Practice Essentials
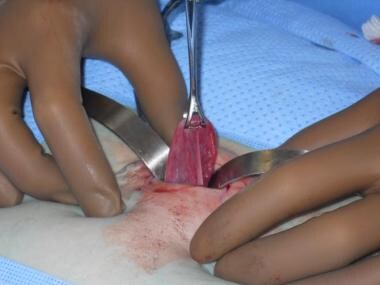
Tubal sterilization results in mechanically blocking or interrupting the fallopian tubes to prevent sperm from fertilizing the egg. (See the image below.) The procedure is indicated for women who want a permanent method of contraception and are free of any gynecologic pathology that would otherwise dictate an alternate procedure. Tubal sterilization is also indicated for women in whom a pregnancy could represent a significant clinical and medical risk.
Background
Prior to the 1960s, female sterilization in the United States was generally performed only for medical indications (when additional pregnancies would be hazardous to the mother). Many centers used a formula (endorsed by the American College of Obstetricians and Gynecologists until 1969) in which age multiplied by parity had to be greater than or equal to 120 before elective sterilization could be considered. The changing cultural climate in the 1960s encouraged women to reduce family size. The popularization of sex steroid hormone contraception (oral contraception) is credited for beginning the sexual revolution and allowing women to use safe, reversible contraception that also provided noncontraceptive benefits.
During the same decade, surgical advances resulted in safe, less invasive female sterilization procedures when childbearing was no longer desired. Most importantly, insurance companies began to cover female sterilization procedures, making the procedure accessible to millions of women in the United States previously unable to afford the surgery.
Currently, approximately 700,000 bilateral tubal sterilizations are performed annually in the United States. Of these, half are performed postpartum and half are ambulatory interval procedures. Eleven million US women aged 15-44 years rely on bilateral tubal occlusion for contraception, and more than 190 million couples worldwide use surgical sterilization as a safe and reliable method of permanent contraception.
History of the Procedure
Key events in the history of tubal sterilization include the following:
-
In 1823, Blundell first suggested tubal ligation for sterilization before the Medical Society of London.
-
In 1876, Porro performed a cesarean hysterectomy with the secondary intention of sterilization.
-
In 1880 in Toledo, OH, Lungren was first to ligate a woman's tubes.
-
In 1885, Thomas suggested tubal ligation as opposed to Porro's operation for sterilization.
-
In 1895, Dührssen used a double ligature and was the first to perform tubal ligation via colpotomy.
-
In 1897, Kehrer and Buettner divided the tubes between the sutures.
-
In 1898, Ruhl cut the tube 5 cm from the uterus and sutured the ends to a vaginal incision.
-
In 1898, Rose removed the tubes at the cornua.
-
In 1919, Madlener crushed and ligated the tubes with nonabsorbable suture.
-
In 1924, Irving published his method in which the proximal portion of the severed tube is buried in a small myometrial tunnel on the anterior uterine surface.
-
In 1930, colleagues posthumously published the Pomeroy technique in the New York State Journal of Medicine.
-
In 1936 in Switzerland, Bosch performed the first laparoscopic tubal occlusion as a method for sterilization.
-
In the 1940s, Hajime Uchida developed his technique, which can be performed as an interval or puerperal procedure. He subsequently reported on his personal experience with more than 20,000 tubal sterilizations over 28 years without a known failure. [1]
-
In the 1960s, the era of laparoscopy began with unipolar electrocoagulation of the fallopian tube. Failure rates and safety concerns associated with both unipolar and bipolar electrosurgery led to the development of laparoscopic devices that do not require radiofrequency energy.
-
In 1973, Jaroslav Hulka devised a spring clip that could be applied laparoscopically. [2]
-
In 1981, Filshie introduced a titanium and silicone clip that was widely used in Europe. It was not introduced into the United States until the 1990s. [3]
-
In 2002, the Essure hysteroscopic sterilization procedure was approved for use in the United States.
