Overview
Radiation therapy is an essential component in Gynecology in the primary nonsurgical management and the adjuvant postoperative treatment of selected malignancies arising in the female reproductive tract. Gynecologic cancers were among the first malignancies treated with ionizing radiation, more than a century ago. (See the image below.)
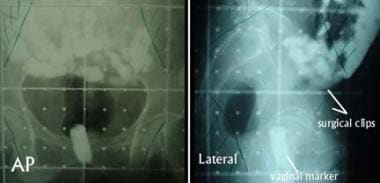 Images show initial anteroposterior (AP, left) and lateral (right) fields used for postoperative adjuvant pelvic external-beam radiotherapy in a patient with stage IC grade 2 adenocarcinoma of the endometrium who underwent hysterectomy. Additional high-risk features included extensive lymphovascular invasion and a serosal resection margin of about 2 mm. Visible are contrast material in the small bowel and the vaginal marker, which is a tampon soaked with radiopaque material. Areas to be blocked in the parallel, opposed AP-posteroanterior and lateral beams are indicated in the corners of these simulation images. The surgical clips marking the sampled region of the external iliac lymph nodes also facilitate design of the lateral fields.
Images show initial anteroposterior (AP, left) and lateral (right) fields used for postoperative adjuvant pelvic external-beam radiotherapy in a patient with stage IC grade 2 adenocarcinoma of the endometrium who underwent hysterectomy. Additional high-risk features included extensive lymphovascular invasion and a serosal resection margin of about 2 mm. Visible are contrast material in the small bowel and the vaginal marker, which is a tampon soaked with radiopaque material. Areas to be blocked in the parallel, opposed AP-posteroanterior and lateral beams are indicated in the corners of these simulation images. The surgical clips marking the sampled region of the external iliac lymph nodes also facilitate design of the lateral fields.
Current strategies for treating cancers of the uterine corpus, uterine cervix, vulva, and vagina are tailored to the clinical and pathologic stage of disease. Early stage lesions of the lower genitourinary tract can be treated surgically if resection can be accomplished without substantial tissue disruption. Postoperative radiotherapy is reserved for cases in which histopathologic analysis of the removed specimen reveals features suggesting a high risk for local recurrence.
Primary radiotherapy can provide an opportunity for cure for women with unresectable, locally advanced disease; for women with resectable disease in whom the risk of surgical morbidity is unacceptably high; and for women with medical risk factors that contraindicate primary surgical therapy. However, for women with distant metastatic disease at presentation, cure is unlikely, although palliative radiotherapy frequently improves a patient's quality of life when used to relieve symptoms.
Although adjuvant radiotherapy is commonly used to manage advanced or metastatic cervical and endometrial cancer, it is sometimes administered as adjuvant therapy for ovarian cancer. Radiation therapy may also be used for hormonal ablation.
In this article, clinical indications and common techniques for radiation therapy in the management of common gynecologic cancers are described.
Staging
Carcinoma of the uterine corpus
Staging guidelines from the International Federation of Gynecology and Obstetrics (FIGO) and the American Joint Committee on Cancer (AJCC) are based on surgical findings. The depth of tumoral invasion and the histopathologic grade of the tumor influence the recommendations for adjuvant postoperative therapy. The grade of the tumor is based primarily on the amount of solid growth of its glandular component.
In grade 1 tumors, less than 5% of the neoplasm has a solid growth pattern. Grade 2 and 3 tumors consist of 6-50% and greater than 50% solid growth, respectively. Papillary serous, clear-cell, and mixed mesodermal tumors are all considered grade 3 lesions.
The FIGO/AJCC staging system for endometrial carcinoma is as follows:
-
Stage IA - Tumor confined to uterus, no or less than half myometrial invasion
-
Stage IB –Tumor confined to uterus, greater than half myometrial invasion
-
Stage II - Cervical stromal invasion, but not beyond uterus
-
Stage IIIA - Tumor involving the serosa and/or adnexa
-
Stage IIIB - Vaginal and/or parametrial involvement
-
Stage IIIC1 - Metastasis to the pelvic lymph nodes
-
Stage IIIC2 – Para-aortic node involvement
-
Stage IVA - Tumor invasion of the bladder and/or bowel mucosa
-
Stage IVB - Distant metastasis, including metastasis to the inguinal lymph nodes and/or intra-abdominal (other than para-aortic) lymph nodes
Carcinoma of the uterine cervix
FIGO/AJCC staging guidelines for cervical cancer are as follows:
-
Stage IA1 - Invasive carcinoma diagnosed only on microscopy and not deeper than 3mm or horizontally spreading more than 7 mm
-
Stage IA2 - Invasive carcinoma diagnosed only on microscopy, with stromal invasion of 3-5 mm and horizontal spread of not more than 7 mm
-
Stage IB - Clinically visible lesion confined to the cervix or a microscopic lesion larger than a IA tumor (deeper than 5 mm or horizontally spreading more than 7 mm)
-
Stage IB1 - Clinically visible lesion 4 cm or smaller in greatest dimension
-
Stage IB2 - Clinically visible lesion larger than 4cm in greatest dimension
-
Stage IIA1 - Tumor with vaginal involvement not exceeding the upper two thirds of the vagina, without parametrial invasion, smaller than 4 cm in greatest dimension
-
Stage IIA2 - Larger than 4 cm in greatest dimension
-
Stage IIB - Tumor with parametrial invasion
-
Stage IIIA - Tumor involving the lower one third of the vagina
-
Stage IIIB - Tumor extending to the pelvic sidewall and/or causing hydronephrosis or a nonfunctioning kidney
-
Stage IVA - Invasion of the bladder or rectal mucosa
-
Stage IVB - Distant metastasis (the para-aortic lymph nodes are considered a distant metastatic site)
Vulvar cancer
The current FIGO/AJCC staging guidelines for vulvar cancer are as follows:
-
Stage IA - Tumor confined to the vulva or perineum and 2 cm or smaller with stromal invasion 1 mm or less, negative nodes
-
Stage IB – Tumor confined to the vulva or perineum, larger than 2 cm or with stromal invasion less than 1 mm, negative nodes
-
Stage II - Tumor of any size with adjacent spread (one third lower of the urethra, one third of the lower vagina, anus), negative nodes
-
Stage IIIA – Tumor of any size with positive inguinofemoral lymph nodes
-
Stage IIIAi - One lymph node metastasis greater than or equal to 5 mm
-
Stage IIIAii - One or 2 lymph node metastasis(es) of less than 5 mm
-
Stage IIIBi – Two or more lymph nodes metastases greater than or equal to 5 mm
-
Stage IIIBii - Three or more lymph nodes metastases less than 5 mm
-
Stage IIIC – Positive node(s) with extracapsular spread
-
Stage IV – Tumor invades other regional structures (two thirds of the upper urethra, two thirds of the upper vagina), bladder mucosa, rectal mucosa, or fixed to pelvic bone
-
Stage IVB – Any distant metastasis including pelvic lymph nodes
Vaginal cancer
The FIGO/AJCC staging system for vaginal cancer stipulates that any cancer involving the vagina that also involves either the cervix or the vulva is automatically classified as originating within one or the other of those structures, although the original epicenter of the tumor cannot always be established with certainty.
