Overview
Infertility affects approximately 13-14% of reproductive-aged couples. It is defined as the inability to conceive after 1 year of properly timed, unprotected intercourse. This definition is based on the cumulative probability of pregnancy.
Table 1. Cumulative Probability of Pregnancy in Couples With Normal Fertility (All Reproductive-aged Women) (Open Table in a new window)
Month |
Monthly Probability |
Cumulative Probability |
1 |
0.2 |
0.20 |
2 |
0.2 |
0.36 |
3 |
0.2 |
0.49 |
4 |
0.2 |
0.59 |
5 |
0.2 |
0.67 |
6 |
0.2 |
0.74 |
7 |
0.2 |
0.79 |
8 |
0.2 |
0.83 |
9 |
0.2 |
0.86 |
10 |
0.2 |
0.89 |
11 |
0.2 |
0.91 |
12 |
0.2 |
0.93 |
Cycle fecundability is the probability that a single menstrual cycle will result in pregnancy (see Table 1 above). Cycle fecundity is the probability that a single cycle will result in a live birth. Assuming a constant monthly probability of conceiving (fecundability) of 20%, the theoretical cumulative pregnancy rate after 12 months is 93%. However, studies show that this number is actually lower. A famous study conducted in the 1950s showed that 50% conceived within 3 months, 72% within 6 months, and 85% within 12 months. [1] Other cohort studies have produced similar results.
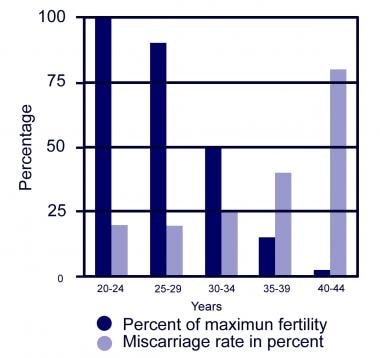
Fecundability and fecundity are dependent on multiple factors as described below, but one of the most important is the female partner's age. Cycle fecundability decreases as the number of oocytes decreases. Cycle fecundity also decreases, largely due to an increase in miscarriages. See the image below.
The number and quality of a woman’s oocytes declines with age. The decline in the number of oocytes begins at 20 weeks' gestation when the female fetus has approximately 6-7 million oogonia (largest lifetime endowment). The number of oocytes decreases to approximately 2-3 million at birth and decreases again to 300,000 by the time of puberty. Interestingly, the human female has lost most of her eggs before she is even capable of reproducing. At present, the pool of eggs is believed to be nonregenerable.
After the onset of puberty and menses, the female human ovary recruits at least 30-50 oocytes during each menstrual cycle. The oocytes compete with one another to become the dominant follicle and eventually ovulate to be released as an egg capable of being fertilized. Prior to the onset of menopause (10-15 y), menstrual cycles shorten and rapid follicular loss occurs because more oocytes are being recruited.
The birth rate among US women has dropped from 106.2/1000/year in 1950 to 62.9/1000/year in 2014 [2] . This has coincided with several social trends including later marriage, increased divorce rates, improvement in contraception and access to family planning, increased educational level achieved among women, and a greater number of women in the work force. Interestingly, the birth rates by age have dramatically shifted, with birth rates in women aged 15-29 years decreasing and birth rates among women aged 30-44 years increasing from the years 1990–2014.
Though not as abrupt or noticeable as menopause in women, changes in fertility and sexual functioning do occur in men as they grow older. One review of the literature shows that, currently, there is not enough data to show a clear effect of advancing paternal age on ART outcomes. [3] A recent retrospective analysis of 2,627 intracytoplasmic sperm injection (ICSI) cycles showed paternal age had no effect on pregnancy outcomes and parameters of embryo quality. Both studies point out to the need for more studies in this topic with long term follow up [4] .
For related information, see Medscape's article Infertility.
Cause of Infertility
LenThe cause of infertility can be easily identified in some couples. In other couples the cause is much less clear, and multiple factors may contribute. The major recognized causes of infertility are listed in Table 2.
Table 2. Causes of Infertility (Open Table in a new window)
Cause |
Couples |
Women |
Male |
35% |
-- |
Ovulatory |
15% |
40% |
Tubal |
35% |
40% |
Other |
5% |
10% |
Unexplained |
10% |
10% |
Obtaining a thorough history and physical examination is essential in evaluating an infertile couple.
The questions asked during the evaluation should include the following topics:
-
History of prior pregnancues and outcomes of each pregnancy
-
Length of time of sexual activity without conctraception
-
Current and past medical/surgical problems and recent changes in health
-
Employment and potential chemical/environmental exposures
-
Family history of miscarriages
-
Personal and family history of birth defects or inheritable disorders
-
Updated medicine list (including any over-the-counter medicine or herbs)
-
Tobacco, alcohol, and drug use
-
Coital frequency and any sexual dysfunction
-
Thorough gynecologic history, including pelvic pain, discomfort with intercourse, menstrual cramps, cycle length, and duration of flow
-
Prior pelvic infections
-
Use of prior contraception
-
Prior infertility work up or treatments
Diagnostic Tests
When evaluating an infertile couple, diagnostic studies should be selected as indicated. If the history is unclear, then tests that address the above-mentioned major categories of infertility should be obtained.
Male factor testing
After a medical history and physical examination, semen analysis is the single best test for evaluating for male factor infertility.
For optimum and consistent results, abstinence is required 3-5 days prior to semen collection. The World Health Organization (WHO) has established methods for semen analysis, but methods may vary among facilities. Additionally, the WHO has established normal reference values.
