Overview
The diagnosis of pregnancy requires a multifaceted approach using three main diagnostic tools. These are history and physical examination, laboratory evaluation, and ultrasonography. Currently, physicians may use all of these tools to diagnose pregnancy at early gestation and to help rule out other pathologies.
History and Physical Examination
The diagnosis of pregnancy has traditionally been made based on history and physical examination findings. Important aspects of the menstrual history must be obtained. The woman should describe her usual menstrual pattern, including date of onset of last menses, duration, flow, and frequency. Items that may confuse the diagnosis of early pregnancy are an atypical last menstrual period, contraceptive use, and a history of irregular menses. Additionally, as many as 25% of women bleed during their first trimester, further complicating the assessment. [1]
In a patient with menses of regular frequency, the classic presentation of pregnancy is amenorrhea as well as any combination of nausea, vomiting, generalized malaise, and breast tenderness.
Upon physical examination, one may find an enlarged uterus after bimanual examination, breast changes, and softening and enlargement of the cervix (Hegar sign; observed at approximately 6 weeks). The Chadwick sign is a bluish discoloration of the cervix from venous congestion and can be observed by 8-10 weeks. A gravid uterus may be palpable low in the abdomen if the pregnancy has progressed far enough, usually by 12 weeks. Currently, through the use of chemical assays and ultrasonography, physicians are capable of making the diagnosis of pregnancy before many of the physical signs and symptoms are evident. [2]
Laboratory Evaluation
Several hormones can be measured and monitored to aid in the diagnosis of pregnancy. The most commonly used assays are for the beta subunit of human chorionic gonadotropin (hCG). Other hormones that have been used include progesterone and early pregnancy factor.
The cytotrophoblast and syncytiotrophoblast each secrete a variety of hormones that include, but are not limited to, corticotropin-releasing hormone, gonadotropin-releasing hormone, thyrotropin-releasing hormone, somatostatin, corticotropin, human chorionic thyrotropin, human placental lactogen, inhibin/activin, transforming growth factor-beta, insulin-like growth factors 1 and 2, epidermal growth factor, pregnancy-specific beta-1 glycoprotein, placental protein 5, and pregnancy-associated plasma protein-A. To date, no commercially feasible tests that use these hormones have been made available to aid in the diagnosis of pregnancy.
Beta-human chorionic gonadotropin
hCG is a glycoprotein similar in structure to follicle-stimulating hormone (FSH), luteinizing hormone (LH), and thyrotropin. hCG is composed of alpha and beta subunits. The alpha subunit of hCG is similar to the alpha subunit of FSH, LH, and thyrotropin. The free beta subunit of hCG differs from the others in that it has a 30–amino acid tailpiece at the COOH terminus. Free beta subunits are degraded by macrophage enzymes in the kidney to make a beta subunit core fragment, which is primarily detected in urine samples.
The beta-hCG subunit is present in the syncytial layer of the blastomere. Hyperglycosylated hCG is a form of hCG produced by invasive cytotrophoblast cells in early pregnancy and implantation. hCG messenger RNA is detectable in the blastomeres of 6- to 8-cell embryos at 2 days but cannot be isolated in culture medium until 6 days. Detection in maternal serum and urine is evident only after implantation and vascular communication has been established with the decidua by the syncytiotrophoblast 8-10 days after conception.
hCG is present in the maternal circulation as either an intact dimer, alpha or beta subunit, and degraded form, or beta core fragment. Intact and free beta subunit are initially the predominant forms of hCG, with the beta core fragment emerging as the predominant form in the fifth week after conception. Additionally, intact and free beta subunit have the most day-to-day variability and are transiently undetectable even 10 days after detection of pregnancy. [3] Optimally, tests used for early pregnancy detection should be able to recognize all forms of intact hCG, including the free beta subunit and the beta core fragment.
Currently, 4 main hCG assays are used: (1) radioimmunoassay, (2) immunoradiometric assay, (3) enzyme-linked immunosorbent assay (ELISA), and (4) fluoroimmunoassay. These assays are highly specific for hCG with antibodies directed against 2 or more isotopes on the intact hCG molecule. Time of detection is related to the sensitivity of the assay being used. Most current pregnancy tests have sensitivity to approximately 25 mIU/mL, but can also have lower thresholds of 100 mIU/mL depending on the test. Urine devices must be formulated to detect hyperglycosylated hCG, which is the key molecule in early pregnancy.
