Overview
Pneumonia is defined as "new lung infiltrates plus clinical evidence that the infiltrate is of an infectious origin, which include the new onset of fever, purulent sputum, leukocytosis, and decline in oxygenation." [1]
Hospital-acquired pneumonia (HAP), or nosocomial pneumonia, is a lower respiratory infection that was not incubating at the time of hospital admission and that presents clinically 2 or more days after hospitalization. Pneumonia that presents sooner should be regarded as community acquired pneumonia. VAP refers to nosocomial pneumonia that develops among patients on ventilators. [1, 2] Ventilator-associated pneumonia (VAP) is defined as pneumonia that presents more than 48 hours after endotracheal intubation.
The term healthcare-associated pneumonia (HCAP) was defined as pneumonia in nonhospitalized patients who had significant experience with the healthcare system and were believed to be at an increased risk for infection with multidrug-resistant (MDR) organisms because of such contact [2] ; however, more recent studies have indicated that many individuals who met the criteria for HCAP were not infected with MDR pathogens. [3] Retrospective studies actually have suggested a worse outcome when broad-spectrum antibiotics were used in these cases. The risk for infection with MDR organisms appears to depend much more on specific risk factors of the given patient than on contact with various aspects of the healthcare system. Patients who would have met the criteria for HCAP should not be empirically treated with antibiotics to cover MDR bacteria unless they have valid risk factors for acquiring MDR organisms. [4, 5]
HAP is a common nosocomial bacterial infection and is most prevalent in medical and surgical intensive care units (ICUs). As such, HAP adds significantly to the cost of hospital care and to the length of hospital stays.
Pathophysiology
Inhalation, aspiration, and hematogenous spread are the 3 main mechanisms by which bacteria reach the lungs. The primary route by which organisms enter the lower airways is aspiration of oropharyngeal secretions into the trachea.
Primary inhalation pneumonia develops when these organisms bypass normal respiratory defense mechanisms or when the patient inhales aerobic gram-negative organisms that colonize the upper respiratory tract or respiratory support equipment.
Aspiration pneumonia results from aspiration of colonized upper respiratory tract secretions. In healthy individuals, roughly 45% of the population is estimated to aspirate during sleep, with critically ill patients likely aspirating more frequently. [6, 7]
The stomach appears to be an important reservoir of gram-negative bacilli that can ascend and colonize the respiratory tract. A prospective observational study found that patients who used acid-suppressive medications were more likely to develop hospital acquired pneumonia (HAP) than were patients who did not (5% vs 2%). The risk for pneumonia was significantly increased with proton pump inhibitors, but not with histamine 2-blocking agents. [8]
Hematogenously acquired infections originate from a distant source and reach the lungs via the bloodstream.
Etiology
The development of hospital-acquired pneumonia (HAP) represents an imbalance between normal host defenses and the ability of microorganisms to colonize and then invade the lower respiratory tract.
Because aerobic gram-negative bacilli (eg, Pseudomonas aeruginosa) are the major pathogens associated with HAP, the pathophysiology of nosocomial pneumonia relates to the destructive effect on lung tissue. Aerobic gram-negative pathogens may be divided into 2 categories. The first category includes organisms that cause necrotizing pneumonia with rapid cavitation, microabscess formation, blood-vessel invasion, and hemorrhage (eg, P aeruginosa). [9] Alternatively, other non-necrotizing gram-negative bacilli (eg, Serratia marcescens) may be responsible for nosocomial pneumonia.
Common causes of hospital-acquired pneumonia
Common bacteria involved in hospital-acquired pneumonia (HAP) include the following [10] :
-
P aeru ginosa
-
Staphylococcus aureus, including methicillin-susceptible S aureus (MSSA) and methicillin-resistant S aureus (MRSA)
-
Klebsiella pneumoniae
-
Escherichia coli
-
Non-Enterobacteriaceae bacteria such as S marcescens, Stenotrophomonas maltophilia, and Acinetobacter species are less common causes. Acinetobacter species commonly colonize respiratory tract secretions in patients in the ICU. HAP caused by Acinetobacter species or B cepacia may be associated with outbreaks. Streptococcus pneumoniae and Haemophilus influenzae are recovered only in early-onset HAP.