FIGO/AJCC staging for vaginal carcinoma is as follows:
-
Stage I - Tumor confined to the vagina
-
Stage II - Tumor invading the paravaginal tissues but not the pelvic wall
-
Stage III - Tumor extending to the pelvic wall and/or metastasis to an inguinal or pelvic lymph node
-
Stage IVA - Tumor invading the bladder or rectal mucosa or extending beyond the true pelvis
-
Stage IVB - Distant metastasis
Ovarian cancer
See the list below:
-
Stage IA– Growth limited to 1 ovary, no tumor on the external surface, capsule intact, no ascites present containing malignant cells
-
Stage IB – Growth limited to both ovaries, no tumor on the external surfaces, capsules intact, no ascites present containing malignant cells
-
Stage IC – Tumor either stage IA or IB, but with the tumor on surface of 1 or both ovaries with capsule ruptured, with ascites present containing malignant cells, or with positive peritoneal washings
-
Stage IIA - Extension and/or metastases to the uterus and/or tubes
-
Stage IIB - Extension to other pelvic tissues
-
Stage IIC - Tumor either stage IIA or IIB, but with tumor on surface of 1 or both ovaries, with capsule(s) ruptured, with ascites present containing malignant ovaries, or with positive peritoneal washings
-
Stage III - Tumor involving 1 or both ovaries with histologically confirmed peritoneal implants outside pelvis and/or positive retroperitoneal or inguinal nodes; superficial liver metastasis; tumor limited to true pelvis, but with histologically proven malignant extension to small bowel and omentum
-
Stage IIIA - Tumor grossly limited to the true pelvis, with negative nodes, but with histologically confirmed microscopic seeding of abdominal peritoneal surfaces or histologically proven extension to small bowel mesentery
-
Stage IIIB - Tumor of 1 or both ovaries with histologically confirmed implants, peritoneal metastasis of abdominal peritoneal surfaces of 2 cm or less in diameter; nodes are negative
-
Stage IIIC - Peritoneal metastasis beyond the pelvis of more than 2 cm in diameter and/or positive retroperitoneal or inguinal nodes
-
Stage IV - Growth involving 1 or both ovaries with distant metastases; if pleural effusion is present, positive cytology must be apparent to allot a case to stage IV; parenchymal liver metastasis qualifies as stage IV disease
Carcinoma of the Uterine Corpus
When hysterectomy is medically contraindicated, primary radiotherapy can offer 5-year disease-specific survival rates of 80-90%, approaching those achieved with surgery.
Indications for adjuvant radiation after surgery for endometrial cancer are somewhat controversial. Whole-pelvis external-beam radiotherapy (XRT, or EBRT) and intravaginal brachytherapy are potential adjuvant postoperative therapies for patients with stage I disease. [1] Recommendations are based on the stage and grade of disease. The American Brachytherapy Society has published guidelines for postoperative treatment (see Table 1, below). These reflect the lack of absolute consensus regarding optimal therapy after surgery.
Table 1. American Brachytherapy Society Guidelines for the Postoperative Treatment of Pathologic Stage I Endometrial Carcinoma (Open Table in a new window)
Stage |
Grade |
||
1 |
2 |
3 |
|
IA |
Observation only |
Observation only |
Observation, intravaginal brachytherapy, or intravaginal brachytherapy plus pelvic XRT |
IB |
Observation or brachytherapy |
Observation, intravaginal brachytherapy, or intravaginal brachytherapy plus pelvic XRT |
Intravaginal brachytherapy or intravaginal brachytherapy plus pelvic XRT |
IC |
Observation or intravaginal brachytherapy plus pelvic XRT |
Intravaginal brachytherapy plus pelvic XRT |
Intravaginal brachytherapy plus pelvic XRT |
Data supporting the use of intravaginal brachytherapy in stage IB grade 1 or 2 lesions came from randomized studies conducted in the 1960s and 1970s at the Roswell Park Institute. In those studies, intravaginal brachytherapy significantly reduced the incidence of vaginal recurrence to less than 1%.
Several randomized trials have now examined the role of postoperative adjuvant radiation in endometrial cancer. The first, a randomized study conducted at the Norwegian Radium Hospital, demonstrated a statistically significant reduction in the risk of vaginal and pelvic recurrence in patients who received more than the usual amount of external radiation treatment compared with those who did not (1.9% vs 6.9%, respectively) but no difference in overall 5-year survival. In the study, 540 patients with stage I endometrial cancer were treated between 1968 and 1974 with 6000 cGy of vaginal radium. These patients were then randomized to receive no further treatment versus an additional 4000 cGy of XRT. Surgical staging was not performed. [2]
A second study (Postoperative Radiotherapy in Endometrial Cancer [PORTEC]) conducted in the Netherlands at numerous radiation oncology centers between 1990 and 1997 found that radiation reduced locoregional recurrence of endometrial cancer but not 5-year overall survival and cancer-specific death rates. In the study, more than 700 patients who had undergone hysterectomy for intermediate-risk endometrial cancer were randomized to receive 4600 cGy of XRT versus no further treatment. No vaginal brachytherapy was used and routine surgical staging was not performed. Radiation reduced locoregional recurrence (4% vs 14%), but 5-year overall survival and cancer-specific death rates were not statistically different in the 2 treatment groups. [3]
In a trial by the Gynecologic Oncology Group, GOG 99, conducted between 1987 and 1995, in which 448 patients with intermediate-risk endometrial cancer were randomized to receive postoperative XRT (5040cGy) versus no further therapy, the cancer recurrence rate was lower in the radiation group. Unlike the PORTEC trial, in which patients with deeply invasive grade 3 tumors were excluded, the definition of intermediate risk for enrollment into the GOG trial included any degree of myometrial invasion of any grade in the absence of lymph node metastases. Patients in the GOG trial underwent routine surgical staging with lymphadenectomy. [4]
Of 392 patients who were eligible for the study, 6.8% had recurrences in the radiation group versus 15.3% in the group that had no further therapy, reflecting predominantly a reduction of vaginal recurrences (2 vs 13). The rate of extrapelvic recurrence was not statistically different between the treatment groups.