-
In 2018, Bayer announced that they will stop selling and distributing the Essure device in the United States.
Procedures to block the fallopian tube may be divided into those performed at the time of delivery or shortly thereafter, and those performed at another time. The latter are referred to as interval sterilization procedures. Minilaparotomy (Uchida, Pomeroy, or Parkland technique) is the most common procedure in the immediate postpartum period, performed via periumbilical incision following vaginal delivery. The proximity of the uterine fundus in relation to the umbilicus during the immediate postpartum period facilitates this approach. However, there is a much higher incidence of poststerilization remorse associated with procedures performed immediately following delivery.
The laparoscopic approach may be used at any time other than the postpartum period and involves either a single umbilical 10 mm port, or a smaller umbilical camera port and a secondary suprapubic port through which the various devices are introduced.
Although local anesthetic techniques have been described and used for transabdominal approaches, the vast majority of intraperitoneal tubal interruption procedures in the United States are performed using general or spinal anesthesia. And by definition, all require incisions that invade the peritoneal cavity, thereby introducing complications related to general or spinal anesthesia as well as injury to intra-abdominal structures.
Several attempts have been made in the past to achieve transcervical tubal blockage using radiofrequency, chemical scarring with quinacrine, and the injection of liquid silicone. However, these have all failed for safety or efficacy reasons.
In November 2002, the Food and Drug Administration approved the use of Essure microinserts (Conceptus, Inc, Mountain View, Calif) for hysteroscopic sterilization. The devices consist of polyethylene terephthalate (PET) fibers wrapped around a stainless steel core, surrounded by 24 coils of nickel-titanium alloy. After the microinserts are deployed, the PET fibers induce the tubal epithelium to undergo fibrosis, which results in proximal tubal occlusion. This process takes approximately 3 months to form complete occlusion, which is then documented by a hysterosalpingogram.
In April of 2018, the U.S. Food and Drug Administration ordered restrictions to the sale and distribution of the Essure permanent contraceptive device in order to ensure that all women are provided with adequate risk information so that they can make informed decisions whether to use the permanent contraceptive. [4]
In July 2018, Bayer announced that they will stop selling and distributing the Essure device in the United States after December 31, 2018 due to declining sales of the product. [5] Ultimately, the Essure system was removed from the US market on December 31, 2018. As of December 31, 2019, all unused Essure units should have been returned to Bayer so that they are no longer available for implantation. [6]
Relevant Anatomy
The relevant anatomy is as follows:
-
The 2 fallopian tubes (oviducts) lie on either side of the uterus in the upper margin (mesosalpinx) of the broad ligament. Each tube is divided into 4 parts. From lateral to medial, the parts are as follows:
-
The fimbriated end (infundibulum) is a bugle-shaped extremity with a fimbriated ostium that overlies the ovary, to which an elongated appendage (the fimbria ovarica) adheres.
-
The ampulla is wide, thin-walled, and somewhat tortuous and is the largest portion of the tube, both in length and caliber.
-
The isthmus is a narrow, straight, thin-walled portion of the tube immediately adjacent to the uterus. The ampullaryisthmic junction is the site where the fertilized egg pauses in its transit to the endometrial cavity, waiting for the progesterone produced by the corpus luteum to create a favorable environment for implantation. The isthmic portion of the fallopian tube is the site for all sterilization procedures that depend on intra-abdominal tubal occlusion. When a segment of tube is removed, as in the Pomeroy or Uchida technique, the isthmus is the preferred site of excision because of the relative ease of reanastomosis should the procedure be reversed in the future.
-
In the intrauterine or intramural portion of the tube, the lumen narrows to approximately 1 mm or less as it pierces the uterine wall, terminating in the tubal ostium, which is located on the superolateral aspect of the uterine cavity.
Although the mesonephric (wolffian) ducts degenerate in females, duct remnants may be sites of cyst formation.