Characteristics of each hCG assay are listed as follows:
Radioimmunoassay
-
Sensitivity - 5 mIU/mL
-
Time to complete - 4 hours
-
Postconception age when first positive - 10-18 days
-
Gestational age when first positive - 3-4 weeks
Immunoradiometric assay
-
Sensitivity - 150 mIU/mL
-
Time to complete - 30 minutes
-
Postconception age when first positive - 18-22 days
-
Gestational age when first positive - 4 weeks
Immunoradiometric assay
-
Sensitivity - 1500 mIU/mL
-
Time to complete - 2 minutes
-
Postconception age when first positive - 25-28 days
-
Gestational age when first positive - 5 weeks
Enzyme-linked immunosorbent assay
-
Sensitivity - 25 mIU/mL
-
Time to complete - 80 minutes
-
Postconception age when first positive - 14-17 days
-
Gestational age when first positive - 3.5 weeks
Enzyme-linked immunosorbent assay
-
Sensitivity - Less than 50 mIU/mL
-
Time to complete - 5-15 minutes
-
Postconception age when first positive - 18-22 days
-
Gestational age when first positive - 4 weeks
Fluoroimmunoassay
-
Sensitivity - 1 mIU/mL
-
Time to complete - 2-3 hours
-
Postconception age when first positive - 14-17 days
-
Gestational age when first positive - 3.5 weeks
Dimeric hCG and both the alpha and beta subunits are produced in the pituitary gland of nonpregnant females and are released in association with LH. Although levels are much higher in postmenopausal patients (110 pg/mL vs 10 pg/mL), they are still below the sensitivity of the most sensitive clinical assays (approximately 1 mIU/mL) used in pregnancy monitoring.
Home pregnancy tests use a sandwich model of monoclonal antibodies in an enzyme-linked immunosorbent assay (ELISA). These are used largely in a qualitative fashion. False-negatives are possible when the concentration of hCG is below the detection threshold. False-positives are rare but also possible (discussed below in further detail).
Serial hCG monitoring
hCG is detectable in the serum of approximately 5% of patients 8 days after conception and in more than 98% of patients by day 11. At 4 weeks' gestation (18-22 d postconception), the dimer and beta subunit hCG doubling times are approximately 2.2 days (standard deviation ± 0.8 d) and fall to 3.5 days (standard deviation ± 1.2 d) by 9 weeks' gestation. Levels peak at 10-12 weeks' gestation and then begin to decline rapidly until another, more gradual rise begins at 22 weeks' gestation, which continues until term.
The initial rate of rise, measured by serial quantitative hCG testing, is important in the monitoring of early complicated pregnancies that have yet to be documented as viable and/or intrauterine. Failure to achieve the projected rate of rise may suggest an ectopic pregnancy or spontaneous abortion. hCG doubling times are subject to fluctuations of intact hCG during early pregnancy, so interpretation of these values must take into account the assays used and the clinical picture.
In one study, 200 women who received a diagnosis of ectopic pregnancy by serial hCG were evaluated. Of no surprise, the rise in hCG values in women with ectopic pregnancies was slower than those with viable pregnancies and the decline of hCG values in women with ectopic pregnancies was slower than for those with completed spontaneous abortion. However, 20.8% of women with ectopic pregnancies presented with a rise in hCG values similar to the minimal rise for women with a viable gestation, and 8% of women presented with a fall in hCG values similar to women with a completed spontaneous abortion. [4] Several other studies such as this one demonstrate that a single pattern of hCG does not exist for abnormal early pregnancy, so caution must be taken in interpreting serial hCG values in the evaluation of early pregnancy prior to making a diagnosis of ectopic pregnancy or early pregnancy loss.
On the other hand, an abnormally high level or accelerated rise can prompt investigation into the possibility of molar pregnancy, multiple gestations, or chromosomal abnormalities.