The addition of pelvic irradiation decreased the 2-year cumulative incidence of recurrence from 12% to 3%, which was statistically significant. Greatest reductions occurred in the subgroup of patients at high intermediate risk. For women younger than age 50 years, high intermediate risk was defined as a grade 2 or 3 tumor, invasion of the outer third or the myometrium, and/or lymphovascular invasion. For patients aged 51-69 years, high intermediate risk was defined as 2 of the 3 criteria listed, and for those aged 70 or older, it was any 1 of the criteria.
In patients at high intermediate risk, 2-year cumulative incidences of recurrence were 26% for surgery alone versus only 6% with the addition of whole-pelvis radiation. The difference in 4-year overall survival for all patients was not statistically significant (92% in the irradiation arm vs 86% in the surgery-only arm). Intravaginal brachytherapy was not used in either treatment arm.
A meta-analysis by Kong has concluded that while postoperative radiotherapy reduces locoregional relapse, the risk of endometrial cancer-related death or distant site recurrence was not reduced. [5]
XRT protocol
Whole-pelvis XRT may be administered by using a 4-field setup involving parallel opposed anteroposterior (AP), posteroanterior (PA), right lateral, and left lateral fields. Patients are positioned prone on a partially depressed surface that allows for anterior displacement of the small intestines away from the lateral fields. A radiopaque marker is placed in the vagina. During simulation, this marker facilitates identification of the vaginal cuff.
Typical field borders are the following:
-
Superior (all fields) - L4-L5 or L5-S1 interspace
-
Inferior (all fields) - Middle or inferior aspect of the obturator foramen or at least 5 cm below the vaginal cuff
-
Lateral (AP and PA fields) - 1.5-2 cm lateral to bony pelvis
-
Anterior (lateral fields) - Anterior pubic symphysis, with portions of small bowel anterosuperior to the external iliac nodal chain blocked out
-
Posterior (lateral fields) - Posterior aspect of S3, covering the region of insertion of the uterosacral ligament, with a 2 cm or greater margin posterior to the vagina inferiorly
See the image below for an example of typical fields for whole-pelvis XRT.
 Images show initial anteroposterior (AP, left) and lateral (right) fields used for postoperative adjuvant pelvic external-beam radiotherapy in a patient with stage IC grade 2 adenocarcinoma of the endometrium who underwent hysterectomy. Additional high-risk features included extensive lymphovascular invasion and a serosal resection margin of about 2 mm. Visible are contrast material in the small bowel and the vaginal marker, which is a tampon soaked with radiopaque material. Areas to be blocked in the parallel, opposed AP-posteroanterior and lateral beams are indicated in the corners of these simulation images. The surgical clips marking the sampled region of the external iliac lymph nodes also facilitate design of the lateral fields.
Images show initial anteroposterior (AP, left) and lateral (right) fields used for postoperative adjuvant pelvic external-beam radiotherapy in a patient with stage IC grade 2 adenocarcinoma of the endometrium who underwent hysterectomy. Additional high-risk features included extensive lymphovascular invasion and a serosal resection margin of about 2 mm. Visible are contrast material in the small bowel and the vaginal marker, which is a tampon soaked with radiopaque material. Areas to be blocked in the parallel, opposed AP-posteroanterior and lateral beams are indicated in the corners of these simulation images. The surgical clips marking the sampled region of the external iliac lymph nodes also facilitate design of the lateral fields.
For the 4-field setup, photon energies of 6MV or greater provide a homogeneous dose distribution. An AP-PA setup is also acceptable, but it is not preferred unless high-energy photons are available. A total dose of 45Gy is typically given in 25 fractions administered 5 days per week.
Brachytherapy
Although neither GOG 99 nor the PORTEC study incorporated intravaginal brachytherapy, the vaginal cuff is well recognized to be a common site of recurrence in many patients with early stage disease. The PORTEC 2 study concluded that vaginal cuff brachytherapy is as effective as XRT, but showed fewer gastrointestinal side effects. [6] Intravaginal brachytherapy may be administered with an afterloading device to deliver a low dose rate (LDR) or a high dose rate (HDR).
The HDR technique has become popular because of its convenience as a well-tolerated outpatient regimen. When intravaginal brachytherapy is given after pelvic XRT, a typical dosage schedule for intravaginal brachytherapy is 15 Gy prescribed to a depth of 0.5 cm over 3-4 cm of the upper vagina, given in 3 weekly fractions of 5 Gy each. When intravaginal brachytherapy is administered without pelvic XRT, a dose of 21 Gy to a depth of 0.5cm given in 3 fractions is commonly prescribed. Equivalently effective schedules of 2-5 fractions, prescribed at depth or at the surface of the cylinder, are administered according to the individual practitioner's preference. See the image below for an example of an HDR intravaginal brachytherapy device.
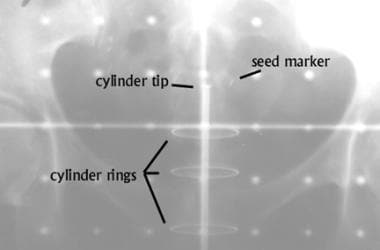 Anteroposterior view of an intravaginal cylinder for administering high–dose rate intravaginal brachytherapy. A nonradioactive gold seed marker was placed submucosally at the vaginal cuff to allow verification of the position of the device during weekly treatments. The visible metallic rings indicate the location of individual removable segments of the particular type of cylinder in use.
Anteroposterior view of an intravaginal cylinder for administering high–dose rate intravaginal brachytherapy. A nonradioactive gold seed marker was placed submucosally at the vaginal cuff to allow verification of the position of the device during weekly treatments. The visible metallic rings indicate the location of individual removable segments of the particular type of cylinder in use.
Additional considerations
Two important pathologic findings not specifically addressed in the FIGO/AJCC staging system are extensive invasion of the lymphovascular space and a close (less than a few millimeters) margin of resection. When either feature is found, consideration of postoperative radiotherapy is warranted. Also, adjuvant treatment should be considered in certain patients with pathologic stage I disease in whom surgical staging was incomplete.