Pathogens infrequently associated with hospital-acquired pneumonia
The following infrequently are implicated pathogens in nosocomial pneumonia clusters/outbreaks, usually affecting severely immunocompromised patients:
-
Le gionella species
-
Influenza A virus
-
Respiratory syncytial virus (RSV)
-
Human parainfluenza virus 3 (HPIV-3)
-
Human metapneumovirus (hMPV)
Nosocomial Legionella pneumonia occurs often in outbreaks or clusters. Influenza A, RSV, hMPV, or HPIV-3 may cause hospital-acquired pneumonia (HAP) from person-to-person spread.
Organisms associated with ventilator-associated pneumonia
Organisms associated with ventilator-associated pneumonia (VAP) include the following:
-
P aeruginosa
-
S Aureus, including MSSA and MRSA
-
S maltophilia
-
Acinetobacter species
-
Enterobacteriaceae are less commonly seen in VAP than in hospital-acquired pneumonia (HAP)
These organisms commonly are recovered from respiratory secretions in patients with VAP. [11] The recovery of a respiratory pathogen from respiratory secretions does not establish it as the cause of nosocomial pneumonia. MSSA/MRSA frequently colonize respiratory secretions in intubated patients but rarely, if ever, cause nosocomial pneumonia/VAP. In contrast, MSSA/MRSA may cause community-acquired pneumonia (CAP) in those with influenza. Anaerobic organisms are not important pathogens in nosocomial pneumonia. (See Differentials in Nosocomial Pneumonia.)
Risk factors
The stomach appears to be an important reservoir of gram-negative bacilli that can ascend and colonize the respiratory tract. A prospective observational study found that patients who used acid-suppressive medications were more likely to develop hospital acquired pneumonia (HAP) than were patients who did not (5% vs 2%). Further evaluation by drug class showed that the risk for pneumonia was significantly increased with proton pump inhibitors, but not with histamine 2-blocking agents. [8]
Endotracheal intubation is an independent risk factor with multiple associated factors such as:
-
Micro aspiration around endotracheal tube
-
Endotracheal intubation
-
Prolonged duration of ventilation
-
Abnormal swallowing function
-
Secretions pooled above endotracheal tube
The endotracheal tube, which acts as a foreign body, the pooling of secretions and the resultant need for suctioning can damage the tracheal mucosa. This can further facilitate tracheal colonization. Additionally, pathogenic bacteria can form a glycocalyx biofilm on the tube’s surface that protects them from both antibiotics and host defenses. This can become a contributing factor to recurrence of infection and treatment failure as well.
Other risk factors include prior antibiotic use, cross contamination with other patients on the unit and malnutrition.
Epidemiology of Nosocomial Pneumonia
Incidence in the United States
Nosocomial pneumonia accounted for 22% of all hospital infections in the United States. [12] It is the second most common infection in hospitalized patients, and the most common infection in the intensive care unit responsible for one-fourth of all ICU infections. [13, 14]
International incidence
The international incidence and prevalence of nosocomial pneumonia is similar to that in the United States, with comparable rates of responsible microorganisms.
Racial and sexual predilections
Nosocomial pneumonia has no racial or sexual predilection.
Age predilection
Nosocomial pneumonia is most common in elderly patients; however, patients of any age may be affected.
Morbidity and mortality
Morbidity and mortality in hospital-acquired pneumonia and ventilator-associated pneumonia
Intubation and ventilatory support bypass the normal host defense mechanisms, predisposing patients with ventilator-associated pneumonia (VAP) to infection.
In addition, hospital-acquired pneumonia (HAP)/VAP that develops in ICU patients is associated with high morbidity and mortality rates, because these patients are already critically ill. Estimated all-cause mortality is between 25-50%. [15]
Compromised cardiac and lung function may further decrease their cardiopulmonary reserve.
Ventilator-associated barotrauma often decreases already compromised lung function. In addition, it may alter chest radiographic appearances.