False-positive hCG results
As with most tests, hCG test results can be either falsely negative or positive. The prevalence of false-positive serum hCG results is low, with estimates ranging from 0.01-2%. False-positive serum hCG results are usually due to interference by non-hCG substances or the detection of pituitary hCG. Some examples of non-hCG substances that can cause false-positive results include human LH, anti-animal immunoglobulin antibodies, rheumatoid factor, heterophile antibodies, and binding proteins. Most false-positive results are characterized by serum levels that are generally less than 1000 mIU/mL and usually less than 150 mIU/mL. The median serum concentration for patients with false-positive results reported to the US Food and Drug Administration (FDA) between 1985 and 2001 was 75 mIU/mL. Also notable was that only 2 (0.74%) of 271 separate hCG determinations in undiluted sera in both the FDA database and the literature were greater than 1000 mIU/mL.
Several methods are available to help detect false-positive serum hCG results. The first step is to check urine hCG levels. The free beta-hCG subunit is further degraded in the kidney to a beta subunit core fragment that has less than half the molecular weight of the free beta subunit. Some of the substances that can cause serum false-positive results have much higher molecular weights that are not easily filtered through the renal glomeruli; therefore, they do not produce a positive urine hCG result. Other steps to verify or disprove a positive serum hCG result include retesting the same specimen, testing a new specimen, taking serial measurements to look for a rise, performing serial dilutions to look for linearity, and testing using a different method.
The five potential sources of positive hCG results outside of pregnancy are described below [5, 6] :
Phantom hCG
-
Caused by heterophilic antibodies that bind the capture and labeled antibodies together without hCG being present
-
Antibody production results from exposure to animals used to produce antibodies used in assay
-
Rule out with sensitive urine assay, as these antibodies do not cross into urine
Pituitary hCG
-
Stimulated by gonadotropin-releasing hormone; suppressed by gonadotropin-releasing hormone agonist and estrogen/progestin therapy
-
Can be detected in postmenopausal patients due to increased GnRH secretion (Snyder et al proposed that postmenopausal women should have a higher cutoff for a negative hCG of 14 IU/L [7] )
-
Diagnosed by administering oral contraceptive pills, which should suppress hCG levels
Exogenous administration of hCG
-
Used by some centers to aid in weight loss by intramuscular or oral administration
-
Repeat hCG assays should be negative if exogenous administration is discontinued for at least 24 hours
Trophoblastic neoplasm - Consists of pregnancy, gestational trophoblastic neoplasia (GTN), and placental site trophoblastic tumors (PSTTs)
-
Gestational trophoblastic neoplasia
- Quiescent - Constant, low levels of hCG without evidence of primary or metastatic malignancy; premalignant state; resistant to chemotherapy and surgery; follow with frequent hCG levels and if found to be rising, consider active gestational trophoblastic neoplasia
- Active - Invasive cytotrophoblasts produce hyperglycosylated hCG found only in early pregnancy and invasive gestational trophoblastic neoplasia; thus, hyperglycosylated hCG or invasive trophoblastic antigen can be measured to rule in active disease
-
Placental site trophoblastic tumors - Diagnosed with low-level hCG in combination with intramyometrial lesions on imaging
Nontrophoblastic neoplasm - Can be secreted by different cancers, (eg, testicular, bladder, uterine, lung, liver, pancreas, stomach)
False-negative hCG results
False-negative hCG test results usually involve urine and are due to the qualitative nature of the test. Reasons for a negative test result may include an hCG concentration below the sensitivity threshold of the specific test being used, a miscalculation in the onset of the missed menses, or delayed menses from early pregnancy loss. Delayed ovulation or delayed implantation are other reasons for low hCG concentrations at the time of testing, which yields a false-negative result.
A single case report in the literature is notable when considering false-negative urine hCG test results. A 37-year-old woman presented to an emergency department in hypovolemic shock 13 weeks after her last menstrual period. She had a dilation and curettage for an intrauterine pregnancy 8 weeks before presentation. Two different samples yielded negative qualitative urine pregnancy test results. The third urine hCG test result was weakly positive, and, at that time, her serum hCG level was 22,430 mIU/mL. She was diagnosed with an interstitial ectopic pregnancy and underwent surgery, during which approximately 2000 mL of free blood was found in her peritoneal cavity. Interstitial pregnancies make up less than 3% of tubal pregnancies, but they can be present in conjunction with negative urine pregnancy test results.