-
Epoophoron (homologous to the epididymis) - Constantly lies in the lateral portion of the mesosalpinx and mesovarium
-
Paroophoron (homologous to the paradidymis) - Variably lies more medially in the mesosalpinx
-
Hydatid cysts of Morgagni (homologous to the appendix of epididymis) - Represent the most cranial remnant of the mesonephric duct
Contraindications
Patient ambivalence regarding sterilization is an absolute contraindication. Even though surgically reversing the tubal occlusion at a later date or becoming pregnant through in vitro fertilization is technically feasible, the decision to proceed with sterilization should be considered permanent and irreversible. The cost and disability associated with tubal occlusion is miniscule compared with the expense and time involved in either tubal reanastomosis or assisted reproductive technologies. While the patient should make the request herself, be of sound mind, and not act under external duress; the physician should provide patients who have decided that their families are complete with information regarding the various sterilization options. Many patients may not be aware that a nonincisional, in-office, hysteroscopic method is available. If there is any doubt whatsoever, other long-term but not irreversible methods of contraception, such as the IUD, should be considered when not contraindicated.
Special legal and ethical criteria must be met in cases where the patient undergoing sterilization has a physical, psychological, or intellectual disability.
Any gynecologic malignancy or symptomatic gynecologic pathology (eg, pelvic relaxation, uterine tumors, ovarian tumors) in which a hysterectomy is indicated obviates the need for a tubal occlusion.
In the puerperium, defer sterilization if maternal or infant complications exist. While sterilizations performed in the immediate postpartum are accompanied by a high incidence of regret, even when both mother and baby are healthy, many physicians advocate performing all sterilizations as an interval procedure.
The laparoscopic approach is relatively contraindicated in patients with a diaphragmatic hernia (through the foramen of Morgagni). Limit the Trendelenburg position to 15°, limit intra-abdominal pressure to a maximum of 10 mm Hg, and perform the operation under endotracheal anesthesia.
The laparoscopic approach is also contraindicated in patients with severe cardiopulmonary disease or dysfunction. The pneumoperitoneum may compress the vena cava and azygous system and diminish cardiac return, leading to cardiac decompensation. The diaphragm may be splinted (thus reducing respiratory tidal flow) both by the weight of the intraabdominal organs, which fall cephalad with the patient in the Trendelenburg position, and by the pneumoperitoneum. Absorption of gas from the pneumoperitoneum may further aggravate the build up of carbon dioxide. The resultant hypercarbia may cause cardiac arrhythmias.
Special consideration must be given to obese patients, who make up an increasingly large percentage of the population. The presence of morbid obesity and/or a history of multiple abdominal surgeries with adhesion formation takes the laparoscopic approach to sterilization out of the realm of elective, low-risk surgery. It would be difficult to justify the selection of a laparoscopic approach in such patients when lower-risk procedures are available.
A publication by the FDA reviewed the incidence of trocar injuries that were reported to the FDA. Between 1997 and 2002, 1300 trocar injuries and 30 deaths were reported, yielding a trocar-related injury rate of 3%. Not counting bowel injury from electrosurgery, wound infection, urinary tract infection, or anesthetic complications, with 300,000 laparoscopic sterilizations performed in the United States each year, that translates to a minimum of 9000 complications from laparoscopic sterilization each year. [7]
According to the United States Collaborative Review of Sterilization, the odds ratio of complications from general or regional anesthesia is approximately 3 times that of local anesthesia.
The hysteroscopic insertion of microinserts for tubal occlusion may be safely performed in a physician's office under local anesthesia. It does not carry the operative risks incident to laparoscopy such as injury to bowel, bladder, or major vessels caused by trocar insertion or energy sources. The hysteroscopic approach may be safely used in cases of morbid obesity, abdominal scarring, and pelvic adhesions that would otherwise impede the abdominal approach to the fallopian tube. The hysteroscopic microinsert is contraindicated in patients allergic to nickel or contrast media, patients with active PID, patients in whom only 1 microinsert may be inserted, pregnancy, suspected pregnancy, or within 6 weeks of delivery or pregnancy termination.