As mentioned above, early-onset HAP/VAP pneumonia (ie, hospital-onset of CAP) expectedly has a better prognosis than late onset nosocomial pneumonia because the latter tends to be associated with multidrug-resistant (MOR) organisms. [16, 17, 18, 19, 20]
Patient Prognosis
In HAP/ventilator-associated pneumonia (VAP), outcomes usually depend on risk factors/comorbidities, on preexisting underlying cardiopulmonary function, and host defenses. [21, 22, 23]
The causative pathogen also plays a role with MDR pathogens associated with significantly greater attributable mortality than non-MDR pathogens.
Differential Diagnoses of Nosocomial Pneumonia
All patients with presumed nosocomial pneumonia should undergo testing to rule out conditions that mimic nosocomial pneumonia. The diagnosis of nosocomial pneumonia is difficult because it may present in a very nonspecific fashion.
Many conditions other than nosocomial pneumonia mimic pulmonary infiltrates (eg, fluid, atelectasis) on chest radiographs.
Clinical findings in ventilated patients with fever and/or leukocytosis may have alternative causes, including antibiotic-associated diarrhea, central line–associated infection, sinusitis, urinary tract infection, pancreatitis, and drug fever. Noninfectious inflammation may produce fever.
The most common causes of infiltrates in ventilated patients with fever and/or leukocytosis include the following conditions:
-
Congestive heart failure
-
Pulmonary embolus or infarction
-
Acute respiratory distress syndrome
-
Pulmonary drug reactions
-
Collagen vascular diseases with pulmonary manifestations
-
Alveolar hemorrhage
-
Pulmonary contusion
-
Bronchiolitis obliterans-organizing pneumonia (BOOP)
-
Hypersensitivity pneumonitis.
-
Interstitial lung disease
-
Bronchogenic carcinomas
ARDS usually is readily diagnosable based on the appearance of small lung volumes due to microatelectatic changes on the chest radiograph and the progressive and severe hypoxemia. Little or no fever accompanies ARDS, unless it is due to acute pancreatitis.
Electrocardiography (ECG) and ventilation-perfusion scans help to exclude pneumonia mimics. ECGs, cardiac enzymes, and Swan-Ganz readings may rule out left ventricular failure caused by exacerbation of heart failure or new myocardial infarction.
Diagnostic Concerns
Below is a summary of the recommendations of the recent IDSA/ATF guidelines for the management of hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP). [4]
General approach:
Noninvasive sampling with semiquantitative culture can be performed much more rapidly, with fewer resources and fewer complications compared to invasive sampling techniques. For patients with suspected pneumonia, antibiotic treatment can be discontinued if the quantitative culture results are below the diagnostic threshold, including the following:
-
Endotracheal aspirates 1,000,000 colony forming units
-
Bronchoscopic or mini-BAL culture 10,000 CFU/ml
-
PSB 1000 CFU/mL (Shebl and Gulick 2020)
Quantitative-Culture:
Though controversial, quantitative culture can help differentiate between colonization and true infection through bacterial load estimation; the threshold of CFU decreases and specificity increases with site of sampling further down the respiratory tract. Thus, protected specimen brush (PSB) samples have the highest specificity, but sensitivity declines as more distal secretions are obtained. Furthermore, recent antibiotic use decreases sensitivity as well. [2] Additional tests that may increase the diagnostic yield include Gram’s staining, differential cell counts, staining for intracellular organisms, and procalcitonin levels. Antibiotic tailoring based on quantitative culture approach has been used in research and clinical settings with varying success. More importantly, colony counts above the diagnostic threshold during antibiotic therapy may support treatment failure diagnosis.
Microbiologic approach to diagnosing hospital-acquired pneumonia:(weak recommendation, very low-quality evidence).
IDSA recommends that patients with suspected HAP (non-VAP) be treated according to microbiologic results obtained from noninvasively-collected respiratory samples and routine blood culture. It is very important to accurately target antibiotic therapy and deescalate as appropriate. Of note, noninvasive respiratory sampling includes spontaneous expectoration and sputum induction.