Progesterone
Measuring serum progesterone may be a useful adjunct for evaluating abnormal early pregnancy. Serum progesterone is a reflection of progesterone production by the corpus luteum, which is stimulated by a viable pregnancy. Measurement of serum progesterone is inexpensive and can reliably predict pregnancy prognosis. Currently, radioimmunoassays and fluoroimmunoassays are available that can be completed in 3-4 hours. A dipstick ELISA that can determine a serum progesterone level of less than 15 ng/mL is also on the market. ELISA is helpful as a screening tool for at-risk populations because progesterone levels of greater than 15 ng/mL make ectopic pregnancy unlikely. [8]
Viable intrauterine pregnancy can be diagnosed with 97.5% sensitivity if the serum progesterone levels are greater than 25 ng/mL (>79.5 nmol/L). Conversely, finding serum progesterone levels of less than 5 ng/mL (< 15.9 nmol/L) can aid in the diagnosis of a nonviable pregnancy with 100% sensitivity. Finding serum progesterone levels of less than 5 ng/mL allows diagnostic evaluation of the uterus in a stable patient, even if an ectopic pregnancy cannot be distinguished from a spontaneous intrauterine abortion beforehand. In the event that the serum progesterone level is 5-25 ng/mL, further testing using sonography, additional hormonal assays, or serial examinations is warranted to establish the viability of the pregnancy. Algorithms using serum progesterone are available for the evaluation and management of patients with abnormal early pregnancy.
Early pregnancy factor
The early pregnancy factor (EPF) assay may be useful in the future. EPF is a poorly defined immunosuppressive protein that has been isolated in maternal serum shortly after conception and is the earliest available marker to indicate fertilization. It is detectable in the serum 36-48 hours after fertilization, peaks early in the first trimester, and is almost undetectable at term. EPF also appears within 48 hours of successful in vitro fertilization embryo transfers. EPF cannot be detected 24 hours after delivery or at the termination of an ectopic or intrauterine pregnancy. EPF is also undetectable in many ectopic pregnancies and spontaneous abortions, indicating that an inability to identify EPF during pregnancy heralds a poor prognosis.
EPF has limited clinical applications at this time because the molecule is difficult to isolate. Detection of EPF currently relies on a complex and unwieldy assay termed the rosette inhibition test. EPF may play a more prominent role in the future as the diagnosis of conception prior to implantation elucidates new strategies for contraception, highly accurate dating, and advanced genetic studies.
Home pregnancy tests
At least 25 different home pregnancy tests are currently marketed in the United States. [9] These tests now use the modern immunometric assay. Most of these tests claim "99% accuracy" or some other similar statements on the packaging or product insert. Most of the tests also now advertise that they can be used "as early as the day of the missed menstrual period." Several home pregnancy tests actually instruct that they may be used 3-4 days before the time of the missed period.
The accuracy claims are derived from an FDA guideline that refers to the test's ability to identify approximately 100 nonpregnant urine samples supplemented with intact hCG from a similar number of urine samples not supplemented with hCG. The broad 99% accuracy statement is made for tests with sensitivities for hCG concentrations ranging from 25 mIU/mL (fairly sensitive) to tests with sensitivities of 100 mIU/mL (less sensitive). The 99% accuracy statement in reference to the FDA guideline is misleading in that it has no bearing on the ability of the home pregnancy test to detect early pregnancy.
Home pregnancy tests are most commonly used in the week after the missed menstrual period (fourth completed gestational week). Urine hCG values are extremely variable at this time and can range from 12 mIU/mL to greater than 2500 mIU/mL. This variability continues into the fifth week, when values have been shown to range from 13 mIU/mL to greater than 6000 mIU/mL. Both weeks have a percentage of urine hCG values that is below the sensitivities of detection for common home pregnancy tests (range, 25-100 mIU/mL).
