Practice Essentials
Key observations and recommendations for alveolar echinococcosis (AE) in clinical practice are as follows:
-
Alveolar echinococcosis is a rare pseudotumor zoonotic parasitic disease of the liver that is distinct from cystic echinococcosis. A recent consensus on the terminology in the field of echinococcosis has fixed the names of the diseases, the various species of Echinococcus involved and several terms to be used in the scientific literature on the subject. [1] This terminology is used in this article.
-
The incidence of alveolar echinococcosis is increasing because of the urbanization of its animal hosts and the growing use of therapeutic immune suppression, which increases susceptibility.
-
The diagnosis of alveolar echinococcosis relies on imaging and may be confirmed with specific serology and/or molecular identification of the parasite Echinococcus multilocularis in the lesions.
-
The treatment of alveolar echinococcosis is multidisciplinary and may (1) combine surgery (radical resection of alveolar echinococcosis lesions, possible in 30% of cases) with albendazole anti-infective treatment for 2 years or (2) consist entirely of very long-term continuous albendazole treatment.
-
Palliative surgery should be avoided; anti-scolecidal agents should never be injected into alveolar echinococcosis lesions.
-
Percutaneous drainage is indicated in advanced cases with bacterial infection of a pseudocystic central necrotic cavity.
-
Perendoscopic lavage, drainage, and stenting (with multiple plastic stents) via endoscopic retrograde cholangiopancreatography (ERCP) is indicated in complicated cases with biliary obstruction and/or cholangitis.
-
Disease prognosis has markedly improved over the past 30 years; however, the only drug alternative to albendazole in patients who experience life-threatening hepatic or hematologic adverse effects is the parent drug mebendazole, which often results in similar adverse effects and is unavailable in many countries.
Background
Infection with the larval form of E multilocularis causes alveolar echinococcosis (AE). The infection behaves as a slow-growing malignant tumor. Initially, it is located in the liver and then may spread to any other organ through metastases. Without appropriate therapeutic management, the infection is lethal.
Echinococci are platyhelminths of the cestode genus. The parasitic cycle of the organism involves definitive hosts and intermediate hosts, each harboring different stages of the parasite life cycle.
Carnivores are the definitive hosts for the adult form of the parasite, which is an intestinal worm (which should not be called 'taenia', since Taenia designates another genus). Numerous (ie, tens to thousands) adult worms that average 2-5 mm in length live in the small bowel of carnivores and are attached to the small bowel mucosa by hooks and suckers. After 25-40 days, the worm's last gravid segment, each containing hundreds of microscopic eggs (6-hooked oncospheres or hexacanth embryos, 30-40 mm in diameter), detaches from the nonfertile segments. The egg-containing segments are then dispersed through the feces of the carnivore.
Various species act as intermediate hosts, serving the larval form of the parasite (ie, metacestode). The metacestode is a continuously growing tumorlike multicystic mass that is not clearly separated from host tissues. The larval-stage parasite is composed of microcysts that become fertile by producing a form termed the protoscolex, which is able to recreate the adult worm in the definitive host. The protoscoleces that fill the microcysts transform into adult worms once ingested into the intestine of the carnivore host.
The cycle of E multilocularis in Europe is predominantly sylvatic, involving red foxes (see image below) as definitive hosts and small mammals (rodents or small lagomorphs) as intermediate hosts. In some countries, dogs and cats have been identified as definitive hosts; however, all definitive host species acquire the infection from the sylvatic cycle by consuming small mammals infected with metacestodes of E multilocularis. In Alaska and in the People's Republic of China, the domestic cycle, involving family or stray dogs, is particularly important. In Canada, a peri-urban cycle that involves coyotes has been identified. [2]
 Foxes are the definitive hosts of the cestode Echinococcus multilocularis. Courtesy of Dominique A. Vuitton, MD, PhD.
Foxes are the definitive hosts of the cestode Echinococcus multilocularis. Courtesy of Dominique A. Vuitton, MD, PhD.