For stage II endometrial carcinoma, preoperative or postoperative radiotherapy may be administered. The goals of preoperative treatment are to facilitate hysterectomy by reducing tumoral bulk, by clearing microscopic infiltration from the upper vaginal mucosa, or by rendering cells incapable of local implantation. Pelvic XRT may be combined with brachytherapy in this setting. For stage II disease recognized during postoperative histopathologic analysis, pelvic XRT and intravaginal brachytherapy may be indicated.
For stage III-IV disease, numerous institutions have reported outcomes for patients treated with combinations of pelvic XRT, whole-abdomen radiotherapy (WAR), intravaginal brachytherapy, and chemotherapy; however, no clear consensus has emerged.
A study (GOG 122) comparing WAR with combination chemotherapy involving doxorubicin and cisplatin to treat advanced endometrial carcinoma found that chemotherapy was apparently the more effective treatment. Of 396 assessable patients, 202 were randomized to receive WAR and 194 to receive chemotherapy. At 60 months, 50% of patients who received chemotherapy were predicted to be alive and disease-free as compared with 38% of patients who received radiotherapy, a survival advantage that reached statistical significance. Treatment was thought to have contributed to the death of 8 patients (4%) treated with chemotherapy and 5 patients (2%) treated with radiation. [7]
Histologic variants of endometrial carcinoma require special consideration. Uterine papillary serous cancer (UPSC) is characterized by a propensity for local and distant recurrence.
Carcinosarcoma, formerly called malignant mixed müllerian tumor (MMMT), is associated with a particularly high rate of pelvic failure after hysterectomy. Patients may benefit from postoperative pelvic XRT in all stages of disease. The rate of distant recurrence is higher with carcinosarcomas and other uterine sarcomas than with endometrial adenocarcinomas. As for soft tissue sarcomas arising in other sites, no benefit has been proven for the addition of chemotherapy or other adjuvant systemic therapy.
Carcinoma of the Uterine Cervix
For stage IA1, simple type 1 extrafascial hysterectomy is potentially curative. For stage IB1, the procedure of choice is a radical hysterectomy with resection of the parametria and dissection of the pelvic lymph nodes. Para-aortic lymph nodes should be examined and sampled if findings are suggestive of metastatic disease. If parametrial extension or regional nodal metastasis is identified intraoperatively, many gynecologic oncologists abort hysterectomy and proceed to pelvic radiotherapy.
In a study by the Gynecology Oncology Group (GOG), reported by Sedlis et al, patients who underwent postoperative radiotherapy following radical hysterectomy for early stage cervical cancer demonstrated a statistically significant (47%) reduction in local recurrence of cervical cancer; however, this occurred at the cost of a 4% increase in grade 3/4 (6% vs 2.1%) toxicity, including 1 patient death. In the study, 277 patients had 2 or more of the following risk factors identified after surgery: greater than one third cervical stromal invasion, capillary lymphatic space invasion, and large clinical tumor diameter. Patients were randomized to receive 4600-5040cGy of whole-pelvic radiotherapy postoperatively versus no further treatment. [8]
In the followup of this study, reported by Rotman et al in 2006, a similar statistically significant (46%) reduction in recurrence was confirmed in patients receiving postoperative radiation, along with a prolongation in recurrence-free survival. [9] A statistically significant improvement in overall survival was not observed. Interestingly, much of the benefit of postoperative radiation seemed to be confined to nonsquamous histologies.
Treatment combining chemotherapy with radiotherapy
Patients who have undergone radical hysterectomy with histopathologic evidence of tumoral spread to regional lymph nodes, tumor at the surgical margin, and/or with microscopic involvement of the parametrium are considered to be at high risk for recurrence, and postoperative adjuvant therapy is generally indicated. Traditionally, whole-pelvic radiotherapy alone was offered in this setting, but a randomized trial by GOG, reported by Peters et al (see Table 2) demonstrated a survival advantage for patients who received chemoradiation as compared with radiation therapy alone. [10]
Similarly, postoperative pelvic chemoradiation is recommended for patients with cancers of stage IB1 or higher that are incidentally found after simple hysterectomy for presumed benign disease. Alternatively, such patients may be offered completion surgery consisting of radical upper vaginectomy, parametrectomy, and pelvic lymphadenectomy.
The addition of concurrent chemotherapy to radiation in the treatment of cervical cancer, both in the postoperative adjuvant setting for early stage disease and as definitive primary therapy for advanced disease, emerged as one of the major breakthroughs in the treatment of gynecologic cancer in the last decade.
Table 2, below, summarizes the results of a number of randomized trials showing dramatic survival advantages for chemoradiotherapy versus radiotherapy alone. Meta-analysis would indicate a survival advantage of approximately 30% when chemotherapy is incorporated into treatment.
Given these findings, in 1999 the National Cancer Institute issued a rare clinical announcement that "strong consideration should be given to the incorporation of concurrent cisplatin-based chemotherapy with radiation therapy in women who require radiation therapy for treatment of cervical cancer."
Table 2. Relative Risk of Death Estimates in 5 Clinical Trials (Open Table in a new window)
Study |
FIGO Stage * |
Treatment |
Relative Risk in of Death in Comparison Group |
|
Control Group |
Comparison Group |
|||
Keys et al, 2004 [4] |
IB2 |
Radiotherapy followed by extrafascial hysterectomy |
Radiotherapy plus weekly cisplatin followed by extrafascial hysterectomy |
0.54 |
Rose et al, 1999 [11] |
IIB-IVA |
Radiotherapy plus hydroxyurea |
(1) Radiotherapy plus weekly cisplatin or (2) radiotherapy plus cisplatin, 5-fluorouracil, and hydroxyurea |
(1) 0.61, (2) 0.58 |
Morris et al, 1999 [12] |
IB2-IVA |
Extended radiotherapy (pelvis plus para-aortic lymph nodes) |
Pelvic radiotherapy plus cisplatin and 5-fluorouracil |
0.52 |
Whitney et al, 1999 [13] |
IIB-IVA |
Radiotherapy plus hydroxyurea |
Radiotherapy plus cisplatin and 5-fluorouracil |
0.72 |
Peters et al, 2000 [10] |
IB (postoperative) or IIA (postoperative) |
Radiotherapy |
Radiotherapy plus cisplatin and 5-fluorouracil |
0.5 |
* FIGO = International Federation of Gynecology and Obstetrics |
||||
The treatment of bulky stage I (stage IB2, clinical tumor diameter >4 cm) cervical cancer remains controversial. Common treatment strategies consist of primary radical hysterectomy, with postoperative adjuvant chemoirradiation administration tailored to histopathologic findings, or definitive primary chemoradiotherapy. Proponents of definitive primary chemoradiation point to the fact that many (or in some series, most) patients undergoing radical surgery demonstrate surgical-pathologic risk factors that may ultimately require them to receive adjunctive postoperative chemoradiation, with an attendant increase in morbidity.