Several studies have tested different home pregnancy tests for sensitivity and accuracy claims:
-
One study tested 18 different home pregnancy tests at 5 different hCG concentrations (0, 12.5, 25, 50, and 100 mIU/mL). No difference in sensitivity was detected between tests that had longer reading times (usually approximately 5 min) compared with those with shorter reading times (1 min). Clearly positive results were only found in 44% of the brands when tested at the highest hCG concentration (100 mIU/mL). The sensitivity was improved to 83% of brands tested at 100 mIU/mL when a faintly discernible line was also considered a positive result. Test sensitivity was also increased when reading times were extended to 10 minutes. Overall, 100% accuracy was only achieved in all 18 brands tested when the highest hCG concentration (100 mIU/ml) was used, an extended reading time was used, and faintly discernible results were included as positive.
-
Another study evaluated 7 home pregnancy tests and found that despite the claims, the detection of pregnancy on the day of missed period varied from 16-95%, and some devices were faulty (defined as devices failing to yield a band in the control window).
-
Other studies have found that home pregnancy tests with digital reading may offer significant benefits over traditional nondigital tests.
The limitations of these tests must be understood so that pregnancy detection is not significantly delayed. Early pregnancy detection allows for the commencement of prenatal care, potential medication changes, lifestyle changes to promote a healthy pregnancy (appropriate diet; avoidance of alcohol, tobacco, and certain medications), or early pregnancy termination if desired.
Serum hCG values in infertility
Serum hCG values for the diagnosis of early pregnancy in patients undergoing in-vitro fertilization–embryo transfer (IVF-ET) have been studied. [10] Serum hCG levels 14 days after embryo transfer correlate with pregnancy outcome. In a study of 111 patients with positive quantitative hCG levels 14 days after embryo transfer, the following pregnancy outcomes were observed:
-
Levels < 300 mIU/mL, ongoing pregnancy rate was 9%
-
Levels 300-600 mIU/mL, ongoing pregnancy rate was 50%
-
Levels >600 mIU/mL, multiple pregnancy rate was 100%
Therefore, in this particular population, quantitative assay results can be used to guide counseling and further evaluation.
Ultrasonography
With the advent of transvaginal ultrasonography (TVUS), the diagnosis of pregnancy can be made even earlier than is possible with transabdominal ultrasonography (TAUS). US has long been used in uncomplicated pregnancies for dating and as a screening examination for fetal anomalies. According to the American College of Obstetricians and Gynecologists, US is the preferred modality to confirm the presence of a viable intrauterine gestation. [11] US is not typically used to diagnose pregnancy unless the patient presents with vaginal bleeding or abdominal pain early in gestation or is a high-risk obstetric patient. TVUS is the most accurate means of confirming intrauterine pregnancy and gestational age during the early first trimester. [12]
TVUS has several advantages over TAUS during early pregnancy. TVUS can help detect signs of intrauterine pregnancy approximately 1 week earlier than TAUS. Patients are not required to have a full bladder and are not required to endure uncomfortable pressure on the abdominal wall from the external probe. TVUS is also better for patients who are obese or those who guard during TAUS. One disadvantage is that some patients are anxious about the transvaginal probe and may object to its insertion.
Vaginal probes are typically of higher frequency (5-8 MHz) than abdominal probes (3-5 MHz). The higher frequency allows for better resolution of the image but less penetration of the signal. Also, practice is necessary for familiarization with the orientation on the US monitor when performing TVUS.
The yolk sac is usually identified before the gestational sac (GS) is larger than 10 mm. Likewise, if the yolk sac is larger than 7 mm without signs of a developing fetal pole, the chance of an abnormal pregnancy is increased.
The earliest structure identified is the GS. The GS can be seen on TVUS images by 4-5 weeks' gestation and grows at a rate of 1 mm/d in early gestation. By 5.5-6 weeks' gestation, a double-decidual sign can be seen, which is the GS surrounded by the thickened decidua. The presence of an early GS can be confused with a small collection of fluid or blood or the pseudo GS of an ectopic pregnancy. Because of this, the diagnosis of intrauterine pregnancy should not be made on the basis of visualization of the GS alone.
