Background
Chagas disease, also known as American trypanosomiasis, is caused by infection with the protozoan parasite Trypanosoma cruzi. The organism T cruzi and infection in humans were first described in 1909 by the Brazilian physician Carlos RJ Chagas. [1] T cruzi mostly is found in blood-sucking triatomine insects (kissing bugs) and small mammals in a sylvatic cycle that is enzootic from the southern and southwestern United States to central Argentina and Chile. T cruzi infection in humans occurs in a spotty distribution throughout the range of the sylvatic cycle.
New cases of vector-borne T cruzi infection usually occur in persons who live in primitive houses in areas where the sylvatic cycle is active. The living quarters are invaded by infected triatomines, which become domiciliary. Infected insects take blood meals from humans and their domestic animals and deposit parasite-laden feces. The parasites then are transmitted via contact with breaks in the skin, mucosal surfaces, or the conjunctivas. Transmission also can occur congenitally, via blood transfusion and organ transplantation, and by ingestion of food and drink contaminated with feces from infected bugs. A couple dozen cases of T cruzi transmission via laboratory accidents have been reported, but none recently. [2]
T cruzi infection is life-long. A minority of persons with long-standing T cruzi infection develop the serious cardiac and gastrointestinal problems that characterize chronic symptomatic Chagas disease.
The parasite
T cruzi is a member of the family Trypanosomatidae in the order Kinetoplastida and belongs to a special section called Stercoraria. The infective forms of T cruzi are contained in the feces of the insect vectors and gain entry into its mammalian hosts through contamination. This mechanism of transmission contrasts with that of the two subspecies of African trypanosomes that cause human disease, Trypanosoma brucei gambiense and Trypanosoma brucei rhodesiense, which are transmitted via the saliva of their vectors, and with the mechanism by which a nonpathogenic trypanosome found in the Americas, Trypanosoma rangeli, is transmitted to its mammalian hosts.
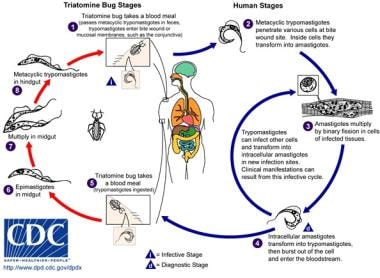
As with other parasites that infect both mammalian and insect hosts, the life cycle of T cruzi is complex (see image below).
The T cruzi life cycle consists of 3 main developmental forms. Epimastigotes are an extracellular and noninfective form of the parasite found in the midgut of insect vectors, where they multiply by binary fission. As epimastigotes (depicted in the first image below) move to the hindgut, they differentiate into metacyclic trypomastigotes (depicted in the second image below), which are nondividing forms resistant to mammalian complement that have the capacity to infect mammalian cells. They enter local cells through breaks in the skin, mucous membranes, or the conjunctivas and transform into the third morphologic form, amastigotes. Amastigotes multiply intracellularly until the host cell is overwhelmed, at which point they transform into bloodstream trypomastigotes.
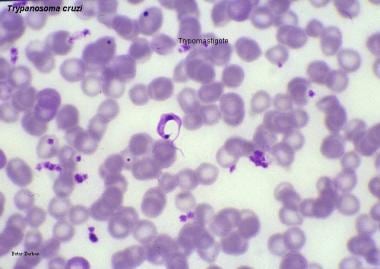 Chagas disease (American trypanosomiasis). The trypomastigote is the infective flagellated form of the parasite found in the blood of the mammalian hosts (blood trypomastigote) and in the hindgut of vectors (metacyclic trypomastigote). Image courtesy of Peter Darben, MD.
Chagas disease (American trypanosomiasis). The trypomastigote is the infective flagellated form of the parasite found in the blood of the mammalian hosts (blood trypomastigote) and in the hindgut of vectors (metacyclic trypomastigote). Image courtesy of Peter Darben, MD.