When no respiratory secretions can be obtained and blood cultures are negative, bronchoalveolar lavage should be considered in patients who are not responding to initial antibiotic therapy. [24]
For patients who are treated empirically for HAP and either have a risk factor for MRSA, including previous IV antibiotic use within 90 days, hospitalization in a unit with >20% MRSA, unknown MRSA prevalence, or who have high mortality risk are recommended for empiric coverage of MRSA. If the patient with HAP does not have MRSA risk factors, then empiric coverage should include MSSA. Double coverage for Pseudomonas aeruginosa and other strong gram-negative bacilli is only suggested for high mortality risk patients with prior IV antibiotic use within 90 days given very low-quality evidence. A single agent often is enough, though it is strongly recommended against using an aminoglycoside as the sole antipseudomonal agent.
Microbiologic approach to diagnosing ventilator-associated pneumonia
For patients with suspected VAP, sampling of the lower airways for quantitative cultures can be obtained through the following methods:
-
Blind tracheobronchial aspiration (TBAS) can be done by inserting a flexible catheter into the distal trachea; however, since it is blind sampling, there is no direct sampling of the lung in which radiographic evidence shows infiltrates. Furthermore, as the catheter is inserted through the endotracheal tube, there is a risk for contamination leading to false-positive results.
-
Bronchoscopy with bronchoalveolar lavage (BAL) allows for direct sampling of the lung segments with radiographic evidence of infiltrates. Limitations of the technique include operator-skill, contamination, and risk of worsening hypoxemia.
-
Protected specimen brush (PSB) can be advanced through a bronchoscope to avoid upper airway contamination. [25]
For patients with suspected HAP/VAP, the IDSA strongly recommends using clinical criteria alone to make the diagnosis. Biomarkers, such as procalcitonin, soluble triggering receptor expressed on myeloid cells (obtained via bronchoalveolar lavage), or C-reactive protein combined with clinical criteria should not be used to diagnose HAP/VAP or in the decision to initiate antibiotic treatment. (IDSA practice guidelines 2016). The biomarker procalcitonin (PCT) usually is unhelpful in the diagnosis of nosocomial pneumonia in ICU patients, who often have elevated PCT levels due to hypotension, renal failure, hepatic insufficiency, pancreatitis, drug reactions, or lung cancer. [26, 27] In addition, there is low sensitivity (67%) and specificity (83%) of clinical criteria plus PCT approach in diagnosing HAP or VAP. [28] Furthermore, even the use of the Modified Clinical Pulmonary Infection Score combined with clinical criteria should not be used to diagnose HAP/VAP. Clinical criteria alone is adequate to make the diagnosis.
Physical Examination
Physical findings in nosocomial pneumonia relate to the pneumonia’s distribution in the chest. Physically, lobar lesions caused by nosocomial pneumonia mimic those caused by any other type of pneumonia (eg, rales in the location of the pneumonic process). A 10-year retrospective study by Qi et al found that the most common clinical manifestation and signs of pneumonia were fever/cough and pulmonary rales, occurring in 491/543 and 344/543 patients. [29] Symptoms can include components of the CURB-65 scoring system, including confusion, blood urea >7 mmol/L, respiratory rate >30 breaths/min, systolic blood pressure < 90 mmHg or diastolic blood pressure < 60 mmHg, and age >65. A score of less than or equal to 2 classified a patient as low-risk, whereas a score of greater or equal to 3 indicated a high-risk patient.
White Blood Cell Count and Blood Cultures
WBC
The WBC count may be normal or elevated in nosocomial pneumonia or disorders that mimic nosocomial pneumonia/ventilator-associated pneumonia (VAP). A left shift reflects the stress and neither rules out nor confirms infection. The degree of left shift indicates the degree of stress in the host. Neither leukocytosis nor leukopenia count favors the diagnosis of nosocomial pneumonia over the diseases that mimic nosocomial pneumonia, as these can produce similar changes.
Blood cultures
Obtain blood cultures as early as possible, although they infrequently are positive, except in cases of hematogenous nosocomial pneumonia. The recovery of MSSA/MRSA in blood cultures in patients with nosocomial pneumonia/VAP without the clinical findings of MSSA/MRSA pneumonia (eg, rapid cavitation, high fevers, cyanosis) is likely due to skin contamination of blood cultures.