E multilocularis eggs, which are the infectious agents for humans, are dispersed in the environment via the feces of carnivores. The eggs may contaminate various types of food, including fruits and vegetables collected from gardens or infected meadows, and drinking water. An oncosphere membrane protects Echinococcus eggs, making them extremely tolerant of environmental conditions. E multilocularis eggs may remain infectious at temperatures ranging from -30°C to +60°C. They are easily destroyed by heat but may survive months or years at low temperatures, especially if they are protected against drying. Freezing the eggs at -20°C does not affect their infectious potency.
Simultaneous occurrence of both alveolar echinococcosis and cystic echinococcosis resulting from Echinococcus granulosus sensu lato infection is extremely rare but has occurred in endemic areas where both species are present in the environment (eg, western China).
Pathophysiology
Alveolar echinococcosis is a chronic disease with a presymptomatic stage that may last for years before signs and symptoms develop. The variability of the signs and symptoms depends on the location of the lesions (see image below), which may develop in the liver and/or in various organs or tissues, especially the lungs, brain, and bones.
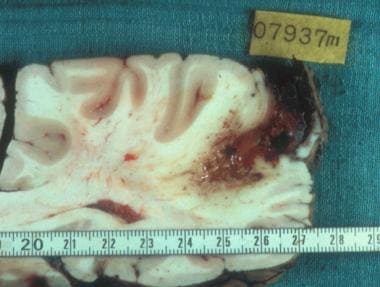
 Macroscopic aspect of alveolar echinococcosis lesions in the liver. Courtesy of Bernadette Kantelip, MD.
Macroscopic aspect of alveolar echinococcosis lesions in the liver. Courtesy of Bernadette Kantelip, MD.
E multilocularis larvae grow as tumorlike buds that transform into multiple microcysts filled with fluid and, in 15% of cases, with protoscoleces. The parasitic microcysts are lined with a germinal layer and a laminated layer, which are immediately surrounded by an exuberant granulomatous response generated by the host's immune system. This reaction has 2 main consequences, fibrosis and necrosis (see image below). Both reactions protect the host against larval growth but may also be deleterious.
Fibrosis in alveolar echinococcosis is extremely active from the beginning of the infection. Irreversible acellular fibrosis composed of cross-linked collagens ensues and isolates the parasitic lesions from the host but also compresses and obstructs major vessels and bile ducts. Noncaseous necrosis in the center of the lesions may be superinfected by bacteria and fungi, possibly leading to complications (eg, liver abscesses, septicemia).
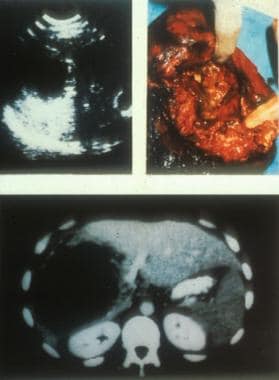 Ultrasonographic, CT scan, and perioperative aspect of a typical lesion of alveolar echinococcosis with central necrosis. Courtesy of Jean-Philippe Miguet, MD.
Ultrasonographic, CT scan, and perioperative aspect of a typical lesion of alveolar echinococcosis with central necrosis. Courtesy of Jean-Philippe Miguet, MD.