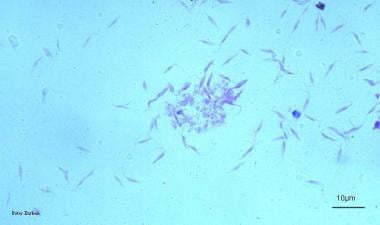 Chagas disease (American trypanosomiasis). The epimastigote form of Trypanosoma cruzi is the multiplying stage of the parasite that grows in the gut of the insect vector and also in cell-free culture medium as shown here. Image courtesy of Peter Darben, MD.
Chagas disease (American trypanosomiasis). The epimastigote form of Trypanosoma cruzi is the multiplying stage of the parasite that grows in the gut of the insect vector and also in cell-free culture medium as shown here. Image courtesy of Peter Darben, MD.
As the host cells rupture, the trypomastigotes are released into the lymphatics and bloodstream, through which they spread to distant sites and invade new host cells. See image below.
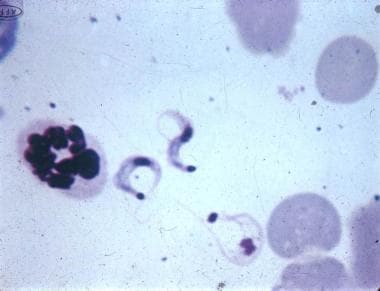 Trypanosoma cruzi trypomastigotes in a mouse blood smear (Giemsa, x625). Courtesy of Dr. Herbert B Tanowitz, New York, NY.
Trypanosoma cruzi trypomastigotes in a mouse blood smear (Giemsa, x625). Courtesy of Dr. Herbert B Tanowitz, New York, NY.
This process continues asynchronously for the life of the host. Small numbers of trypomastigotes may be ingested in blood meals taken by uninfected triatomines. The trypomastigotes then transform into epimastigotes in the midgut of these insects, thus completing the cycle.
T cruzi also can be transmitted when mammalian hosts ingest infected insects, and this mechanism of transmission may play a major role in maintaining the sylvatic cycle.
Numerous biological, biochemical, and molecular studies have shown that the population of T cruzi is highly diverse. [3, 4, 5, 6, 7, 8, 9] Although T cruzi is a diploid organism in which some genetic exchange may occur in insect vectors, [10] its genetic and phenotypic diversity is thought to result from the clonal multiplication of the epimastigote and amastigote forms. The consensus is that T cruzi can be divided into 6 discrete taxonomic units (DTUs; TcI through TcVI). The strains in each DTU show variability in geographic, epizootic, epidemiologic, and pathogenic characteristics. [11] Unfortunately, no clear associations have been found between the strain groups and pathogenicity or drug susceptibility. In general, the extensive genetic and, more recently, proteomics data generated so far, including the framework of the DTUs, have not yet led to new tools to reduce transmission, advances in the management of clinical disease, new drugs, or a vaccine. In the area of diagnosis, however, the results of molecular work on T cruzi have led to the development of highly accurate assays that are used widely to detect T cruzi infection.
Vectors of T cruzi
Triatomines, which transmit T cruzi, belong to the family Reduviidae in the order Hemiptera. Reduviidae has 22 subfamilies, including the Triatominae. [12, 13] Although the vectors of T cruzi occasionally are referred to as reduviids, this term is not appropriate since the vast majority of the species in the family Reduviidae are phytophagous or insectivorous and do not transmit the parasite.
All triatomine species are able to transmit T cruzi to humans, but only a handful become domiciliary to any great extent and are important as T cruzi vectors. Many more triatomine species are involved in the widespread sylvatic cycles. Triatomines have 5 nymphal stages (instars), all of which can harbor and transmit T cruzi.
The three vector species most important in the transmission of T cruzi to humans include Triatoma infestans, Rhodnius prolixus (see image below), and Triatoma dimidiata. Historically, T infestans has been the most important by far, as it has been the primary vector in the sub-Amazonian endemic regions. Since the early 1990s, under the aegis of the Southern Cone Initiative (SCI), which is supported by the World Health Organization (WHO) and the Pan American Health Organization (PAHO), Chagas disease control programs in Argentina, Bolivia, Brazil, Chile, Paraguay, and Uruguay have focused on eliminating domiciliary T infestans.