Radiography
Radiographic Findings
Serial chest radiography is most useful in ruling out progression of the pneumonia and serves as an indicator of the efficacy of appropriate antimicrobial therapy. Serial imaging is not indicated in documenting infection resolution since radiographic improvement lags far behind microbiological cure, often by several months. Similarly, as found in the study by Qi et al, among patients with diagnosed pneumonia, a majority (530/543) had some sort of pulmonary consolidation whereas a small subset (73/543) had pleural effusion seen on chest X-ray or on computed tomography.
Computed tomography (CT) scanning or spiral CT scanning may be useful in differentiating mimics from actual nosocomial pneumonia. For example, blood cultures that are positive for MSSA/MRSA in a febrile intubated patient are not diagnostic of MSSA/MRSA nosocomial pneumonia/ventilator-associated pneumonia (VAP). The diagnosis of MSSA/MRSA community-acquired pneumonia/nosocomial pneumonia is based on the clinical presence of fever, cyanosis, hypotension, and rapid cavitation of infiltrates (< 72 hours) on chest radiographs plus MSSA/MRSA in respiratory secretions.
Since only approximately 25% of changes documented on portable chest radiographs of ventilated patients represent an infectious process, follow-up CT scanning should be obtained in this situation.
Go to Imaging Atypical Pneumonia and Imaging Typical Pneumonia for more complete information on these topics.
Bronchoscopic Techniques
These techniques have variable sensitivities and specificities, although there are accepted criteria for semiquantitative cultures to improve the diagnostic reliability of bronchoscopically derived cultures.
Protected brush/cultures from bronchoalveolar lavage (BAL) reflect airway colonization (eg, Aspergillus, MSSA/MRSA, P aeruginosa) rather than nosocomial pneumonia/ventilator-associated pneumonia (VAP) pathogens in the lung parenchyma.
Multiple organisms as a cause of nosocomial pneumonia
Multiple pathogens obtained via nontissue biopsy culture methods usually indicate lower airway colonization.
Histologic Findings
Histologic study of lung tissue reveals either necrotizing or nonnecrotizing pneumonia, depending on the pathogen. P aeruginosa produces a necrotizing pneumonia with vessel invasion, local hemorrhage, and microabscess formation. [9] Other aerobic gram-negative bacilli produce a polymorphonuclear response at the site of invasion, but microabscess formation and vessel invasion are absent.
Other Tests
Electrocardiograms (ECGs) and ventilation-perfusion scans help to eliminate pneumonia mimics. ECGs, cardiac enzymes, and Swan-Ganz readings may rule out left ventricular failure caused by exacerbation of heart failure or new myocardial infarction.
Obtain other tests that are related to the possible underlying causes of the pulmonary infiltrates; for example, if lupus pneumonitis is suspected, ask the patient about a history of SLE pneumonitis. Afterward, serologic tests should be performed to assess for SLE.
ABG studies are useful in assessing the degree of severity of lung dysfunction but not in determining the specific etiology. In general, bacterial nosocomial pneumonia/ventilator-associated pneumonia (VAP) have low A-a gradients (< 35).
Treatment Considerations
In 2016, the Infectious Disease Society of America and American Thoracic Society published guidelines on management of adults with hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP). [4] .The treatment discussion is congruent with these guidelines.
-
In order to tailor optimal choice of antibiotics and guide healthcare professionals, it is advised that each hospital generate facility specific antibiograms.
-
In an effort to minimize patient harm and exposure to unnecessary antibiotics and reduce the development of antibiotic resistance, the guidelines recommend that the antibiogram data be used to decrease the unnecessary use of dual gram-negative and empiric methicillin-resistant Staphylococcus aureus (MRSA) antibiotic treatment.
-
Suggest noninvasive sampling with semiquantitative cultures to diagnose VAP, rather than invasive sampling with quantitative cultures or noninvasive sampling with quantitative cultures.
-
However, the panel recognizes that invasive quantitative cultures occasionally will be performed by some clinicians. For patients with suspected VAP whose invasive quantitative culture results are below the diagnostic threshold for VAP, the guidelines suggest that antibiotics be withheld rather than continued.
-
Suggest that patients with suspected HAP (non-VAP) be treated according to the results of microbiologic studies performed on respiratory samples obtained noninvasively, rather than being treated empirically.
