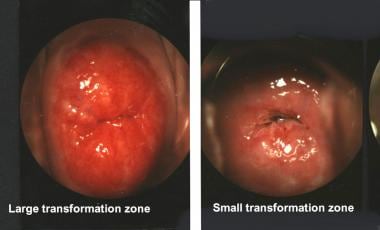 The exocervical incision should be placed 2-3 mm beyond the limits of the transformation zone and must be adjusted to its size.
The exocervical incision should be placed 2-3 mm beyond the limits of the transformation zone and must be adjusted to its size.
CIN usually arises in the transformation zone and usually extends to a depth of less than 7 mm. The blood supply of the cervix originates mainly from the cervical branches of the uterine artery and from branches of the vaginal and pudendal arteries.
Contraindications
Conization should be avoided during pregnancy if at all possible because it commonly causes significant (>500 mL) bleeding. Approximately 30% of pregnant patients who undergo conization develop delayed postoperative hemorrhage, and fetal loss has been reported in as many as 10%. Rare indications for performing this procedure include the possible presence of invasive cancer discovered during the first or second trimester.
-
Conization site as related to uterine anatomy.
-
The exocervical incision should be placed 2-3 mm beyond the limits of the transformation zone and must be adjusted to its size.
-
In cases of histologically diagnosed cervical intraepithelial neoplasia grades 2 and 3, prebiopsy cytology results were reported as high-grade squamous intraepithelial lesions, low-grade squamous intraepithelial lesions, and atypical squamous cells of undetermined significance in nearly equal proportions (data from 6 independent studies).
-
Site of preconization cerclage.
-
Laser conization is initiated by outlining the exocervical margins with 0.5- to 1-mm dots produced by laser energy at a power setting of 20-50 W. A laser incision is then performed to connect the dots and is extended to a depth of 3-5 mm.
-
Loops for the loop electrosurgical excision procedure.
-
Loop electrosurgical excision procedure.