 Rhodnius prolixus, a common vector of Trypanosoma cruzi. Eggs, first- and second-stage nymphs, and adult.
Rhodnius prolixus, a common vector of Trypanosoma cruzi. Eggs, first- and second-stage nymphs, and adult.
These efforts have been widely successful, so much so that Uruguay (1997), Chile (1999), and Brazil (2006) have been declared free of vector‑borne transmission. [14, 15]
Major progress in vector control also has been achieved in Argentina, Paraguay, and Bolivia. Programs similar to the SCI have been implemented in the Andean nations and Central America, where R prolixus typically is found. [16, 17] The range of T dimidiata is similar but also extends far into Mexico. Other domiciliary species occupy more restricted areas and play less-important roles in the transmission of T cruzi to humans. The sylvatic species can colonize human dwellings and thus present a potential risk for transmission.
Mammalian hosts of T cruzi
T cruzi infection has been found in more than 100 mammalian species throughout the range mentioned above, which includes the southern and southwestern United States. Mammals typically involved in sylvatic cycles of transmission include opossums, armadillos, raccoons, monkeys, wood rats, and coyotes, among many others. [18, 19, 20, 21, 22, 23, 24]
Pets such as dogs and cats can become infected in enzootic regions, likely as they eat parasitemic prey or ingest infected insects. [25, 26, 27] It is of interest that Carlos Chagas observed T cruzi in the blood of wild marmosets and a domestic cat before he discovered the parasite in the blood of his first infected patient, Berenice. In some situations, dogs have been shown to be an important link in the maintenance of the domiciliary cycle and consequent transmission to humans. [28]
Livestock occasionally have been found to be infected with T cruzi, but the parasite is not known to adversely affect their health. Birds, amphibians, and reptiles are naturally resistant to T cruzi infection. In some situations, however, birds may be important sources of blood meals for triatomines.
The modes of transmission of T cruzi to humans
Historically, most transmission of T cruzi to humans has resulted from the contamination of vulnerable surfaces (eg, breaks in the skin, mucosae, and the conjunctivas) with the feces of infected vectors. However, as noted, vector-borne transmission has been reduced markedly in many endemic countries. Transfusion transmission was a major public health problem in endemic countries for decades, but, as accurate serologic assays for T cruzi infection were developed and screening of blood donors became mandatory and were implemented throughout the endemic range, this problem essentially has been eliminated. [29]
Not surprisingly, T cruzi can be transmitted via transplantation of organs obtained from persons with chronic infection, and occasional reports of this in Latin America [30] and in the United States [31, 32, 33, 34] have appeared.