Similar to several other parasitic diseases, alveolar echinococcosis appears as a polar disease (as defined in leprosy). The ability of the organism to infect a host and the severity of disease once successfully inoculated depend on the receptivity of the host (ie, host immune defenses). This explains the particular susceptibility of immunocompromised patients to develop alveolar echinococcosis. [3, 4]
Mass screenings prove that abortive forms (see image below) exist and may occur in most cases, explaining the relatively low prevalence of this disease. Experimental studies in infected mice and immunologic studies in humans reveal the importance of cell-mediated immunity in the control of larval growth. Immune responses, characterized by a helper T cell TH 1 profile of cytokine secretion, can kill the larvae, thus protecting the host. The development of the periparasitic granuloma and the tolerance state to the parasite are associated with a sequential secretion of cytokines and chemokines. [5, 6] The progressive forms of the disease are characterized by a TH 2 profile consisting of increased interleukin (IL)–10, transforming growth factor (TGF)–beta, and IL-5 secretion. In addition, T-cell exhaustion may play a major role in disease progression in advanced cases, and immune "check-points" such as PD1-PDL-1 or TIGIT could be used as targets for biotherapeutic intervention. [7, 8, 9, 10]
Epidemiology
Frequency
United States
Foxes infected with E multilocularis are present in most of the northern and central states. The organism has been observed in all or parts of 11 contiguous states and 3 adjacent Canadian provinces in an area centered by southern Manitoba and North Dakota. However, only 2 cases involving humans living in this area were described in the 20th century. Transport of infected foxes from endemic areas to eastern and southern states for hunting purposes could create new areas at risk of becoming endemic. In Alaska, alveolar echinococcosis is observed in Eskimos, especially on St. Lawrence Island, where 30 of 53 cases were diagnosed in Alaska from 1947-1990. Recent studies in the United States and Canada suggest that the risk in humans is currently increasing in the 2 countries. [2] This was confirmed by the publication of the first AE human case east to the Mississipi river in 2021, and the discovery of a European haplotype of E multilocularis in that patient, as was previously found in Canada. [11] Diagnosis of alveolar echinococcosis (ie, the same disease as observed in humans) in dogs in North-Western USA also supports emergence of the disease in the United States. [12]
International
Alveolar echinococcosis occurs only in the northern hemisphere, in geographically limited foci (endemic areas) of west-central Europe, the Baltic countries, Turkey, most areas of the former Soviet Union, Iran, Iraq, central Asian republics, western and central China, and northern Japan (Hokkaido Island). [13] If considering only at-risk rural populations in regions in central Europe that are endemic for alveolar echinococcosis, the incidence is 1-20 cases per 100,000 persons per year, despite an overall country prevalence that may be very low. In endemic foci of China, prevalence averages 5% but may reach 10% in villages with specific risk factors. The prevalence of E multilocularis infection in foxes is 15-70% in endemic areas.
Recent trends are related to increasing percentages of infected foxes and increased distribution of those foxes. The presence of infected foxes in large cities of Europe and northern Japan is now well documented; 10% (in city centers) to 50% (in the suburbs) may be found in cities of the European endemic areas (such as Zurich or Geneva in Switzerland, Stuttgart in Germany, or Nancy in France). This and a newly recognized trend of infection in dogs and cats in endemic areas in Europe may lead to major changes in the human populations at risk in the near future. [14]
Mortality/Morbidity
Major complications leading to death include biliary obstruction with bacterial and/or fungal superinfections (eg, cholangitis, septicemia), secondary biliary cirrhosis, bleeding from esophageal or duodenal varices due to portal hypertension, Budd-Chiari disease, obstruction of the vena cava, and complications of heart, lung, or brain metastases (see image below).
Alveolar echinococcosis may markedly impair patients' quality of life, and the economic costs associated with treatment are high because the disease is chronic and requires life-long treatment and follow-up care.
The outcome of treatment is unpredictable; however, since the beginning of the 1980s, a combination of surgery, interventional radiology, and benzimidazole treatment has improved patient survival rates and quality of life. In developed countries, earlier diagnosis and well-managed treatment have improved average life expectancy at diagnosis from 3 years in the 1970s to 20 years in 2005.
A French study revealed an improvement in prognosis in most patients treated with benzimidazole; the mortality rates merged with that of the general population. [15]
Race
No known racial predilection exists; however, the genetic background, including human lymphocyte antigen (HLA) characteristics, linked to the intensity and/or to the TH 1/TH 2 balance of the patient's immune response may be associated with the occurrence and/or severity of the disease.
Sex
Older reports indicate a male predilection for infection; however, these reports are not accurate. In endemic areas in Europe, the male-to-female ratio is approximately equal. In the endemic areas of China, women are affected more commonly than men. Sex differences in prevalence seem to reflect epidemiologic rather than strictly sex-related risk factors, such as caring for dogs in Western China. However, the influence of slightly impaired immune defenses resulting from repeated pregnancies cannot be excluded.