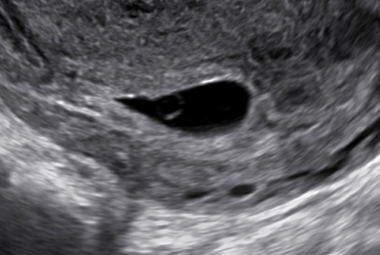
The yolk sac can be recognized by 4-5 weeks' gestation and is seen until approximately 10 weeks' gestation. The yolk sac is a small sphere with a hypoechoic center and is located within the GS (see the image below). Visualization of a yolk sac confirms the presence of an intrauterine pregnancy (IUP). It does not, however, confirm whether a pregnancy is viable. If an IUP is visualized but the patient’s clinical presentation is concerning for a ruptured ectopic pregnancy, heterotopic pregnancy may also be on the differential diagnosis. These are uncommon with a frequency of 1 in 30,000 spontaneously conceived pregnancies. However, with the advent of assisted reproductive technology (ART), the incidence of heterotopic pregnancies is on the rise, with the frequency of 1 in 2000 of all pregnancies conceived via ART.
Observing a GS that is larger 10 mm without a yolk sac is rare, and if this is observed, it most likely represents an abnormal pregnancy (see the image below). In addition, a gestational sac that is eccentric (versus smoothly ovoid or round) or located in the lower uterine segment may be suspicious for nonviable pregnancy.
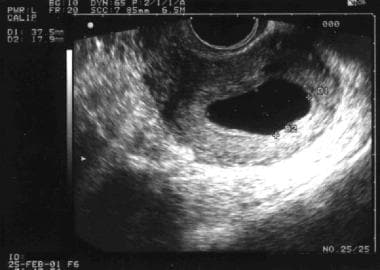 Pregnancy diagnosis. This is a gestational sac (GS) that measures approximately 2 X 3 cm, without evidence of a yolk sac. When the GS is larger than 10 mm and no yolk sac is identified, an abnormal pregnancy is likely. This particular situation is referred to as a blighted ovum or an anembryonic pregnancy.
Pregnancy diagnosis. This is a gestational sac (GS) that measures approximately 2 X 3 cm, without evidence of a yolk sac. When the GS is larger than 10 mm and no yolk sac is identified, an abnormal pregnancy is likely. This particular situation is referred to as a blighted ovum or an anembryonic pregnancy.
Likewise, a yolk sac larger than 7 mm without evidence of a developing fetal pole suggests a nonviable pregnancy.
The fetal or embryonic pole is first seen on TVUS images at approximately 5-6 weeks' gestation. It should always be seen by TVUS when the GS is larger than 18 mm or by TAUS when the GS is larger than 2.5 cm. The fetal pole is a linear hyperechoic structure that grows at approximately 1 mm/d.
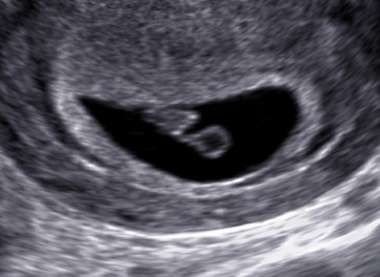 Pregnancy diagnosis. The image shows an approximately 6 week pregnancy with a fetal pole and yolk sac visualized within the gestational sac.
Pregnancy diagnosis. The image shows an approximately 6 week pregnancy with a fetal pole and yolk sac visualized within the gestational sac.
Cardiac motion can sometimes be identified in a 2- to 3-mm embryo but is almost always present when the embryo grows to 5 mm or longer. At 5-6 weeks' gestation, the fetal heart rate ranges from 100-115 beats per minute. The heart rate will steadily increase to a mean of 140 beats per minute by 9 weeks' gestational age.
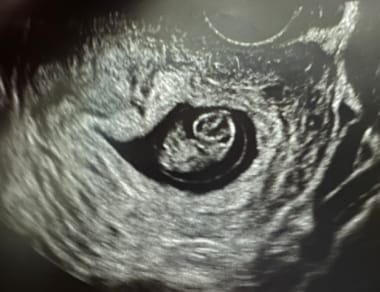 Pregnancy diagnosis. At 9 weeks, a embryo is clearly seen in the gestational sac. A fetal heart rate should also be visible.