The rate of congenital (transplacental) transmission from mothers with chronic T cruzi infection to their newborns is about 5%, with a range of 2-10% in various studies. [35] To date, no measures have been defined to reduce or eliminate this form of transmission. As transmission by vectors and through transfusion of contaminated blood have been reduced, the proportion of new T cruzi infections that result from congenital transmission has increased. Nonetheless, the total number of instances of congenital transmission certainly has decreased as seroprevalence rates have fallen. [36, 37, 38, 39, 40]
In contrast to Toxoplasma gondii, vertical transmission of T cruzi is possible with successive pregnancies. Transmission of T cruzi via human milk appears to be extremely rare, and chronic T cruzi infection is not a contraindication to breastfeeding. [41] Several instances of transmission of T cruzi to groups of people via ingestion of food or drink presumably contaminated with the feces of infected vectors have been reported. [42, 43, 44] Finally, the facility of producing infective forms of T cruzi in the laboratory has resulted in occasional accidental transmissions in this context, but none have been reported in many years. [2, 45]
Pathophysiology
An inflammatory lesion called a chagoma caused by T cruzi may appear at the site of entry in patients with acute Chagas disease. Histologic changes may include interstitial edema, lymphocytic infiltration, and reactive hyperplasia of adjacent lymph nodes due to intracellular parasitism of muscle and other subcutaneous tissues. When parasitized host cells rupture, trypomastigotes are released and often can be detected by microscopic examination of anticoagulated blood. As the infection spreads systemically, muscles, including the myocardium, and various other tissues become parasitized. [46]
Acute myocarditis, consisting of patchy areas of necrosis and infected cells, may develop (see image below). [47, 48] The pseudocysts occasionally seen in sections of infected tissues are intracellular aggregates of amastigotes. The patent parasitemias of the acute illness may be accompanied by lymphocytosis, and transaminase levels may be elevated. The cerebrospinal fluid may contain parasites. [49]
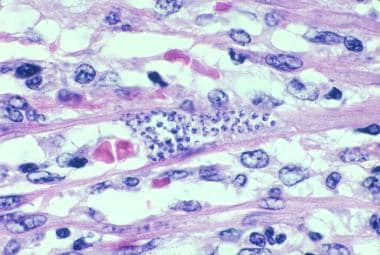 Trypanosoma cruzi in heart muscle of a child who died of acute Chagas disease in Texas. (H&E, x900).
Trypanosoma cruzi in heart muscle of a child who died of acute Chagas disease in Texas. (H&E, x900).
The heart is the most commonly affected organ in persons with chronic Chagas disease. [50, 51, 52, 53] Autopsy may reveal marked bilateral ventricular enlargement, often involving the right side more than the left, in the heart of patients who die of chagasic heart failure (see image below). The ventricular walls often are thin, and mural thrombi and apical aneurysms may be present. In addition, diffuse interstitial fibrosis, widespread lymphocytic infiltration, and atrophy of myocardial cells all may be present.
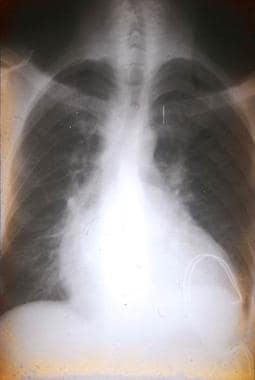 Chest radiograph of a Bolivian patient with chronic Trypanosoma cruzi infection, congestive heart failure, and rhythm disturbances. Pacemaker wires can be seen in the area of the left ventricle.
Chest radiograph of a Bolivian patient with chronic Trypanosoma cruzi infection, congestive heart failure, and rhythm disturbances. Pacemaker wires can be seen in the area of the left ventricle.
T cruzi parasites rarely are found during microscopic examination of stained sections of myocardial tissue; however, in numerous studies, T cruzi ‑specific polymerase chain reaction (PCR) assays have demonstrated parasites in areas of focal inflammation. [54, 55] Pathologic changes in the conduction systems of chronic chagasic hearts are common and often correlate with dysrhythmias. [56] Chronic inflammatory lesions and dense fibrosis frequently involve the right branch and the left anterior branch of the bundle of His, but lesions also may be found in other segments of the conduction system.
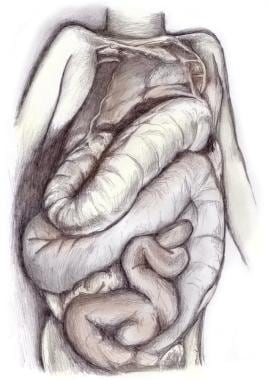
Salient features on gross examination of the colon or esophagus in patients with chronic chagasic gastrointestinal disease (megadisease) include dilatation and muscular hypertrophy of the affected organs (see images below). [57, 58, 59] Focal inflammatory lesions with lymphocytic infiltration are visible on microscopy. The number of neurons in the myenteric plexus often is markedly reduced, and periganglion and intraganglion fibrosis with accompanying Schwann cell proliferation, along with lymphocytosis, are present in most patients with megadisease. The functional effects of this parasympathetic denervation generally are limited to the esophagus or colon, although clinically manifest dysfunction of the ureters, biliary tree, and other hollow viscera has been reported. [60]
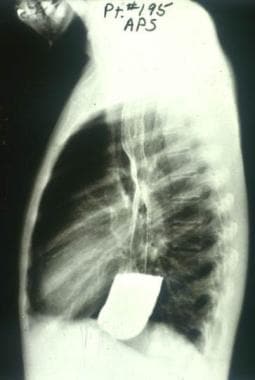 Barium swallow radiographic study of a Brazilian patient with chronic Trypanosoma cruzi infection and megaesophagus. The markedly increased diameter of the esophagus and its failure to empty are typical findings in patients with megaesophagus caused by Chagas disease. Courtesy of Dr. Franklin A. Neva, Bethesda, MD.