Age
The typical age at onset is 55 years. However, the average age at diagnosis is younger in western China (age 40 years), and mass screenings have identified symptomatic and asymptomatic infections in patients ranging in age from 6 years to elderly persons.
Prognosis
Untreated alveolar echinococcosis usually is fatal, with an average 5-year survival rate of 40%. Therapeutic approaches that have been developed since the early 1980s have markedly improved the prognosis of the disease. In Europe, the actuarial survival rate at 5 years was 88% in a series of 80 patients observed from 1983-1993. [16] Beyond the first year following diagnosis, the mortality rate in patients with alveolar echinococcosis tends to equal that of the general population. [15, 17] In some parts of the world, especially China, the prognosis is far less favorable despite innovative surgical treatments because of late diagnosis and lesser access to treatment. [18]
Major complications that lead to death include biliary obstruction with bacterial and/or fungal superinfections (eg, cholangitis, septicemia), secondary biliary cirrhosis, bleeding from esophageal or duodenal varices due to portal hypertension, Budd-Chiari disease, obstruction of the vena cava, and complications of heart, lung, or brain metastases (see image below).
Alveolar echinococcosis may markedly impair the patient’s quality of life, and the economic costs associated with treatment are high because the disease is chronic and requires life-long treatment and follow-up care.
The outcome of treatment is unpredictable; however, since the beginning of the 1980s, a combination of surgery, interventional radiology, and benzimidazole treatment has improved survival rates and quality of life. In European patients who survive more than 1 year after diagnosis, life expectancy does not significantly differ from that observed in the general population. [15, 17]
In a French series of 117 patients, the actuarial survival rate at 5 years improved from 67% in patients diagnosed from 1972-1982 to 88% in patients diagnosed from 1983-1993. Of the 34 deaths with a clearly identified cause, 28 were related to the parasitic disease and/or its treatment. [15] In France, the life expectancy in patients with alveolar echinococcosis does not significantly differ from that in the general population. In a Swiss series of 155 patients, for an average 54-year-old patient diagnosed in 1970, the life expectancy was estimated to be reduced by 18.2 for men and 21.3 years for women. By 2005, this was reduced to approximately 3.5 and 2.6 years, respectively.
Patients undergoing radical surgery typically have a better outcome, whereas older patients have a poorer prognosis than younger patients. Alveolar echinococcosis-related mortality is mostly observed in the first 2 years after diagnosis and in symptomatic patients. [15]
Costs of treatment in Western Europe amount to approximately US $160,000 per patient. Assuming the improved life expectancy of patients is due to modern treatment, the cost per disability-adjusted life years (DALY) saved is approximately US $8,800.
Liver transplant recipients for AE have a 5-year survival rate of 46%; in 1 per 6 of such patients, the survival rate exceeded 20 years; even patients with progressing residual lesions or recurrence due to anti-rejection immune suppression may thus survive long-term [19] ; however, adherence to albendazole treatment is crucial. Aydinli et al (2015) reported an overall mortality rate of 22.2% in Turkey after a mean follow-up of 16 months after liver transplantation. [20] Ex-vivo liver resection with autotransplantation (ELRA) has been proposed as an alternative to liver allotransplantation, also reserved for very advanced cases. With this procedure, the 30-day mortality rate and overall mortality rate (>90 days) were 7.24% (5/69) and 11.5% (8/69), respectively; no recurrence was observed within a 22.5-month (14-89) follow-up period in surviving patients. [18]
Patient Education
Patient education focuses on the following:
-
Precautions regarding albendazole treatment (strict adherence to a necessary long-term treatment, intake with fat-containing meal, possible adverse effects, and importance of biological measurements, including blood testing and alanine amino transferase studies)
-
Possible occurrence of bacterial infection in advanced cases, potentially resulting in chills, fever, and/or jaundice
-
Necessary long-term medical follow-up
Despite the rarity of family-clustered cases, consider offering serology and/or liver ultrasonographic examinations to family members and relatives who share the same risk factors and immunogenetic background as the patient.