Another commonly used method for evaluating morphology is the strict Kruger method. Although no particular measurements can be used to discriminate between fertile and infertile men, odds of male infertility increase with increases in the number of semen parameter abnormalities.
-
Volume - At least 2 mL or greater
-
Sperm concentration - 20 million/mL or more
-
Motility - At least 50% or more with forward progression
-
Morphology - At least 30% or more normal forms (14% strict Kruger criteria)
-
White blood cells - Fewer than 1 million/mL
-
Round cells - Fewer than 5 million/mL
More recently, other tests have been devised to evaluate sperm. The Halosperm test and the Sperm Chromatin Structure Assay (SCSA) have been devised to evaluate the DNA fragmentation of sperm. Some studies suggest that a DNA fragmentation of greater than 30% is associated with a lower probability that the male partner will be able to initiate a pregnancy that carries to delivery.
Ovulatory function testing
Women of reproductive age who have regular menstrual cycles lasting from 21-35 days are likely ovulatory. However, for patients to become more accustomed to predicting ovulation so that they can appropriately time intercourse, they may wish to initiate basal body temperature monitoring or use luteinizing hormone (LH) detection kits.
Basal body temperature (BBT) monitoring is largely a historical method for determining the correct timing of intercourse. A 0.5–1.0ºF rise in temperature is noted 2 days after the luteinizing hormone (LH) peak, which occurs after the day of ovulation. This results from progesterone production from the corpus luteum. This should be done with a special mercury thermometer before rising from bed. Since most studies show that the best day to introduce sperm into the female reproductive tract is either the day of ovulation or the day before ovulation, BBT monitoring -is not useful for coital timing in a current cycle but best serves as a method to confirm the time of ovulation and helps the patient to predict future cycles based on data she has gathered over prior cycles. This method is inexpensive but time-consuming and cumbersome. Urine LH kits are a more useful method to prospectively predict the day of ovulation; however, they can be expensive and result in confusing results, particularly in thesetting of the use of clomiphene citrate, patients with oligo-ovulation, or if pregnancy has occurred.
A deficiency in progesterone production by the corpus luteum (CL) has historically been attributed to infertility and recurrent pregnancy loss in many women with otherwise unexplained miscarriages. The significance and presence of an inadequate luteal phase (also referred to as luteal phase defect [LPD]) has been questioned throughout the literature. Traditionally, diagnosis of an LPD has been determined histologically (a lag of >2 days of an endometrial biopsy compared with the day of the cycle, based on the actual day of ovulation). Some physicians prefer to use low luteal phase progesterone levels (< 10 ng/mL) 6 days after ovulation as their method of diagnosis, with good correlation to histologic findings if repeated in 3 separate menstrual cycles. Isolated LPD (by histological criteria) are observed in 30-40% of healthy fertile couples as well as infertile couples, implying that this defect mustbearepetitive event to be a true cause of infertility or miscarriage. Little evidence suggests that the addition of exogenous progesterone benefits pregnancy outcomes, suggesting a potential defect in endometrial receptivity during the implantation stage.
Ovarian reserve testing
Several simple tests for ovarian reserve exist. Initial testing usually includes cycle day 3 laboratories including follicle stimulating hormone (FSH), estradiol (E2), and leuteinizing hormone (LH). Typically, if the FSH level is greater than 15 mIU/mL or the estradiol level is greater than 75 pg/mL, the prognosis is poor. Day 3 antral follicle scans and ovarian volume may also be used to evaluate ovarian reserve and are simple and accurate.
In patients older than 40 years or for whom poor ovarian reserve is suspected, a clomiphene citrate challenge test may be performed. Clomiphene citrate (100 mg PO qd) is administered on cycle days 5-9. FSH and estradiol levels are drawn on days 3 and 10. If the day-3 or day-10 FSH level is greater than 15 mIU/mL or the day-3 estradiol level is greater than 75 pg/mL, the test results are considered abnormal. The rationale is that if the woman has an elevated day-10 estradiol level due to the clomiphene, yet her FSH level is not suppressed (estrogen suppresses FSH by a negative feedback mechanism), she has significant decreased ovarian reserve.
Tubal disease testing
In patients with unremarkable history or examination findings, a hysterosalpingogram (HSG) performed 2-5 days after the cessation of menstrual flow is the procedure of choice to evaluate tubal anatomy and patency. The risk of infection is extremely low, and most patients do not require antibiotic prophylaxis unless the patient has a history of pelvic infection. Additionally, if distal tubal occlusion is found, treatment should be provided because the risk of infection increases and treatment has been show to prevent infection in these cases. Pretreatment with nonsteroidal anti-inflammatory drugs is recommended with the rare patient requiring a mild sedative.
The HSG is a radiographic technique in which a dye is injected into the cervix. This dye fills the uterus and eventually the tubes. If the tubes are patent, dye spills out into the abdominal cavity. The test requires approximately 10 minutes to complete. This procedure is primarily diagnostic, but it may possibly be therapeutic (for approximately 6 mo), primarily when using an oil-based dye. Additionally, it provides imaging of the uterine cavity.
A history of pelvic inflammatory disease, septic abortion, ruptured appendix, tubal surgery, or ectopic pregnancy should alert the physician to the possibility of tubal damage. In these patients or in patients with significant pelvic pain during the physical examination, proceeding to a diagnostic laparoscopy rather than an HSG may be prudent given the probability of pelvic pathology. In this case, the tubes and the rest of the pelvis may be directly inspected and a chromopertubation may be performed. During this procedure, dye is injected through the cervix and into the uterus. If the dye is seen to spill from both of the tubal openings, the fallopian tubes are presumed patent.