Barium swallow radiographic study of a Brazilian patient with chronic Trypanosoma cruzi infection and megaesophagus. The markedly increased diameter of the esophagus and its failure to empty are typical findings in patients with megaesophagus caused by Chagas disease. Courtesy of Dr. Franklin A. Neva, Bethesda, MD.
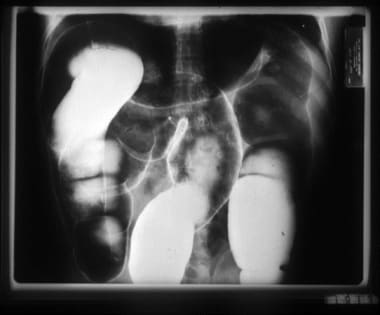 Air-contrast barium enema of a Bolivian patient with chronic Chagas disease and megacolon. The markedly increased diameters of the ascending, transverse, and sigmoid segments of the colon are readily apparent.
Air-contrast barium enema of a Bolivian patient with chronic Chagas disease and megacolon. The markedly increased diameters of the ascending, transverse, and sigmoid segments of the colon are readily apparent.
The pathogenesis of cardiac and gastrointestinal lesions of chronic Chagas disease was a focus of debate for decades. Beginning more than 20 years ago, however, convincing evidence has shown that low levels of parasites in chronically affected tissue, detectable with molecular methods, provoke a chronic inflammatory response that eventually leads to the pathologic changes observed microscopically and organ dysfunction. [54, 55, 61]
Epidemiology
Frequency
United States
Despite the presence of the sylvatic cycle of T cruzi transmission in the southern and southwestern United States, only 23 cases of autochthonous transmission of the parasite have been reported. [47, 62] Although some cases of T cruzi infection probably go unnoticed or unreported, autochthonous acute Chagas disease is rare in the United States. This concept is supported by the extreme rarity of T cruzi infection among US blood donors who were not born in or who have not traveled extensively in endemic countries. [63, 64, 65] The rarity of vector‑borne transmission of T cruzi to humans in the United States likely is due to the overall sparsity of vectors and the generally higher housing standards, which help prevent the vectors from becoming domiciliary. Despite this, triatomine insects have recently been reported as far north as Delaware [66] and the Carolinas. [67]
In contrast, the epidemiology of chronic T cruzi infection in the United States has changed markedly in the last few decades owing to the large number of people who have moved here from endemic countries. According to one recent estimate, 23 million persons from the endemic countries now live in the United States, 288,000 of whom have chronic T cruzi infection. [68, 69] Approximately two thirds of these immigrants are from Mexico, where the overall prevalence of T cruzi infection is 0.5-1%. [70, 71] Imported cases of acute Chagas disease are extremely rare. [72]
Five cases of transfusion-associated transmission of T cruzi were reported in the United States prior to the implementation of donor screening in 2007, [73] all of which occurred in immunocompromised patients. [74] Two additional such cases were found through trace-back studies after screening started. [75]
Two FDA‑approved tests are available for screening US blood donors for Chagas disease: the Ortho T cruzi ELISA Test System (Ortho Clinical Diagnostics, Rochester, NY) [76, 77] and the Abbott Prism/Alinity Chagas assay (Abbott Laboratories, Abbott Park, IL). [78] Donor samples positive in either of the two screening assays generally undergo confirmatory testing with the Abbott Enzyme Strip Assay (ESA) Chagas, which is the only FDA‑approved option for this purpose. [79, 80] The Chagas RIPA, [81, 82] which was used with FDA approval for confirmatory testing from 2007-2014, no longer is available for this purpose.