Neoadjuvant chemotherapy administration (to reduce tumor volume preoperatively) followed by radical hysterectomy and chemoradiotherapy followed by type I extrafascial hysterectomy are additional treatment strategies that are less-commonly used. Randomized trials defining the optimal treatment strategy of bulky cervical cancer are not available. Regardless of treatment, patients with bulky stage I cervical cancers have significantly higher rates of regional lymph node metastases, locoregional and distant-site relapse, and poor outcome.
Because direct extension of tumor into adjacent structures limits the ability of surgery to gain clear surgical margins, and the presence of the uterus is critical for the anatomy of brachytherapy insertion in this setting, primary chemoradiotherapy is generally indicated for the management of stage IIB-IVA locally advanced cervical cancer. Tumoral resection with anterior and/or posterior pelvic exenteration is technically possible in some cases. However, this procedure entails diversion of the urinary tract and/or bowel, and it is usually reserved for patients with isolated central pelvic disease that persists or recurs after definitive chemoradiotherapy.
Brachytherapy
Brachytherapy involves the temporary placement of intrauterine tandem and intravaginal ovoid that are loaded with radioactive material. The devices are placed with the patient under general anesthesia or heavy sedation. Radiopaque vaginal gauze is applied to secure the devices in place and fix their position relative to the bladder and rectum. Intraoperative radiographs or digital fluoroscopic images document appropriate device positioning. Contrast material in a Foley catheter and a rectal tube can be used to identify the International Commission of Radiological Units (ICRU) reference points of interest.
ICRU reference points for gynecologic brachytherapy doses are as follows:
-
Point A = 2 cm cephalic to the external os along the tandem and 2 cm perpendicular to the plane of the tandem
-
Point B = 2 cm cephalic to the os and 5 cm lateral to the patient's midline
-
Bladder point = Most posterior point in the bulb of the Foley catheter along a direct AP line through the center of the bulb
-
Rectal point = 0.5 cm posterior to the vaginal mucosa in the patient's midline at the level of the posterior aspect of the ovoid
Doses are prescribed to ICRU points A and B. The reference point of origin for points A and B is the cervical os, which is identified by using a radiopaque flange adjusted and fixed at the time of tandem placement. Placing a nonradioactive gold seed marker beneath the epithelial surface near the os is also helpful to corroborate localization of the cervical os using radiography.
Brachytherapy may be performed with low dose rate (LDR) or high dose rate (HDR) applications. LDR is defined as a dose of 0.4-2 Gy per hour and HDR is defined as a dose of greater than 12Gy per hour. HDR brachytherapy typically involves 5-7 weekly outpatient procedures in which the radioactive source is iridium-192 (192 Ir) of high activity. Point A is given a dose of 5-7 Gy during each treatment, which is typically accomplished in less than an hour. LDR brachytherapy usually involves 1 or 2 placement procedures in which the radioactive source is cesium-137 (137 Cs). Each time, the sources are left in place for approximately 2 days, during which a dose of 20-40 Gy is administered to point A. See the image below for an example of the placement of an LDR tandem and ovoid.
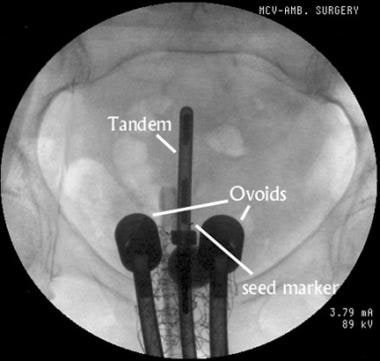 Anteroposterior view of an intrauterine tandem and vaginal ovoids used for low–dose rate brachytherapy. Contrast material is visible in the Foley catheter. A rectal tube is also seen. A nonradioactive gold seed marker was placed near the cervical os to help in verifying that the flange is in contact with the exocervix.
Anteroposterior view of an intrauterine tandem and vaginal ovoids used for low–dose rate brachytherapy. Contrast material is visible in the Foley catheter. A rectal tube is also seen. A nonradioactive gold seed marker was placed near the cervical os to help in verifying that the flange is in contact with the exocervix.
Total combined external-beam and HDR brachytherapy to point A usually is 75-80 Gy, somewhat lower than the total dose of LDR brachytherapy. In the latter, the biologically equivalent total dose to point A is 85-90 Gy. The reason for this difference is related to the anticipated intracellular radiation repair mechanisms active during the prolonged LDR application.
As outlined in a review article by Stewart et al, the advantages of LDR brachytherapy include more than 100 years of safety and efficacy data; standardization of treatment plans, times, and radiation doses; and a requirement for just 2 cesium insertions. [14] Disadvantages of LDR include the need for regional or general anesthesia and inpatient treatment with prolonged bedrest (with a concomitant risk of venous thromboembolism), radiation exposure to staff, and limitations of source strengths.
In the same review, the advantages of HDR were listed as including ease of outpatient treatment with shortened administration times, minimization of staff exposure, the ability to reassess tumor size with each application, and the ability to perform the procedure with the patient under conscious sedation. Disadvantages of HDR, on the other hand, include a higher risk of amplifying dosimetry errors, expense, a need to replace iridium sources frequently, and the need for multiple applications.
In a retrospective review, Petereit et al compared outcomes in patients treated with LDR or HDR brachytherapy, reporting no statistically significant difference in pelvic control or other outcomes in stages IB or II. However, outcomes in some patients with stage IIIB disease worsened. [15]
At least 4 randomized, prospective trials of HDR versus LDR brachytherapy in the treatment of carcinoma of the cervix have now been completed.