Cervical disease testing
Women who have had cervical cone biopsies or trauma to the cervix are at risk for cervical abnormalities and cervical stenosis. If a cervical abnormality is found, the most logical approach is to recommend bypassing the cervix with intrauterine inseminations (IUI), especially if the rest of the findings from the infertility evaluation are normal.
In the past, suspected cervical factor infertility was tested with a postcoital test (PCT), looking at the interaction of cervical mucous and sperm at a specified time after intercourse during the perio-ovulatory phase of the cycle. A lack of consensus exists regarding the accuracy, precision, and use of the PCT in the modern infertility evaluation, and it is now rarely used in practice.
Uterine disease testing
Similar to tubal disease, obtaining a history from the patient is the most important diagnostic tool. A history of repetitive abortions, uterine surgery, postpartum uterine infections, retained products of conception, or postpartum curettage should alert the clinician to a possible uterine factor. A history of abnormal bleeding, such as heavy menses, midcycle spotting, or irregular bleeding, may represent an intrauterine fibroid, polyp, or synechiae. Malpresentation during pregnancy or recurrent pregnancy loss often suggests a uterine anomaly, such as a septum or bicornuate uterus.
A screening transvaginal ultrasonography performed immediately following the cessation of menses may demonstrate a uterine leiomyoma (fibroid) or an endometrial polyp. HSG typically used to evaluate the fallopian tubes can also be used to evaluate the uterine cavity.
If the patient has known blocked tubes and is scheduled for in vitro fertilization (IVF), a sonohysterogram (SHG) or office hysteroscopy (HSC) may be performed. An SHG is performed by placing a small catheter in the uterine cavity and instilling sterile saline to separate the endometrial walls under ultrasonographic guidance. SHG is more sensitive than an HSG in delineating fibroids and polyps. HSC allows for direct visualization of the cavity via an optic fiber.
Testing for endocrine abnormalities
Any evidence of the following should warrant selective hormonal studies.
-
Hirsutism or hair loss
-
Abnormal weight gain
-
Oily skin or acne
-
Purple striae on the abdomen
-
Polyuria/polydipsia
-
Irregular menses
-
Heat or cold intolerance
-
Headaches, visual problems or lactation without prior pregnancy
If the patient displays hirsutism, with or without menstrual irregularity, androgen studies such as dehydroepiandrosterone sulfate (DHEA-S), total testosterone, and 17-hydroxyprogesterone should be performed. If unusual weight gain or fatigue develops, a thyroid-stimulating hormone (TSH) should be obtained. If galactorrhea or irregular menses occurs, measuring the prolactin level should be considered. Acanthosis nigricans suggests hyperinsulinemia. If diabetes is suspected, a glucose tolerance test should be obtained.
Unexplained infertility
By definition, a physician makes the diagnosis of unexplained infertility after all tests are completed, including a diagnostic laparoscopy with or without a hysteroscopy. In modern practice, unexplained infertility is considered after all test results are negative, prior to any surgery, and in a patient with an unremarkable history and physical examination findings.
Treatment & Management
The prevalence of infertility over the last 30 years has been stable, but the treatment and demand for infertility services has increased substantially during that time. This increase is due to several factors: changes in population demographics (older couples trying to conceive), increased patient awareness and access to services, and advances and improvements in fertility treatments.
Treatment of male factor
Confirm any abnormal study result with a repeat semen analysis (SA) at least 4 weeks apart. The average time of sperm turnover is approximately 60-70 days. Since semen parameters can be affected by acute illness and environmental factors, a repeat SA will give a more accurate reflection of overall semen quality. If results remain abnormal, refer the patient to a urologist to evaluate for any genetic, anatomic, hormonal, or infectious causes. If the volume is less than 1 mL, consider retrograde ejaculation and obtain an analysis of the urine. If the sperm concentration is less than 20 million/mL, yet the swim-up extraction yields at least 1 million total motile sperm, intrauterine inseminations (IUI) is the treatment of choice. If sperm counts are extremely low or if motility is poor, in vitro fertilization (IVF) with intracytoplasmic sperm injection (ICSI) may be required.
Treatment of ovulatory dysfunction
If a patient has a normal ovarian reserve, determining the potential cause of the ovulatory defect is prudent. The practitioner should consider the following scenarios prior to initiating treatment. In the presence of obesity and chronic anovulation, polycystic ovarian syndrome (PCOS) or Cushing disease may be evident and in the case of hirsutism, the patient may have elevated androgen levels or hyperinsulinemia, requiring further testing. In a patient with low body mass index and low gonadotropin levels, weight gain and decreased exercise may improve fertility. If oligomenorrhea remains, ovulation induction can be achieved with exogenous gonadotropins. If the physical examination findings are unremarkable, ovulation induction is the next treatment approach to consider.
The following clomiphene citrate (CC) treatment regimens are often used for ovulation induction in patients with idiopathic ovulatory dysfunction or PCOS:
-
Clomiphene citrate (50 mg on days 2-6 or days 5-9)
-
Clomiphene citrate (100 mg on days 2-6) plus follicle stimulating hormone (FSH) (1-2 amps starting on day 8)
-
Clomiphene citrate plus human chorionic gonadotropin (hCG) for ovulation induction [5]
-
Elevated androgen levels – Dexamethasone (0.25 mg/d) plus clomiphene citrate
-
Hyperinsulinemia with elevated androgens -Metformin (500 mg tid) plus clomiphene citrate. Many women spontaneously ovulate on metformin alone; thus, many clinicians allow a trial of 1-3 mo prior to adding clomiphene citrate. However, recent data suggest that clomiphene citrate alone may be more efficacious at achieving a live birth than metformin or the combination of metformin with clomiphene citrate.