-
For patients with suspected HAP/VAP, the guidelines recommend using clinical criteria alone, rather than using serum procalcitonin (PCT) plus clinical criteria, bronchoalveolar lavage fluid (BALF) sTREM-1 plus clinical criteria, or C-reactive protein (CRP) plus clinical criteria to decide whether to initiate antibiotic therapy.
-
In patients with suspected VAP, include coverage for S aureus, Pseudomonas aeruginosa, and other gram-negative bacilli in all empiric regimens.
-
If empiric coverage for MRSA is indicated, either vancomycin or linezolid is recommended.
-
When empiric treatment that includes coverage for MSSA (and not MRSA) is indicated, the guidelines suggest a regimen including piperacillin-tazobactam, cefepime, levofloxacin, imipenem, or meropenem.
-
Oxacillin, nafcillin, and cefazolin are preferred agents for treatment of proven MSSA, but are not necessary for the empiric treatment of VAP if one of the above agents is used.
-
For patients being treated empirically for HAP, prescribe an antibiotic with activity against S aureus.
-
For patients with HAP/VAP due to P aeruginosa, the guidelines recommend that the choice of an antibiotic for definitive (not empiric) therapy be based on the results of antimicrobial susceptibility testing.
-
For patients with VAP or HAP, a 7-day course of antimicrobial therapy is recommended, as well as antibiotic de-escalation. When the final culture and sensitivity results are available, the empiric broad-spectrum regimen should be converted to more narrow and specific coverage.
Antimicrobial therapy
The specific pathogen that causes a given case of nosocomial pneumonia usually is unknown. Therefore, empiric antimicrobial therapy is the only practical approach. These regimens should be based on the local profile of organisms associated with hospital-acquired pneumonia (HAP) and their antibiotic sensitivities. [30] Traditionally, nosocomial pneumonias have been treated for 7-14 days. However, ventilator-associated pneumonia (VAP) (except due to nonfermenting gram-negative rods [eg, P aeruginosa]) can be successfully treated in 7 days. If the patient receives appropriate antimicrobial therapy for 2 weeks and does not respond (ie, improved infiltrates findings on chest radiograph), initiate a diagnostic workup to detect nonbacterial infections (eg, herpesvirus type 1 [HSV-1] pneumonitis) or noninfectious disease mimics (eg, bronchogenic carcinomas).
Cefiderocol is a cephalosporin antibiotic that is capable of penetrating outer cell membranes of Gram-negative bacteria by acting as a siderophore. In September 2020, it gained FDA approval for hospital-acquired bacterial pneumonia (HABP) and ventilator-associated bacterial pneumonia (VABP) caused by the following susceptible Gram-negative microorganisms: Acinetobacter baumannii complex, Escherichia coli, Enterobacter cloacae complex, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Serratia marcescens. Approval was based on the phase 3 APEKS-NP study. Results showed cefiderocol was noninferior to high-dose meropenem for all-cause mortality at Days 14 and 28. [31]
Initial and definitive treatment of hospital-acquired pneumonia
MSSA should be covered unless the patient has risk factors for MRSA, including intravenous antibiotic use within the preceding 90 days, exposure to a hospital unit where more than 20% of S aureus isolates are MRSA, or a high risk for death (eg, need for ventilatory support due to septic shock). Vancomycin or linezolid should be used, guided by local antibiogram, to empirically cover MRSA.
For empiric coverage of MSSA, piperacillin-tazobactam, cefepime, levofloxacin, imipenem, or meropenem are preferred. In cases of proven MSSA infection, oxacillin, nafcillin, or cefazolin is favored.
Double coverage against P aeruginosa should be provided in the empiric treatment of individuals with HAP who are likely to have Pseudomonas and other gram-negative infections or who are at a high risk for mortality (need for ventilatory support and/or septic shock). For all other cases, single coverage of P aeruginosa is indicated. [16]
Initial and definitive treatment of ventilator-associated pneumonia
Empiric treatment of VAP should include coverage of S aureus, P aeruginosa, and other gram-negative bacilli.