A study from Osaka University reported no significant differences in 5-year, cause-specific survival for HDR versus LDR. Moderate to severe complications, however, were higher in the HDR group than in the LDR group (10% vs 4%, respectively). [16] Hareyama and others have pointed out difficulties with randomization with this study. [17]
Patel et al reported no significant differences in 5-year survival or pelvic control for HDR versus LDR brachytherapy, although they noted a statistically significant higher rate of rectal complications for patients in the LDR group (19.9% vs 6.4%). [18]
Hareyama et al, in a study randomizing 132 patients with stage II or stage IIIB cervical cancer to LDR vs HDR, found no statistically significant differences in survival or complication rates. [17] Finally, Lertsanguansinchai et al randomized 237 patients between HDR and LDR brachytherapy and reported comparable outcomes. [19]
Intercalated between brachytherapy procedures are additional external-beam boost treatments in which custom blocking is used to shield areas of the bladder and rectum that have received a high dose from brachytherapy. Between LDR brachytherapy implants, boost treatments covering the lateral parametrial and pelvic nodes are commonly administered. These treatments contribute in dosing the volume, which includes ICRU point B but not point A. The total cumulative dose to point B administered with initial whole-pelvis external-beam radiotherapy (XRT), brachytherapy, and boost treatments (if given) is 50-55 Gy for stage IB and as much as 65 Gy or more when extensive parametrial disease or sidewall fixation is present.
Intracavitary brachytherapy devices are sometimes difficult to position because of anatomic distortion due to tumoral infiltration into surrounding structures. In high-stage disease, the vaginal fornices are commonly effaced because tumor erodes the cervix. The lateral distribution of the radiation dose to the parametrium is compromised when ovoids cannot be placed to a level near the cervical os. The net result can be that the devices cannot be appropriately arranged to avoid excessive doses to the bladder and rectal reference points while giving an adequate dose to point A.
One solution is to administer pelvic XRT and brachytherapy in moderately high doses and then perform extrafascial hysterectomy to resect the residual cervical tumor. However, this option is generally not feasible for disease of stage IIB or higher. An alternative is to use interstitial brachytherapy, wherein blind-ended catheters are placed through the perineum into areas of tumor infiltration in the lower pelvis lateral to the cervix.
Thin, plastic threads laced with192 Ir sources then may be loaded into the catheters to provide the necessary lateral dose distribution.
At some institutions, when interstitial brachytherapy is indicated, patients undergo computed tomography (CT) scan ̶ based treatment planning before implantation to determine the amount of192 Ir needed and the expected spatial arrangement of catheters. The present authors have found that laparoscopic visualization can be extremely helpful in verifying the anatomic distortions of the region to be implanted and in recognizing when catheter placement must be adjusted. See the images below for illustrations of a case in which an interstitial catheter was placed in a patient with stage IVA cervical cancer.
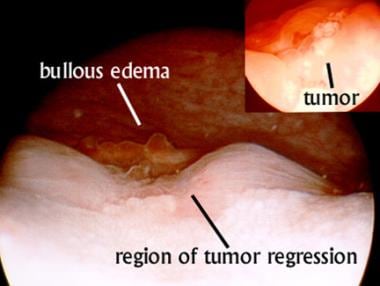 Cystoscopic view of a patient with stage IVA cervix cancer before interstitial implantation. Inset, image obtained before any treatment reveals a whitish exophytic tumor between the ureteral orifices. Main image reveals regression of the tumor after 43-Gy external-beam treatment to the pelvis combined with weekly cisplatin therapy and a single application of intracavitary brachytherapy. Evidence of bullous edema remains despite regression of the tumor. The patient required percutaneous nephrostomy before treatment; however, at this point, urine flow through both ureteral orifices was visible.
Cystoscopic view of a patient with stage IVA cervix cancer before interstitial implantation. Inset, image obtained before any treatment reveals a whitish exophytic tumor between the ureteral orifices. Main image reveals regression of the tumor after 43-Gy external-beam treatment to the pelvis combined with weekly cisplatin therapy and a single application of intracavitary brachytherapy. Evidence of bullous edema remains despite regression of the tumor. The patient required percutaneous nephrostomy before treatment; however, at this point, urine flow through both ureteral orifices was visible.
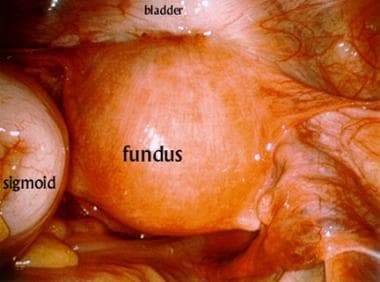 Laparoscopic view of the pelvis during application of the device for interstitial brachytherapy. The anterior uterus is densely adherent to the posterior bladder. The sigmoid colon is retracted to the patient's left side.
Laparoscopic view of the pelvis during application of the device for interstitial brachytherapy. The anterior uterus is densely adherent to the posterior bladder. The sigmoid colon is retracted to the patient's left side.
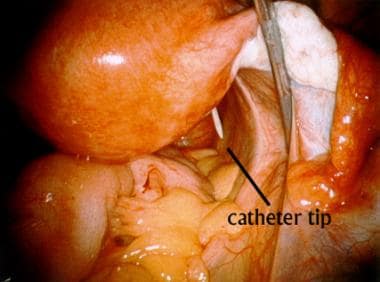 When the right parametrial tissues are retracted, penetration of the catheter into the peritoneal cavity can be observed. The catheter is then repositioned to avoid exposing its sharp end to the mobile bowel in the low pelvis.
When the right parametrial tissues are retracted, penetration of the catheter into the peritoneal cavity can be observed. The catheter is then repositioned to avoid exposing its sharp end to the mobile bowel in the low pelvis.
When the right parametrial tissues are retracted, penetration of the catheter into the peritoneal cavity can be observed.
Treatment combining XRT and brachytherapy
Comprehensive radiotherapy for stage IB-IVA cervical cancer involves both XRT and brachytherapy. Initial external-beam fields encompass a clinical target volume including the primary tumor and the adjacent areas at risk for direct occult invasion or regional lymph-node metastases. The borders of the field are usually similar to those used for endometrial cancer, with particular attention to coverage of the posterior extent of disease. The superior border is typically placed at the L4-L5 interspace.
For patients with gross disease in the para-aortic nodal region, some believe retroperitoneal lymphadenectomy before XRT can improve the likelihood of disease control.
Although acceptable variations to the dose schedule of combined pelvic XRT and brachytherapy for cervical cancer are available, a typical regimen includes an initial external-beam dose of 40-45 Gy to the pelvis in fractions of 1.8-2 Gy. Cisplatin is usually given weekly at a dose of 40 mg/m2 of body surface area, with a maximum weekly dose of 70mg.