-
Aromatase inhibitors (letrozole) (2.5 mg PO on cycle days 3-7) has been shown in early studies to work as well as clomiphene citrate to induce mono-ovulation and to have little or no adverse affect on endometrial thickness.
The goal of therapy is to achieve 3 ovulatory cycles; 40-50% of women should become pregnant in this timeframe in the absence of any other abnormalities. If conception has not occurred after 3 clomiphene citrate cycles, the practitioner should investigate other causes of infertility. No more than 6 consecutive cycles are recommended because of the theoretical risk of borderline ovarian tumors and extremely low pregnancy success rates after this point.
Evidence suggests that starting clomiphene citrate earlier (day 2 or 3 rather than day 5) is more beneficial because this conforms to a more typical cycle length of 28 days. Beginning on cycle day 2 or 3 promotes ovulation around days 12-16, which is more physiologic and may avoid delayed ovulation and excessive maturity of the oocyte. Studies from the 1970s in women with documented delayed ovulation (after cycle day 16) revealed a higher relative miscarriage rate. This was believed to be caused by meiotic dysfunction within the oocyte.
Ovulation (LH) predictor kits are much easier for patients to use when clomiphene citrate is started on day 2 or 3. LH levels are normally elevated after the patient takes clomiphene citrate, and the half-life of the medication is 5 days. Thus, in a cycle day 5-9 regimen, a patient may receive a false-positive reading if she follows the directions on the kit and starts monitoring on cycle days 12-13.
Evidence demonstrates that a day 2 or 3 clomiphene citrate start allows the endometrium to thicken to a more normal, physiologic range. Endometrial thinning is a well-known adverse effect of clomiphene citrate. An endometrium that is thinner than 7-8 mm has been associated with a lower pregnancy rate in IVF cycles.
Ovulation induction can also be initiated with exogenous FSH. During such cycles, FSH is generally started on cycle day 3 and the patient is monitored for development of 1-2 dominant follicles. When 1-2 dominant follicles have grown to the appropriate size, ovulation is hormonally induced and IUI is performed.
Treatment of tubal disease
IVF offers the best chance for conception in patients with significant tubal disease. Often, if only 1 tube is affected, ovarian stimulation with gonadotropins produces mature oocytes in the ovary near the patent tube. In patients with minimal or moderate tubal disease, laparoscopic lysis of adhesions and procedures should be performed to normalize tubal function, with an emphasis on prevention of adhesion recurrence. In patients with an irreparable hydrosalpinx, removing the tube or disconnecting it from the uterus may reduce the risk of a tubal pregnancy and enhance embryo implantation if the patient requires IVF.
Pregnancy rate following treatment can be dependent upon location of tubal disease (see Table 3 below).
Laparoscopic lysis of adhesions offers an opportunity to conceive either naturally or with minimal types of therapy. If only proximal tubal occlusion is present, these obstructions can be fixed with a balloon tuboplasty under fluoroscopic guidance similar to the common angioplastic procedure in cardiology.
Table 3. Treatment of Tubal Pathology (Open Table in a new window)
Procedure |
Pregnancy Rate (3-6 mo) |
Lysis of adhesions |
50% |
Mild distal obstructive disease |
80% |
Moderate distal obstructive disease |
30% |
Severe distal obstructive disease |
15% |
Proximal tubal obstruction |
30% |
Treatment of cervical factor
Intrauterine inseminations may offer a reasonable option for treatment. The presence of antisperm antibodies in the female or male warrants IUI. If the antibodies are on the sperm itself, washing the sperm with a chymotrypsin/galactose preparation may improve sperm motility.
Treatment of uterine factor
An operative hysteroscopy is usually required to lyse adhesions or remove endometrial polyps or submucosal fibroids. Intramural fibroids and subserosal fibroids may be removed by laparotomy, traditional laparoscopy or robotically assisted laparoscopy. At present, none of the commercially available barriers have been proven to be better at adhesion prevention over excellent surgical technique. In severe cases of uterine pathology, the best option for conception may be through the use of a gestational carrier.
Treatment of endocrine abnormalities
Ensure that any endocrine abnormality is normalized prior to attempts at conception. Keep in mind that women with luteal phase defects also have ovulatory dysfunction. Clomiphene citrate, as previously mentioned, and luteal phase progesterone supplementation may be potentially effective treatments. The recommended progesterone is either micronized progesterone in vaginal suppositories (50-100 mg bid) or progesterone vaginal cream (Crinone 8%; 90 mg/d). Oral micronized progesterone may be used but causes significant somnolence and adverse central nervous system effects (eg, depression).
Treatment of unexplained infertility
The choice of treatment protocol depends on how aggressive the couple wants to be with their efforts to conceive. Most physicians start with either clomiphene citrate or gonadotropins in conjunction with IUIs. Patients should completely understand the success rates (see Table 4) and the risks of multiple pregnancy with any treatment protocol.
Table 4. Unexplained Infertility and Pregnancy Rates per Cycle According to Treatment (Open Table in a new window)
Protocol |
Pregnancy Rate, % |
No treatment |
1.3-4.1 |
IUI alone |
3.8 |
Clomiphene with timed coitus |
5.6 |
Clomiphene with IUI |
10 |
Gonadotropins with timed coitus |
7.7 |
Gonadotropins with IUI |
17.1 |
IVF |
35-50 |
In vitro fertilization (IVF)
Modern IVF generally involves controlled ovarian hyperstimulation with exogenous gonadotropins, harvesting the eggs via transvaginal ultrasonographic-guided aspiration, co-culture of eggs and sperm in culture (or intracytoplasmic injection of sperm into the oocyte), and placement of the resultant zygotes (2-5 d later) directly into the uterus.