Prognosis
Mortality
The risk of death from tubal sterilization is 1-2 cases per 100,000 procedures; most of these are complications of general anesthesia. The most common cause of death during laparoscopic BTL appears to be hypoventilation related to anesthesia. Cardiopulmonary arrest and hypoventilation are reported as the leading cause of death in most cases. Sepsis as a cause of death from laparoscopic sterilization is directly related to bowel perforations or electrical bowel burns. The mortality rate is low when compared with the risk of death from hysterectomy (5-25 cases per 100,000 procedures) and from pregnancy (8 cases per 100,000 live births in the United States and 500 cases per 100,000 live births in developing countries).
No deaths have been reported from the hysteroscopic approach.
Complications
Unintended laparotomy
Unintended laparotomy occurs with 1-2% of laparoscopic procedures; most of these conversions are attributable to technical inability to complete the laparoscopic procedure rather than to complications of the procedure.
Bowel injury
Bowel injury can occur during insertion of the insufflation needle or trocar or during electrocoagulation. Small injuries from the needle or trocar with no bleeding or leakage of enteric contents can usually be managed expectantly; otherwise, prompt laparotomy is indicated.
Vascular injury
Vascular injury can occur during insufflation needle or trocar insertion. Injury to a large vessel is a life-threatening emergency. Perform an immediate laparotomy with direct pressure over the injury to control bleeding until repair (usually by a vascular surgeon) can be performed.
Method failure (pregnancy or ectopic pregnancy)
Although sterilization is highly effective and considered the definitive form of pregnancy prevention, it has a failure rate during the first year of 0.1-0.8%. At least one third of these are ectopic pregnancies. Recent findings suggest that pregnancy is somewhat more common than previously estimated, that the risk of pregnancy persists for many years after sterilization, and that the risk varies by method and patient age at sterilization.
In the CREST study, 10,685 women were enrolled from 1978-1986; follow-up continued until 1994. The CREST study reviewed procedures performed at 10 large teaching institutions, and the data may not reflect the experience from the private sector. Whether the findings can be extrapolated to the general population is unclear. In addition, the Filshie clip, which has a lower incidence of failure than the other laparoscopic techniques, was not included in this study. The 10-year cumulative probability of pregnancy varied from 7.5 cases per 1000 procedures for postpartum partial salpingectomy and unipolar coagulation to 36.5 cases per 1000 procedures for spring clip application. The CREST study identified a 10-year cumulative failure rate of 18.5 failures per 1000 patients for all methods combined. Pregnancies occurring in the 10th year after sterilization were identified for all methods of laparoscopic occlusion evaluated.
Rodriquez et al also found decreased efficacy with the titanium clip than partial salpingectomy and does not recommend using the titanium clip during the postpartum period. [8]
The risk of pregnancy varied by patient age at sterilization and by method, with the highest risk among young women sterilized with bipolar coagulation (54.3 cases per 1000 procedures). Overall, women sterilized at age 34-44 years were half as likely to become pregnant after sterilization compared to women sterilized at age 28-33 years and were approximately one third as likely to become pregnant as women sterilized at age 18-27 years. When pregnancy occurs after BTL, the risk of ectopic pregnancy is high. The CREST study reported a 32% rate of ectopic pregnancy following tubal ligation. Several studies suggest that the risk is highest after bipolar coagulation, with more than 50% of pregnancies being ectopic.
BTL failures can be grouped into the following categories:
-
Luteal phase pregnancy is defined as a pregnancy in which conception occurs before the BTL, but pregnancy is diagnosed after an interval tubal sterilization. Strategies to reduce the incidence (reported to occur at a rate of 1-15 cases per 1000 interval sterilizations) include effective contraception, scheduling of BTL during the proliferative phase, and preoperative urine enzyme-linked immunoassay pregnancy testing.