Morbidity associated with combined-modality treatment
Because combined-modality treatment can exacerbate anemia, blood transfusions are commonly administered to maintain a hemoglobin level of 10-12g/dL or higher. Data from several studies of cervical cancer demonstrated that anemia during radiation treatment was an independent poor prognostic factor in multivariate analysis.
A small Southwest Oncology Group study examining the role of recombinant human erythropoietin (EPO) in patients with cervical cancer and hemoglobin levels of less than 12.5 g/dL found that during treatment, although hemoglobin levels responded to EPO, local control and disease-free survival outcomes were poor compared with results in a cohort (GOG 120) treated without EPO. Whether the difference was attributable to a worsened prognosis due to anemia or to an adverse effect on tumoral control by EPO itself was unclear. [20]
Other toxicities of combined-modality treatment include electrolytic irregularities, such as hypokalemia, hypomagnesemia, and hypocalcemia. Patients receiving this treatment should be monitored closely and given electrolyte replenishments when indicated.
Intensity-modulated radiation therapy
Conventional XRT, while highly effective, delivers ionizing radiation not only to the target tumor volume but also to significant areas of adjacent normal tissue, accounting for many of the observed acute (diarrhea, cystitis) and late (small bowel obstruction from luminal narrowing and fibrosis, chronic proctitis, sigmoid strictures, ureteral stricture, chronic hemorrhagic cystitis) tissue toxicities. These potential complications limit the total radiation dose that can be safely delivered by conventional radiotherapy techniques.
In an attempt to increase the ratio of prescribed radiation dose given to the tumor as opposed to surrounding normal tissues, a technique of intensity-modulated radiation therapy (IMRT) has recently been developed.
As outlined in an excellent review article by Salama et al, IMRT uses advanced radiographic imaging and computer software to generate sophisticated 3-dimensional (3-D) treatment planning. [21] Using a multileaf collimator, computer-optimized intensity-modulated beams are delivered to produce highly conformal 3-D dose distributions.
Although IMRT is still in its relative infancy and long-term patient follow-up is not yet available, the use of this modality in the treatment of gynecologic cancer is increasing and has the potential not only to reduce normal tissue toxicity but also to potentially substitute for brachytherapy administration in patients with advanced pelvic malignancies who are not good candidates for conventional brachytherapy.
Vulvar and Vaginal Cancer
Although primary nonsurgical treatment is usually preferable for stage I or II vaginal lesions, partial or total vaginectomy followed by postoperative radiotherapy is sometimes feasible for these. Postoperative radiotherapy for stage I or II is indicated when pathologic evidence suggests inguinal node metastasis or close (< 8 mm) resection margins around the primary site. A dose of 45-50Gy is given in fractions of 1.8-2Gy to regions at risk for residual microscopic disease.
For limited involvement of the ipsilateral nodes with no pathologic nodal involvement identified during contralateral dissection, the external-beam fields are often reduced to cover only the hemipelvis.
Using the proper technique for effective radiotherapy of the regions of the groin nodes is important. The region of concern is anterior to the ischium, and excessive irradiation of the femoral neck should be avoided. Available options include a wide AP photon field prescribed to the depth of the external iliac artery as it crosses the inguinal ligament, supplemented by a narrow PA field to ensure adequate coverage of the pelvic nodes. As an alternative, electrons may be used to cover some or all of the region of the inguinal nodes, but the energy must be carefully selected to achieve a sufficiently deep distribution of radiation dose to the full nodal area.
Citing data from dosimetric analysis, an abstract from a single institution comparing intensity-modulated radiation therapy (IMRT) with traditional planning for treatment of all pelvic and inguinal nodes suggested improved homogeneity (due to elimination of dose modulation across abutting/overlapping fields) and relative sparing of femoral heads with IMRT.
For stage III-IVA disease, the management strategy is tailored to the individual. A common policy is to surgically remove all resectable regional and iliac nodes larger than 1cm in diameter. When resection of the primary tumor entails permanent diversion of the lower urinary or gastrointestinal tract, the authors offer combined-modality therapy. At present, a chemoradiotherapy schedule involving 5-fluorouracil and mitomycin-C is used. This regimen is highly effective as the standard primary treatment of squamous cell carcinomas of the anal canal. (See the image below.)
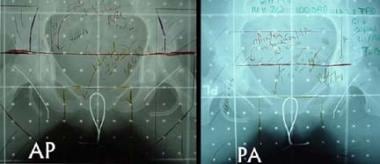 Anteroposterior (AP) (left) and posteroanterior (PA) (right) fields for external-beam radiotherapy in a patient with clinical stage III squamous cell carcinoma of the vulva. Bilateral superficial groin dissection revealed no evidence of regional lymph-node metastasis. Drains are still in place in the surgical wounds. The primary lesion, outlined with a wire during the simulation, involved the vagina and approached to within 1 cm of the anal sphincter, rendering it unresectable without sacrificing bowel continence. Initial treatment included the use of bolus material over the region indicated to ensure that a full dose was administered to the surface of the lesion during 6-MV photon treatment. Asymmetric AP and PA fields were used. 5-fluorouracil and mitomycin-C were currently administered during external-beam radiotherapy. The field was reduced after 36 Gy was given. The total dose to the primary lesion was 45 Gy. Approximately 2 months after treatment, residual fibrosis was found at the primary site. This area was completely excised without injury to the anus. Histopathologic analysis revealed only scar tissue without evidence of tumor. Two years after treatment, the patient had no evidence of recurrence.
Anteroposterior (AP) (left) and posteroanterior (PA) (right) fields for external-beam radiotherapy in a patient with clinical stage III squamous cell carcinoma of the vulva. Bilateral superficial groin dissection revealed no evidence of regional lymph-node metastasis. Drains are still in place in the surgical wounds. The primary lesion, outlined with a wire during the simulation, involved the vagina and approached to within 1 cm of the anal sphincter, rendering it unresectable without sacrificing bowel continence. Initial treatment included the use of bolus material over the region indicated to ensure that a full dose was administered to the surface of the lesion during 6-MV photon treatment. Asymmetric AP and PA fields were used. 5-fluorouracil and mitomycin-C were currently administered during external-beam radiotherapy. The field was reduced after 36 Gy was given. The total dose to the primary lesion was 45 Gy. Approximately 2 months after treatment, residual fibrosis was found at the primary site. This area was completely excised without injury to the anus. Histopathologic analysis revealed only scar tissue without evidence of tumor. Two years after treatment, the patient had no evidence of recurrence.