The first IVF pregnancy was achieved in 1978. Since then, the number of IVF centers and IVF procedures performed has increased dramatically. An intense effort to obtain insurance coverage for these services has also occurred. With the support of organizations such as RESOLVE (ie, the National Fertility Association), 15 US states provide coverage for these services. Currently, 3 states (Massachusetts, New Jersey, and Rhode Island) offer full coverage. Other states exempt health maintenance organization programs, private insurers, or companies with few employees. Other states offer limits to their coverage. In states that provide full coverage, the actual cost per paid subscriber is not substantial. A recent study in Massachusetts calculated that the increase is only $25 per year per subscriber.
As a result of the Fertility Clinic Success Rate and Certification Act, the US Centers for Disease Control and Prevention (CDC) gathers information from 422 of the 475 clinics throughout the United States. Information from 2005 shows that 134,260 assisted reproductive technique (ART) cycles were performed resulting in 38,910 live births (deliveries of one or more living infants) and 52,041 infants.
Complications can arise during IVF. The incidence of first trimester venous thromboembolism in relation to IVF was 0.2%, which is a 10-fold increase over the background population. The 6-7% of IVF pregnancies complicated by ovarian hyperstimulation had a 100-fold increased risk, as opposed to the 5-fold increase in its absence. However, with the advent of certain modifications in IVF treatment protocols, particularly the use of leuprolide injection rather than hCG for final oocyte maturation, the incidence of ovarian hyperstimulation will significantly decrease. No increased risk related to frozen embryo replacement cycles or IVF after the first trimester was noted. Treating women with hyperstimulation with heparin thromboprophylaxis during the first trimester and treating women at high risk for hyperstimulation with frozen embryoreplacementislikelyto lower the venous thromboembolism risk. [6]
Other assisted reproductive techniques
Gamete intrafallopian transfer (GIFT) was developed in 1984 for women with unexplained infertility. GIFT is much less utilized, but to certain religious and ethnic communities (in which fertilization inside the woman's body is the only type allowed), it is considered more acceptable. During this procedure, the patient undergoes a controlled ovarian hyperstimulation. The oocytes are retrieved transvaginally under ultrasonographic guidance, and 3-4 oocytes are placed via laparoscopy into one of the fallopian tubes along with sperm.
During zygote intrafallopian transfer (ZIFT), oocytes are retrieved similar to IVF and GIFT and they are allowed to fertilize in vitro in the laboratory as in IVF. A day after fertilization (2 cell stage), 3-4 embryos are transferred via laparoscopy into one of the fallopian tubes. If the embryos are allowed to develop to greater than a 2-cell stage, the procedure is termed tubal embryo transfer (TET). The only benefit to a ZIFT or TET versus the more traditional IVF is for women who are thought to have compromised embryo quality due to embryo in vitro culture. Placing these zygotes or embryos back into their own natural incubators is thought to enhance subsequent development with improved pregnancy rates.
With the development of enhanced culture media, the success rates for IVF are now comparable, if not better, to those of GIFT and ZIFT, and IVF is less invasive than GIFT and ZIFT.
Interpreting IVF success rates
Comparing one program's success rate to another is difficult because of all the variables involved, including the program’s selection criteria, patient demographics, and insurance coverage. In general, like any statistical analysis, the more IVF cycles a program has performed, the more valid the numbers are. The cancellation rate is a critical number. If the rate is high, the program is possibly very selective for the patients it allows to proceed to egg retrieval. This type of program would rather cancel the patient's procedure than have a low chance for success. The implantation rate refers to the pregnancy rate divided by the number of embryos transferred.
If the implantation rate is low and the pregnancy rate is high, this suggests that the program is transferring a large number of embryos per patient to achieve that success. Chances are good that the program's multiple pregnancy (eg, twins, triplets, and higher order multiples) rate is high. Optimally, the better programs have a low cancellation rates, good pregnancy and implantation rates, and high singleton pregnancy rates compared with multiple pregnancy rates.
The ultimate critical number is the birth rate because this represents the final goal of the patient and the physician. This goal is also less vulnerable to misinterpretation than the pregnancy rate (single positive hCG vs serial increases) or the clinical pregnancy rate (gestational sac vs fetal pole vs fetal pole with heartbeat).
IVF outcomes
2005 data for IVF outcomes are summarized and results can be viewed on the CDC and Society for Assisted Reproductive Technology Web sites. Outcomes are stratified based on cycle type (fresh IVF, frozen embryo IVF, donor IVF, and maternal age). Overall, 134,260 ART cycles were performed in the United States in 2005 resulting in 38,910 live birth deliveries. For reference, in 1996, 14,507 deliveries resulted from 64,681 ART cycles. Because more than 1 infant is born during a live-birth delivery (eg, twins) in some cases, the total number of infants born is larger than the number of live-births. From 1996-2005, the percentage of transfers resulting in live births for fresh–nondonor cycles increased from 28% in 1996 to 34% in 2005.