-
Misidentification of the oviduct because of poor visualization from inadequate exposure, adhesions, adnexal pathology, or poor lighting may result in mistakenly ligating the round ligament, ovarian ligament, infundibular ligament, or dilated broad ligament blood vessels instead of the oviduct. Therefore, initially identifying the fimbriated tubal ends and then tracing the tube medially to the isthmic region is imperative. In postpartum minilaparotomy BTL, Babcock clamps should be placed sequentially along the oviduct until the fimbria is visualized.
-
Incomplete occlusion of the oviduct occurs because of poorly placed mechanical clips or the use of mechanical devices on edematous or dilated tubes. With correct clip application, the mesosalpinx on the surface of the tube is pulled upward to resemble the flat triangular shape of an envelope flap (the Kleppinger envelope sign). When silastic rings are used, the tubal serosa, but not the tubal lumen, may be pulled into the ring, with absence of the vertical crease formed when the entire loop of tube is included in the ring.
-
Incomplete tubal occlusion with electrocoagulation is generally associated with too brief an application of current or with the use of modulated/coagulation current instead of unmodulated/cutting current.
-
Improper technique occurs with the use of the wrong sutures or failure to preserve a 2-cm proximal tubal segment. If a short proximal stump is left, the fluid pressure from uterine contractions could either prevent complete closure of the tubal lumen during healing or cause a fistula to form to relieve pressure after healing is complete.
Pain
After laparoscopy, patients may experience some degree of chest and shoulder pain due to trapped gas. Mechanical blocking devices are believed to cause ischemic pain, but this has not been established in a randomized, controlled trial. Mild analgesics are usually sufficient to control postprocedure pain.
Hysteroscopic sterilization has been reported to be similar to the pain experienced during menses and is generally limited to the procedure and immediate postprocedure time period. [9, 10]
Infection/hemorrhage
Wound infections and hematoma have been associated with minilaparotomy. Pelvic infections and hemorrhage are associated with vaginal approaches. Although prophylactic antibiotics are recommended for women at risk for subacute bacterial endocarditis who are scheduled to undergo a procedure that may lead to bacteremia, the American Heart Association does
not recommend antibiotic prophylaxis for BTL. Hemorrhage is a rare complication (30-90 cases per 100,000 procedures) that usually occurs following major vessel injury during laparoscopic entry and occasionally occurs following mesosalpingeal vessel injury during the occlusion procedure.
Visceral (bowel, bladder, uterus) injuries
Organ injuries can occur from sharp trauma (eg, insufflation needle, trocar, scalpel), blunt trauma (eg, from adhesiolysis), or electrical-thermal trauma. Injuries can also occur during inadvertent application of the occlusion device to the incorrect structure. If recognized at the time of occurrence, injuries to the bowel and bladder (which are more common in the presence of adhesions) are relatively easy to manage and will not result in long-term adverse sequelae. Injuries to the uterus, most often caused by uterine manipulators, do not usually lead to adverse sequelae unless bowel or bladder has been perforated simultaneously.
Patient regret
Sterilization is intended to be permanent, but patient regret is not rare. Poststerilization regret is a complex condition often caused by unpredictable life events. Risk factors for regret that may be useful in presterilization counseling include young age, low parity, and single parent status or being in an unstable relationship. As many as 6% of women who are sterilized report regret or request information about tubal reversal within 5 years of the procedure. Follow-up interviews 14 years postprocedure demonstrate that regrets were expressed by 20.3% of women aged 30 years or younger at the time of BTL and by 5.9% of women older than 30 years at time of procedure.
The proportion of women who actually undergo microsurgical tubal reanastomosis is only 0.2% in the first 5 years after BTL. The most important factor in determining the success of reversal by tubal anastomosis is the length of healthy tube remaining after sterilization. Isthmic-to-isthmic anastomoses are most likely to be successful. Sterilization reversal using a sutureless laparoscopic approach yielded a 59% ongoing pregnancy rate with a 3.9% ectopic rate. Age, previous pregnancy, and sperm quality were major factors affecting the outcome. [11]
-
Elevation of the fallopian tube through the incision.