Interstitial brachytherapy can be used to provide boost treatment to the primary site in selected cases.
For stage I-IVA vaginal disease, combined-modality treatment with radiotherapy and concurrent cisplatin chemotherapy is likely more beneficial than radiotherapy alone because the biologic behavior of vaginal cancer is expected to be similar to that of cervical cancer. After elective regional external-beam radiotherapy (XRT) is administered to fields in doses similar to those used for vulvar cancer, tailored interstitial brachytherapy is generally necessary to deliver potentially curative doses of radiation to the primary site.
Ovarian Cancer
The current regimen for adjuvant therapy after surgery is intravenous combination chemotherapy with carboplatin and paclitaxel or, in optimally debulked patients, intraperitoneal cisplatin and paclitaxel. Whole-abdomen radiation (WAR) has been used, but its popularity has waned because of the favorable toxicity profiles of current chemotherapeutic regimens. Techniques for WAR have included AP-PA field treatment to the entire peritoneal cavity at doses of 25-30Gy given in fractions of 1-1.5 Gy. Renal doses of less than 20Gy and whole-liver doses of less than 30Gy were advisable using shielding techniques.
Boost treatment to the pelvis and para-aortic lymph nodes may be combined with WAR for total doses of 45-50 Gy to these regions. Unfortunately, small bowel obstruction several years following WAR was a common complication.
Palliative radiotherapy
Palliative radiotherapy is frequently offered to patients who have focally symptomatic recurrences of ovarian cancer. The field arrangements and dose schedules are based on the site of recurrence and the patient's overall status. For patients with painful or hemorrhagic pelvic masses refractory to chemotherapy, a hypofractionated schedule of 14.8Gy given in 4 fractions within 2 days may be administered by using AP-PA fields and then repeated once or twice at 2- to 4-week intervals. This regimen has been found to reduce symptoms for most patients. Three monthly fractions of 10Gy are also reasonable, especially in patients with poor performance and a limited life expectancy in whom convenience and expediency are paramount.
Radiotherapy in hormonal ablation
Radiotherapy can also be applied when ovarian hormonal ablation is indicated, most commonly in the treatment of estrogen receptor–positive breast cancer in premenopausal women. [22] In the United States, tamoxifen is widely given to premenopausal women with estrogen receptor–positive breast cancer. [23] However, in many other countries, low-dose radiotherapy is sometimes administered. Radiotherapy can be highly effective and cost-effective.
A large meta-analysis supporting the value of adjuvant hormone therapy for breast cancer included many studies in which radiotherapy was used to achieve ovarian ablation. No superiority has been proven for medical hormonal blockade over radiotherapeutic hormonal ablation or surgical oophorectomy.
A dose of 10-20Gy in 5-10 fractions is usually sufficient to eliminate ovarian hormone production. To limit the size of the radiotherapeutic field, the location of the ovaries is ideally verified by performing CT scanning. As an alternative, a field covering the pelvic soft tissues from 1-2cm below the symphysis to the bottom of the sacroiliac joint includes the ovaries in almost all patients.
-
Images show initial anteroposterior (AP, left) and lateral (right) fields used for postoperative adjuvant pelvic external-beam radiotherapy in a patient with stage IC grade 2 adenocarcinoma of the endometrium who underwent hysterectomy. Additional high-risk features included extensive lymphovascular invasion and a serosal resection margin of about 2 mm. Visible are contrast material in the small bowel and the vaginal marker, which is a tampon soaked with radiopaque material. Areas to be blocked in the parallel, opposed AP-posteroanterior and lateral beams are indicated in the corners of these simulation images. The surgical clips marking the sampled region of the external iliac lymph nodes also facilitate design of the lateral fields.
-
Anteroposterior view of an intravaginal cylinder for administering high–dose rate intravaginal brachytherapy. A nonradioactive gold seed marker was placed submucosally at the vaginal cuff to allow verification of the position of the device during weekly treatments. The visible metallic rings indicate the location of individual removable segments of the particular type of cylinder in use.
-
Anteroposterior view of an intrauterine tandem and vaginal ovoids used for low–dose rate brachytherapy. Contrast material is visible in the Foley catheter. A rectal tube is also seen. A nonradioactive gold seed marker was placed near the cervical os to help in verifying that the flange is in contact with the exocervix.
-
Cystoscopic view of a patient with stage IVA cervix cancer before interstitial implantation. Inset, image obtained before any treatment reveals a whitish exophytic tumor between the ureteral orifices. Main image reveals regression of the tumor after 43-Gy external-beam treatment to the pelvis combined with weekly cisplatin therapy and a single application of intracavitary brachytherapy. Evidence of bullous edema remains despite regression of the tumor. The patient required percutaneous nephrostomy before treatment; however, at this point, urine flow through both ureteral orifices was visible.
-
Laparoscopic view of the pelvis during application of the device for interstitial brachytherapy. The anterior uterus is densely adherent to the posterior bladder. The sigmoid colon is retracted to the patient's left side.
-
When the right parametrial tissues are retracted, penetration of the catheter into the peritoneal cavity can be observed. The catheter is then repositioned to avoid exposing its sharp end to the mobile bowel in the low pelvis.
-
Anteroposterior (AP) (left) and posteroanterior (PA) (right) fields for external-beam radiotherapy in a patient with clinical stage III squamous cell carcinoma of the vulva. Bilateral superficial groin dissection revealed no evidence of regional lymph-node metastasis. Drains are still in place in the surgical wounds. The primary lesion, outlined with a wire during the simulation, involved the vagina and approached to within 1 cm of the anal sphincter, rendering it unresectable without sacrificing bowel continence. Initial treatment included the use of bolus material over the region indicated to ensure that a full dose was administered to the surface of the lesion during 6-MV photon treatment. Asymmetric AP and PA fields were used. 5-fluorouracil and mitomycin-C were currently administered during external-beam radiotherapy. The field was reduced after 36 Gy was given. The total dose to the primary lesion was 45 Gy. Approximately 2 months after treatment, residual fibrosis was found at the primary site. This area was completely excised without injury to the anus. Histopathologic analysis revealed only scar tissue without evidence of tumor. Two years after treatment, the patient had no evidence of recurrence.