Pregnancy diagnosis. At 9 weeks, a embryo is clearly seen in the gestational sac. A fetal heart rate should also be visible.
When performing ultrasonography of the uterus in early pregnancy, it is important to search for and document presence (or absence) of each of these findings (gestational sac, yolk sac, fetal pole presence and length, fetal heart rate presence and rate). When a fetal pole is seen, measurement of the crown-rump length can help confirm the gestational age of the pregnancy, which has important implications both for prenatal care or if the patient is seeking pregnancy termination.
Ultrasonography (not quantitative hCG) is the preferred tool when establishing whether an early pregnancy is viable or not. Sonographic standards for suspecting and diagnosing early pregnancy failures were published by Doubilet et al and are referenced in the table below. [13] Serial ultrasonography is sometimes indicated if the first ultrasound scan is nondiagnostic. For patients who desire pregnancy termination, it is necessary to determine whether a pregnancy is located in the uterus (as standard termination methods are not appropriate to use for an ectopic pregnancy).
Ultrasonography and Human Chorionic Gonadotropin
Ultrasonography becomes even more useful for the diagnosis of early pregnancy and for identifying abnormal pregnancies when it is used in conjunction with assessing quantitative hCG levels. The identification of gestational structures by ultrasonography correlates with specific levels of hCG, termed discriminatory levels. A discriminatory level is the level of hCG at which the structure in question should always be identified in a normal, singleton, intrauterine pregnancy.
The GS has been identified by TVUS with hCG levels as low as 300 mIU/mL, and most experienced TVUS operators should visualize the GS when levels are approximately 1000 mIU/mL. The discriminatory level for the GS is approximately 3600 mIU/mL, and if it is not seen at this point, other pathology must be excluded. Many use a more conservative discriminatory level for the GS, at 2000 mIU/mL by TVUS and 3600 mIU/ml by TAUS, and will begin to rule out pathology if the GS is not seen. The adnexa should be scanned for an ectopic pregnancy, and serial sonography and hCG levels should be performed until a diagnosis is made. Furthermore, one study showed that all viable intrauterine pregnancies had a GS identified by TAUS for hCG levels of greater than 6500 mIU/mL.
Sonographic Standards for Suspecting and Diagnosing Early Pregnancy Failures (Open Table in a new window)
| Sonographic findings diagnostic of pregnancy failure | Sonographic findings suspicious for (but not diagnostic of) pregnancy failure |
|---|---|
| Crown-rump length of ≥ 7 mm with no detectable fetal heart rate | Crown–rump length of < 7 mm and with no detectable fetal heart rate |
| Mean gestational sac diameter of ≥ 25 mm with no fetal pole seen | Mean gestational sac diameter of 16–24 mm and no fetal pole seen |
| Absence of embryo with heartbeat ≥2 wk after a scan that showed a gestational sac without a yolk sac | Absence of embryo with heartbeat 7–13 days after a scan that showed a gestational sac without a yolk sac |
| Absence of embryo with heartbeat ≥11 days after a scan that showed a gestational sac with a yolk sac | Absence of embryo with heartbeat 7–10 days after a scan that showed a gestational sac with a yolk sac |
| Absence of embryo ≥6 wk after last menstrual period | |
| Empty amnion (amnion seen adjacent to yolk sac, with no visible embryo) | |
| Enlarged yolk sac (>7 mm) | |
| Small gestational sac in relation to the size of the embryo (< 5 mm difference between mean sac diameter and crown–rump length) | |
| Adapted from Doubilet et al. [13] | |
The usefulness of TVUS evaluation when the hCG is less than 1000 mIU/mL has been debated. One study showed that valuable information can still be garnered in women presenting for emergent TVUS with an hCG level of less than 1000 mIU/mL. In this study, approximately 13% of the abnormal intrauterine pregnancies and 39% of the ectopic pregnancies were identified by TVUS. Ultrasonography should not be delayed purely on the basis of hCG levels. [14]
Other structures are also anticipated in correlation with specific hCG levels. The yolk sac is commonly observed with an hCG level of approximately 2500 mIU/mL, although it may not be identified until levels are much higher. The embryonic pole usually becomes evident at a level of approximately 5000 mIU/mL, and the fetal heartbeat can be seen in the vast majority of normal gestations when the hCG level reaches 10,000 mIU/mL.