Patients with questions on alveolar echinococcosis may contact the following:
-
Association of Patients with Alveolar Echinococcosis (AIREA; email address, associationairea@gmail.com)
-
National Reference Center for Echinococcosis, University Hospital, F-25030 Besançon (CNR Echinococcoses: https://cnr-echinococcoses-ccoms.univ-fcomte.fr/ ; email address: cnr-echino@chu-besancon.fr)
-
Foxes are the definitive hosts of the cestode Echinococcus multilocularis. Courtesy of Dominique A. Vuitton, MD, PhD.
-
Macroscopic aspect of alveolar echinococcosis lesions in the liver. Courtesy of Bernadette Kantelip, MD.
-
Ultrasonographic, CT scan, and perioperative aspect of a typical lesion of alveolar echinococcosis with central necrosis. Courtesy of Jean-Philippe Miguet, MD.
-
Microtus larvalis (common vole) is one of the most common intermediate hosts of Echinococcus multilocularis in Europe. Courtesy of Patrick Giraudoux, PhD.
-
Sonogram of a typical form of alveolar echinococcosis of the liver, discovered at a screening in China. Courtesy of Dominique A. Vuitton, MD, PhD; Brigitte Bartholomot, MD; and Philip S. Craig, PhD.
-
Sonogram of an abortive form of alveolar echinococcosis of the liver, discovered at a screening in China. Courtesy of Dominique A. Vuitton, MD, PhD; Brigitte Bartholomot, MD; and Philip S. Craig, PhD.
-
Pathognomonic aspect of alveolar echinococcosis lesions invading the adrenal gland (resembling a honeycomb) that shows a necrotic area in the contiguous left liver lesion; MRI showing multiple parasitic cysts smaller than 1 cm in diameter appearing in high signal intensity on T2-weighted sequence. Courtesy of Brigitte Bartholomot, MD.
-
Brain metastasis of alveolar echinococcosis. Courtesy of Jean-Philippe Miguet, MD.
-
Skin metastasis of alveolar echinococcosis. Courtesy of Solange Bresson-Hadni, MD, PhD.
-
Histologic features of alveolar echinococcosis vesicles and periparasitic granuloma in humans, periodic acid-Schiff staining of the laminated layer. Courtesy of Bernadette Kantelip, MD.
-
Fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT scan aspect of active alveolar echinococcosis. White-yellow colors show a very high FDG uptake due to the periparasitic granulomatous infiltration and/or active germinal layer of Echinococcus multilocularis, and green-gray colors show the absence of the FDG uptake by inactive parasitic lesions (mostly necrotic). Courtesy of Solange Bresson-Hadni, MD, PhD, and Oleg Blagosklonov, MD, PhD.
-
Fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT scan aspect of inactive alveolar echinococcosis. No abnormal FDG uptake by the parasitic lesions after several years of albendazole treatment. Courtesy of Solange Bresson-Hadni, MD, PhD, and Oleg Blagosklonov, MD, PhD.
-
Pathognomonic image of alveolar echinococcosis. Multiple microcysts of alveolar echinococcosis, better seen at MRI on T2-weighted sequence. Courtesy of Prof. Eric Delabrousse and Dr. Amel Azizi, Department of Radiology, University Hospital, Besançon, France.
-
T2-weighted MRI. Typical aspect of an advanced liver alveolar echinococcosis case in a young adult presenting with cholestasis. Central necrotic area surrounded by multiple hyperdense microcysts; the lesion invades bile ducts and liver vessels. Courtesy of Laurianne Plastaras, MD, Hôpital Louis Pasteur, Colmar, France, and Stéphane Koch, MD, Centre Hospitalier Régional Universitaire, France.
-
Video of T2-weighted MRI. Typical aspect of an advanced liver alveolar echinococcosis case in a young adult presenting with cholestasis. Central necrotic area surrounded by multiple hyperdense microcysts; the lesion invades bile ducts and liver vessels. Courtesy of Laurianne Plastaras, MD, Hôpital Louis Pasteur, Colmar, France, and Stéphane Koch, MD, Centre Hospitalier Régional Universitaire, France.