The data accumulated during the first 3 years of testing in the United States indicated that about 1 in every 13,000 US blood donors is infected with T cruzi (ie, repeat reactive in the Ortho or Abbott screening assays and positive in the Chagas RIPA or the Abbott ESA), [83] which is consistent with estimates made prior to the initiation of screening by groups familiar with the epidemiology of T cruzi in the United States. No instances of transfusion transmission of T cruzi in the United States are known to have occurred since donor screening was implemented.
Five recipients of organ transplants from 3 donors with T cruzi infection developed acute Chagas disease in the United States, one of whom died of the illness. [31, 32]
International
T cruzi is endemic in Mexico and all the countries of Central America and South America. The Caribbean Islands are not endemic. In 2023, PAHO/WHO estimated that a total of 7 million people are infected with T cruzi and that about 12,000 deaths each year can be attributed to Chagas disease. The total number of new T cruzi infections per year was estimated to be 30,000, fully 9,000 of which result from congenital transmission. Moreover, emigration of millions of people from endemic countries to nonendemic regions has resulted in several hundred thousand T cruzi–infected persons living in the latter areas, particularly in the United States and Europe. [84, 85, 86, 87, 88, 89, 90] To give a country‑specific perspective on the relative prevalence rates, in 2007, PAHO published the following data regarding the countries most affected by Chagas disease: Bolivia (6.8% prevalence); Argentina (4.1%); El Salvador (3.4%); Honduras (3.1%); Paraguay (2.5%); Guatemala (2%); Ecuador (1.7%); French Guyana, Guyana, and Surinam (1.2%); Venezuela (1.2%); Nicaragua (1.1%); Brazil (1%); and Mexico (1%). [91]
As noted above, in recent years, the epidemiology of T cruzi infection has improved markedly in many endemic countries, as blood bank and vector-control programs have been implemented. Because of the success of programs directed at domiciliary vector programs, prevalence rates in younger age groups have been decreasing substantially in many areas. [14, 15, 92, 93, 94, 95] All endemic countries have adopted statutory or regulatory mandates for screening donated blood for T cruzi, the most recent of which was Mexico. [29, 96]
In the author’s view, the enormous progress made in controlling Chagas disease in recent decades clearly indicates that the obstacles hindering the complete interruption of T cruzi transmission to humans are primarily economic and political. In this context, no additional major advances, such as a more detailed understanding of the pathogenesis of Chagas disease, [97, 98] further genetic analyses, [99, 100, 101, 102, 103, 104] novel diagnostic approaches, or breakthroughs in vaccine development, [105] appear to be necessary for its completion.
Mortality/Morbidity
Most of the estimated 12,000 deaths attributable to Chagas disease are due to chronic heart disease [51, 53, 106, 107] or, less frequently, megadisease or meningoencephalitis. [108] In persons with chronic chagasic heart disease, mortality primarily is due to the rhythm disturbances and congestive heart failure that result from the chronic inflammatory cardiomyopathy driven by the persistent presence of parasites in heart tissue. Embolization of intraventricular clots to the cerebrum and lungs can also contribute to mortality.
Persons with severe megaesophagus who do not receive medical attention can die of malnutrition and/or chronic aspiration pneumonitis. Megacolon (depicted in the image below) also can result in death, usually when volvulus develops and is not resolved surgically.
Race
T cruzi infection does not have a racial predilection.
Sex
T cruzi infection does not have a sexual predilection.