In a 2012 study of 612 preterm infants, use of assisted reproductive technologies was not associated with adverse neonatal outcomes. Survival without severe morbidity was significantly higher among infants born using assisted reproductive technologies compared with spontaneously conceived preterm infants, probably because of differences in pregnancy and neonatal characteristics. [7]
In a 2015 systematic review and meta-analysis of the influence of endometriosis on assisted reproductive technologies outcomes from 36 studies (of 1346 articles), investigators found similar outcomes for live births between women with endometriosis who underwent in vitro fertilization and intracytoplasmic sperm injection and women without endometriosis. [8] However, women with severe endometriosis had lower live birth rates, clinical pregnancy rates, and mean number of retrieved oocyte relative to those without endometriosis. The investigators noted there remains not enough evidence to support recommending surgery routinely before women undergo ART. [8]
In another 2015 report that assessed the risk of ectopic pregnancy with assisted reproductive technology over 10 years (2001-2011) in the United States, 9,480 of 553,577 pregnancies were ectopic (1.71%) (including 485 that were heterotopic; these data were combined for analysis). [9] The investigators noted a decrease in ectopic pregnancy rates (2001: 2.0%; 2011: 1.6%) (P< 0.001), and fresh, nondonor cycles had an ectopic pregnancy rate of about 2.0%, whereas it was 1.0% for fresh, donor cycles. However, among fresh, nondonor cycles, the greater the number of embryo transferred resulted in an increased risk of an ectopic pregnancy. [9]
Guidelines for embryo transfer
Multifetal gestations are associated with greater risks for both mothers and infants, including higher rates of caesarean section, prematurity, low birth weight, and infant disability or death. In response to the significant numbers of higher order multiple pregnancies generated from ART, the American Society of Reproductive Medicine (ASRM) released guidelines for the number of embryos transferred in 1999. These were modified in 2006 based on newer data. See Guidelines on number of embryos transferred.
In 2005, the percentage of multiple-infant live births decreased from 38% of all live births in 1996 to 32% in 2005 for fresh-nondonor ART cycles.
Increasing the number of embryos transferred from 1 to 2 not only increases the chance for a live birth but also increases the likelihood of a multiple-infant pregnancy. However, transferring more than 2 embryos may not increase the overall live birth rate. A meta-analysis of randomized trials on single and double embryo transfer found that compared with double embryo transfer, elective single embryo transfer results in a higher likelihood of delivering a term singleton live birth though it also has a lower pregnancy rate. [10]
Many variables affect the decision of how many embryos to transfer. Factors such as the patient's age, embryo quality, number of prior failed IVF cycles, and use of frozen-thawed embryos are important to consider. New data from Europe suggest that a single embryo transfer in the appropriate patient results in approximately a 35% pregnancy rate with a less than 1% multiple pregnancy rate. These patients typically have embryos that are frozen, ensuring that their cumulative pregnancy rate using either fresh or frozen embryos is similar to transferring 2 or more embryos. Single embryo transfer is appropriate in certain situations where the likelihood of a multiple pregnancy is high. This may include women younger than 35 years, women who conceived with first IVF cycle, women with only tubal factor infertility, women with concerns about multiple gestation, and donor egg recipients.
Kresowik et al found that twinning rates were reduced and pregnancy rates maintained after introduction of a mandatory single-embryo transfer policy in women younger than 38 years, with at least 7 zygotes, no prior failed fresh cycles at the center, and with at least one good-quality blastocyst. [11]
Factors contributing to IVF success
The most important factor that determines a successful cycle is the female patient's age. As mentioned previously, decreases in fecundity rates are observed beginning as early as age 30 years. The dramatic effect that age has on fecundability is also observed in ART. Most egg donors are aged 20-35 years, allowing for an optimal control group to observe these differences.
Ultimately, the success of ARTs mimics the overall fecundity trend observed in the general fertile population. That is, pregnancy and live birth rates start to decrease beginning around age 30 years and continue to decrease until the chance of having a live birth is so low that the benefit of ARTs must be evaluated. In women older than 40 years, the chance of having a liveborn infant with a chromosomal abnormality also increases.
Weight may also play a part in IVF success. A retrospective study found that women of normal body weight have a higher chance of pregnancy and live birth following IVF than either underweight or obese women. Women with morbid obesity (BMI >40) have a significantly decreased chance of success with IVF. [12, 13, 14]
A large retrospective study evaluating data from the Society for Assisted Reproductive Technology suggested that the process of ovarian stimulation with gonadotropins was an independent risk factor for low neonatal birth weight compared with women who conceived with a singleton pregnancy. [15]
Oocyte retrieval
Oocyte retrieval is performed approximately 36 hours after 10,000 U of hCG is administered to allow for the resumption of meiosis, cytoplasmic maturation, and loosening of the oocytes within the follicle. This allows for a lower optimal vacuum pressure during aspiration and ultimately less oocyte damage.
The 3 basic methods to retrieve oocytes are laparoscopic, transabdominal, or transvaginal. The laparoscopic approach was used frequently in the 1980s, especially when a GIFT procedure was planned. Often, only the follicles that could be seen on the surface of the ovary were removed, and, if the ovary was very mobile, traction was required to support the ovary as the follicles were aspirated. Associated morbidity occurred with the procedure, which included infection and injury to the pelvic organs. General endotracheal anesthesia was usually used, and the patient's recovery often lasted 2-3 days. As the quality of ultrasonographic images and culture media improved, the need for laparoscopy decreased.
In 1981, ultrasonographic-guided aspiration was first described. Initially, the transabdominal approach was used, usually with the aspirating needle going through the bladder, which, when full, provided a window of visualization for the person operating the abdominal ultrasonographic probe.
Although still used for retrieval of oocytes from ovaries that are adhered high up in the pelvis or to the fundus of the uterus, the transabdominal approach was superseded by the transvaginal approach. The first transvaginal retrieval was performed in 1984 and has now become the procedure of choice because of its ease and low morbidity.