In a study by Connolly et al, discriminatory hCG levels were 3510 mIU/mL for the GS, 17,716 mIU/mL for the yolk sac, and 47,685 mIU/mL for the embryonic pole. Threshold values for hCG levels at which these structures could be visualized on TVUS were 390 mIU/mL for the GS, 1094 mIU/mL for the yolk sac, and 1394 mIU/mL for the embryonic pole. [15]
Ectopic Pregnancy
Ectopic pregnancies are the primary cause of first trimester maternal mortality and should be diagnosed early, before the pregnancy ruptures or the patient becomes unstable. [16]
Ectopic pregnancy may be suspected in a patient with a rising human chorionic gonadotropin (hCG) level, an empty uterine cavity observed on sonogram, pelvic/abdominal pain, and/or vaginal bleeding. [17, 18]
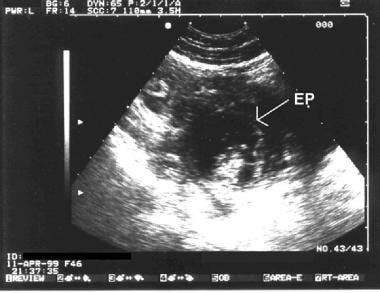 Pregnancy diagnosis. Sonogram showing a complex mass in the adnexa (labeled EP). It was found to be an ectopic pregnancy at the time of surgery.
Pregnancy diagnosis. Sonogram showing a complex mass in the adnexa (labeled EP). It was found to be an ectopic pregnancy at the time of surgery.
Other historical factors related to ectopic pregnancies include prior tubal manipulation, pelvic inflammatory disease, previous ectopic pregnancy, tubal disease, use of an intrauterine device for contraception, fertility therapies, and tubal ligation. However, ectopic pregnancy can occur in a patient with no prior risk factors. [19, 18, 20] See Ectopic Pregnancy for a full description and details.
Conclusion
The diagnosis of pregnancy can be made by several methods. The classic presentation is in a patient with regular menstrual cycles who presents with amenorrhea and typical history and physical examination findings. Ultrasonography can be used to confirm a viable intrauterine pregnancy. Currently, most women are diagnosed with pregnancy after a missed menstrual cycle and a positive urine or serum hCG finding. The pregnancy is diagnosed as viable with serial examinations and normal pregnancy development, a normal result after dating ultrasonography, or a positive finding of fetal heart tones using Doppler studies.
Patients who are considered high-risk or those who present with abdominal pain or vaginal bleeding in early gestation are more likely to be evaluated with ultrasonography and additional hormonal assays. A number of different combinations can aid in the diagnosis of a viable intrauterine pregnancy. The physician must ascertain what is most appropriate at the time of patient presentation.
For excellent patient education resources, visit eMedicineHealth's Pregnancy Center.
-
Pregnancy diagnosis. Sonogram showing a complex mass in the adnexa (labeled EP). It was found to be an ectopic pregnancy at the time of surgery.
-
Pregnancy diagnosis. The arrow is pointing to the yolk sac as seen within the gestational sac (GS). The yolk sac is usually identified before the GS is larger than 10 mm. Likewise, if the yolk sac is larger than 7 mm without signs of a developing fetal pole, the chance of an abnormal pregnancy is increased.
-
Pregnancy diagnosis. This is a gestational sac (GS) that measures approximately 2 X 3 cm, without evidence of a yolk sac. When the GS is larger than 10 mm and no yolk sac is identified, an abnormal pregnancy is likely. This particular situation is referred to as a blighted ovum or an anembryonic pregnancy.
-
Pregnancy diagnosis. A gestational sac with a yolk sac is seen in the uterus.
-
Pregnancy diagnosis. The image shows an approximately 6 week pregnancy with a fetal pole and yolk sac visualized within the gestational sac.
-
Pregnancy diagnosis. At 9 weeks, a embryo is clearly seen in the gestational sac. A fetal heart rate should also be visible.