Age
Morbidity during the acute phase of Chagas disease is more pronounced in children than in adults. The gastrointestinal and cardiac manifestations of chronic T cruzi infection become apparent many years or even decades after initial infection and thus occur almost exclusively in adults. However, the lifetime risk for the development of such symptoms in chronically infected persons is only 10-30%. [109, 110]
-
Chagas disease (American trypanosomiasis). The trypomastigote is the infective flagellated form of the parasite found in the blood of the mammalian hosts (blood trypomastigote) and in the hindgut of vectors (metacyclic trypomastigote). Image courtesy of Peter Darben, MD.
-
Chagas disease (American trypanosomiasis). The epimastigote form of Trypanosoma cruzi is the multiplying stage of the parasite that grows in the gut of the insect vector and also in cell-free culture medium as shown here. Image courtesy of Peter Darben, MD.
-
Chagas disease (American trypanosomiasis). Megacolon.
-
Life cycle of triatomines. Courtesy of the CDC.
-
Trypanosoma cruzi trypomastigotes in a mouse blood smear (Giemsa, x625). Courtesy of Dr. Herbert B Tanowitz, New York, NY.
-
Rhodnius prolixus, a common vector of Trypanosoma cruzi. Eggs, first- and second-stage nymphs, and adult.
-
Trypanosoma cruzi in heart muscle of a child who died of acute Chagas disease in Texas. (H&E, x900).
-
Chest radiograph of a Bolivian patient with chronic Trypanosoma cruzi infection, congestive heart failure, and rhythm disturbances. Pacemaker wires can be seen in the area of the left ventricle.
-
Barium swallow radiographic study of a Brazilian patient with chronic Trypanosoma cruzi infection and megaesophagus. The markedly increased diameter of the esophagus and its failure to empty are typical findings in patients with megaesophagus caused by Chagas disease. Courtesy of Dr. Franklin A. Neva, Bethesda, MD.
-
Air-contrast barium enema of a Bolivian patient with chronic Chagas disease and megacolon. The markedly increased diameters of the ascending, transverse, and sigmoid segments of the colon are readily apparent.
-
Natural course of American trypanosomiasis. Courtesy of Dr. Patricia Paredes, Guadalajara, Jal., Mexico.
-
Footnote 1. Mexico, as well as all nations of Central and South America, are endemic for Chagas disease. Chagas disease is not endemic in any of the Caribbean Islands. Women who were born in Chagas endemic countries or who have resided therein for a substantial period are at geographic risk for having Chagas disease and should be screened serologically.{Edwards MS, Montgomery SP. Congenital Chagas disease: progress toward implementation of pregnancy-based screening. Curr Opin Infect Dis. 2021 Oct 1. 34 (5):538-545}. In addition, women who are not themselves at geographic risk but whose mothers are at such a risk and are not known to be seronegative are in turn considered to be at risk for Chagas disease. Footnote 2. The two approaches useful in this regard are microscopic examination of anticoagulated blood and polymerase chain reaction (PCR). Serologic testing of newborns is not useful because assays for specific immunoglobulin G (IgG) will yield a positive result, reflecting the mother’s chronic infection, and because immunoglobulin M (IgM) assays lack acceptable levels of sensitivity and specificity. Footnote 3. All children born to at risk women should be tested serologically since several studies indicate that the rate of congenital Chagas disease in babies born to infected mothers is 2%-10%. Footnote 4. The latter should include periodic monitoring for signs and symptoms of chronic cardiac and gastrointestinal Chagas disease followed by appropriate interventions, when indicated. The usefulness of specific drug treatment in adults with chronic Trypanosoma cruzi infections has not been clearly demonstrated and is a matter of ongoing debate. Footnote 5. The parasitologic cure rate in babies with congenital Chagas disease approaches 100% when a full course of treatment is given during the first year of life. Footnote 6. The sensitivities of microscopic examination of anticoagulated blood and PCR are less than 100%. Thus, the occasional baby who tests negative in these approaches immediately after birth may actually be infected with T cruzi. Serologic testing for specific IgG should be delayed until maternal IgG has disappeared.