Micromanipulation
Intracytoplasmic sperm injection (ICSI) is the treatment of choice for couples in whom the male partner has azoospermia or severe oligospermia. ICSI is also indicated for men with significant antisperm antibodies, low sperm motility, or significantly abnormal sperm morphology (Kruger strict morphology < 4%). [16]
ICSI is used when poor fertilization occurs with regular insemination techniques in the laboratory. ICSI may be used when a limited amount of sperm is available, such as in couples where the man has stored sperm prior to chemotherapy. ICSI is indicated in certain preimplantation genetic (PGD) procedures—specifically those cases being evaluated for single-gene recessive disorders. This prevents the potential contamination of the specimen with sperm that may be attached to the egg.
Sperm can be obtained from the ejaculate or directly from the epididymis. Recently, success was obtained with spermatids from testicular biopsies.
The potential transmission of a genetic abnormality is a possibility when ICSI is performed. The normal barrier for morphologically abnormal sperm that tend to have genetic abnormalities (ie, zonal pellucida) is bypassed with ICSI. Morphologically normal sperm may also have genetic abnormalities. Approximately 10% of sperm from healthy men have chromosomal abnormalities. Men who are infertile have a 5-7% chance of having a chromosomal abnormality. Chromosomal abnormalities include microdeletions of the long arm of the Y chromosome in areas AZFa, AZFb, and AZFc (DAZ or deleted in azoospermia region). These deletions can be passed on to male offspring, with resulting oligospermia.
Some data suggest a 30% increase in birth defects in children conceived with ICSI. Overall, this implies that the risk of having a child with a birth defect from ART with ICSI goes from a normal baseline of 3% to, at most, 4%.
Approximately 1-2% of men with azoospermia have genetic translocation, Klinefelter syndrome (47,XXY), or a congenital bilateral absence of the vas deferens, which is associated with mutations in the cystic fibrosis transmembrane regulator (CFTR) gene or the 5T allele.
In the situation where the male partner has the CFTR mutation, the female partner should also be screened for cystic fibrosis. In any couple undergoing ICSI for male factor infertility, a karyotype and Y-DNA mapping should be considered if the sperm concentration is less than 5 million/mL, and genetic counseling should be offered. Prenatal testing of ICSI pregnancies has revealed an incidence of 0.83% of sex chromosome abnormalities (higher than those reported for spontaneous pregnancies).
Guidelines
The German Society of Gynecology and Obstetrics (DGGG), in cooperation with the Swiss Society of Gynecology and Obstetrics (SGGG) and the Austrian Society of Gynecology and Obstetrics (OEGGG), have developed guidelines for counselling, diagnostic workup, and treatment of infertility. [17]
Infertility consultation and workup
The initial history should include an inquiry concerning risk factors relevant to infertility, such as age, smoking status, alcohol intake, eating disorders, drug use, and intensive physical exercise. The patient must understand that such factors may not only adversely affect the treatment outcome but also potentially damage gametes and embryos. Patients must also understand that a body mass index (BMI) over 30 kg/m2 or under 19 kg/m2 may predispose to ovulation disorders, which may cause infertility.
Before fertility treatment is initiated, women must be informed that folic acid substitution is required.
Appropriate psychotherapy or counseling should be recommended to patients whose fertility disorder is related to behavior (eg, eating disorder, drug addiction).
Sexuality and sexual disorders
Patients should be asked about sexual issues in the couple’s relationship. Sexual therapy should be recommended to couples who feel that their sexual behavior and experience require treatment.
Psychological factors
Screening tools for psychological vulnerability may be considered, if relevant. In these cases, a psychosomatic diagnosis should be offered; routine psychopathological diagnosis is unnecessary. Psychosocial counseling or psychotherapy is generally not recommended in these cases unless the fertility disorder has a behavioral etiology or the patient has a mental illness that requires treatment.
Diagnosis and treatment of congenital and acquired genital anomalies
Following the gynecological examination, vaginal ultrasonography must be performed to rule out congenital malformation. Three-dimensional vaginal ultrasonography with or without hysteroscopy, possibly combined with laparoscopy, should be performed if a congenital malformation is suspected.
Fibroids must be diagnosed with vaginal ultrasonography. Before fertility treatment is initiated, submucosal fibroids (Federation of Gynecology and Obstetrics [FIGO] type 0 and 1) must be removed hysteroscopically. Laparoscopy may be performed for intramural and subserous fibroids.
Suspected intrauterine polyps and/or adhesions should be evaluated with hysteroscopy. Hysteroscopy should be used to remove intrauterine polyps and adhesions.
If tubal patency evaluation is indicated, either laparoscopy with chromopertubation or hysterosalpingo contrast ultrasonography must be performed. Laparoscopy used to investigate tubal patency must be combined with hysteroscopy.
Women with a septate or subseptate uterus should undergo hysteroscopic septum dissection before fertility treatment is initiated. Bicornuate uterus, duplex uterus, and unicornuate unicollis uteri should not be corrected surgically in women with primary infertility.
Hydrosalpinx must be treated with laparoscopic salpingectomy or laparoscopic proximal tubal occlusion before assisted reproductive treatment (ART) is initiated.
Diagnosis and treatment of endometriosis
Infertile women with suspected endometriosis should undergo laparoscopic diagnostic workup with histological confirmation, chromopertubation, and hysteroscopy.
Peritoneal foci of endometriosis should be removed surgically.
Patients with ovarian endometriosis should be counseled regarding the procedural risks (reduced ovarian reserve) and possible benefits of surgery preoperatively.
Deep infiltrating endometriosis may be treated with surgical resection.
Diagnosis and treatment of vaginal infections
Asymptomatic women should not undergo screening for bacterial vaginosis with vaginal smears, nor should patients undergo acute chlamydia infection screening if asymptomatic. However, screening for chronic chlamydia infection may be performed with serology.
Clindamycin or metronidazole is suggested as treatment for bacterial vaginosis.
Infection prophylaxis is unwarranted in asymptomatic women and in the absence of pathogen confirmation.
Diagnosis and treatment of endocrine factors
The basic hormonal diagnostic workup in women with infertility consists of luteinizing hormone (LH), follicle-stimulating hormone (FSH), prolactin, testosterone, dehydroepiandrosterone (DHEAS), sex hormone–binding globulin (SHBG), free androgen index, estradiol, and anti-müllerian hormone (AMH) on days 3-7 of the menstrual cycle (or when no follicle has a diameter >10 mm). Vaginal ultrasonography and thyroid evaluation are performed along with the basic diagnostic workup. Any additional testing is based on specific findings.
Progesterone levels may be assessed at approximately 7 days following presumed ovulation to determine ovulatory cycle.
Primary or secondary amenorrhea
A pregnancy test is the first step in evaluating for amenorrhea. After a basic diagnostic endocrine workup is performed, additional examinations are based on symptoms.
Women with a high BMI (>30 kg/m2) should be advised to lose weight.
Hyperprolactinemia
Confirmed hyperprolactinemia in women should be treated with dopamine agonists.
Polycystic ovary syndrome or hyperandrogenemia
If polycystic ovary syndrome (PCOS) is suspected, diagnostic criteria for PCOS must be evaluated clinically. Rotterdam criteria include abnormal periods with oligoovulation or anovulation, laboratory-confirmed or clinical hyperandrogenemia, and characteristic PCO sonomorphology findings.
Drug therapy to induce ovulation should be monitored with ultrasonography, especially in women with PCOS, to reduce the likelihood of multifollicular growth, multiple pregnancy, and overstimulation.
In women with PCOS and oligo-ovulation or anovulation, clomiphene stimulation or letrozole stimulation (off-label) is first-line therapy to induce ovulation.
Adrenogenital syndrome and congenital adrenal hyperplasia
If androgenital syndrome (AGS) is suspected, molecular-genetic testing must be performed. Partners with confirmed AGS must be provided with genetic counselling.
Glucocorticoid treatment should be administered to women with classic AGS. An endocrinologist must be consulted for treatment and monitoring.
Anti-müllerian hormone, age, and oocyte quality
Although the AMH level may be used to estimate ovarian activity and responsiveness to hormone stimulation treatment, it is not used for fertility evaluation.
Anovulatory cycle and luteal phase insufficiency
In women with a regular and unremarkable menstrual cycle duration, endometrial biopsy to evaluate the luteal phase is unwarranted.
Cyclical progestogens should not be given to women with spontaneous menstrual cycles.
Diabetes mellitus
Before conception, hemoglobin A1c (HbA1c) testing must be performed in women with diabetes. A planned pregnancy is appropriate only when blood sugar levels are within the reference range or near the reference range.
Thyroid dysfunction
All women who want children should undergo thyroid-stimulating hormone (TSH) testing. A TSH value exceeding 2.5 mU/L should prompt testing of anti-thyroid antibodies.
At least 100-150 µg iodine supplementation/day should be given to women before conception.
L-thyroxine should be used in women with a TSH level of 2.5 mU/L or more to decrease the TSH level to less than 2.5 mU/L.
Definitive thyroid treatment must be completed in women with hyperthyroidism before ART is initiated and prior to conception.
Treatment of immunological factors
Women with antiphospholipid syndrome or systemic lupus erythematosus (SLE) must undergo treatment by an interdisciplinary team prior to conception. Antibody status, disease activity, comorbidities, and an updated treatment approach are components of management.
Rheumatoid arthritis, chronic inflammatory bowel disease (IBD), multiple sclerosis (MS), and other autoimmune or immune disorders must be closely managed by an interdisciplinary team, with treatment initiated before conception.
Vaccination status
The patient’s vaccination status should be evaluated. Rubella and varicella zoster immunity status must be confirmed and vaccination recommended, if necessary. Tetanus, diphtheria, and pertussis vaccinations should be given to women of childbearing age.
-
Female age and fertility.
-
Assisted reproduction technique procedures. Rates from the United States in 2003. Adapted from the Centers for Disease Control and Prevention.
-
Live births per retrieval for different types of assisted reproduction technique procedures (2003). Adapted from the Centers for Disease Control and Prevention.
-
2003 success rates for assisted reproduction technique cycles using fresh nondonor eggs. Adapted from the Centers for Disease Control and Prevention.
-
Outcomes of pregnancies from assisted reproduction technique cycles using fresh nondonor eggs or embryos (2003). Adapted from the Centers for Disease Control and Prevention.
-
2003 multifetal pregnancy rates from assisted reproduction technique cycles using fresh nondonor oocytes or embryos. Adapted from the Centers for Disease Control and Prevention.
-
2003 live birth and multiple-infant birth rates using fresh nondonor oocytes or embryos by number of embryos transferred. Adapted from the Centers for Disease Control and Prevention.
-
Live birth rates per transfer for fresh embryos by age (top image) and from patient or donor eggs (bottom image) (1996, 2002, 2003). Adapted from the Centers for Disease Control and Prevention.
-
2003 success rates using intracytoplasmic sperm injection in couples with male-factor infertility. Adapted from the Centers for Disease Control and Prevention.