MRSA should be covered empirically in patients with any of the following risk factors for antibiotic resistance:
-
Patients located in units where more than 10-20% of S aureus isolates are MRSA
-
Patients in units where the prevalence of MRSA is unknown
The preferred antibiotics for treatment of MRSA infections include vancomycin and linezolid. Linezolid appears to be 15% more efficacious than even adjusted-dose vancomycin. It should be considered over Vancomycin in patients with renal insufficiency and those infected with high-MIC (MIC in the upper range of sensitivity, 1.5–2 μg/mL) isolates of MRSA.The recommended antibiotics for the treatment of suspected MSSA (and not MRSA) infections include piperacillin-tazobactam, cefepime, levofloxacin, imipenem, and meropenem. When the pathogen is confirmed as MSSA, the patient should be switched to oxacillin, nafcillin, or cefazolin.
A single antibiotic with activity against P aeruginosa should be administered, except in patients with risk factors for multidrug-resistant (MDR) organisms, including the following:
-
Intravenous antibiotic use within the preceding 90 days
-
Septic shock or ARDS preceding VAP
-
Five or more days of hospitalization prior to VAP onset
-
Acute renal replacement therapy prior to the onset of VAP
-
The patient is located where more than 10% of gram-negative isolates are resistant
-
Patients in ICUs where antibiotic sensitivity rates are not available
Double-drug coverage of P aeruginosa should combine agents with a high degree of antipseudomonal activity and low resistance potential. Optimal combinations include piperacillin-tazobactam or a cephalosporin (cefepime, ceftazidime) or carbapenems (imipenem, meropenem) or aztreonam plus either a fluoroquinolone (levofloxacin, ciprofloxacin) or aminoglycoside (amikacin, gentamicin, tobramycin) or polymyxins (colistin, polymyxin B).
In general, aminoglycosides should be avoided in the treatment of VAP. This also holds true for colistin. This recommendation is based on poor penetration of these agents in the lung tissue, in addition to the potential nephrotoxicity of aminoglycosides and the challenge in achieving therapeutic blood levels in patients with fluctuating renal function. Unfortunately, VAP due to Pseudomonas has a high failure rate, up to 40%, regardless of the antibiotic regimen. This rate is impacted by the resistance of the pathogen and initial antibiotic selection.
In cases of HAP and VAP, antibiotics should be administered by either extended or continuous infusion. Dosing needs to be based on antibiotic blood levels and also should also be weight-based, when applicable.
The use of inhaled antibiotic therapy should be generally limited to cases of VAP produced by gram-negative bacilli that are sensitive only to aminoglycosides or polymyxins (colistin or polymyxin B). These antibiotics should also be administered systemically. [32]
Sulbactam/durlobactam (Xacduro) is the first antibiotic approved to treat HABP and VABP caused by Acinetobacter. Results from the phase 3 ATTACK trial showed sulbactam/durlobactam was noninferior to colistin in patients with carbapenem-resistant Acinetobacter infections. [33] A carbapenem or ampicillin/sulbactam also are options in treating Acinetobacter HAP/VAP. If there is resistance to these agents, inhaled and intravenous colistin should be substituted. The guidelines recommend against the use of tigecycline in the treatment of Acinetobacter VAP.
Several antibiotic and beta-lactamase inhibitor combinations have been approved by the FDA for VAP caused by susceptible gram-negative microorganisms, including ceftolozane/tazobactam, ceftazidime/avibactam, and imipenem/cilastatin/relebactam. [34]
Caveats
An important caveat is to differentiate P aeruginosa colonization from actual lung infection. P aeruginosa pneumonia is characterized by fever, cyanosis, hypotension, and rapid cavitation (< 72 hours) on chest radiography. Sputum recovered from these cases typically is greenish due to the pyocyanin pigment that is produced by the organism when it invades tissue. This usually is accompanied by an almond odor.
Enterobacter species typically do not cause hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP). S maltophilia and B cepacia are common colonizers of respiratory secretions, but they rarely, if ever, cause nosocomial pneumonia in otherwise healthy hosts; however, they are colonizers/potential pathogens in patients with bronchiectasis or cystic fibrosis.
S aureus (MSSA/MRSA) commonly colonizes respiratory secretions (30-50%) but rarely, if ever, causes necrotizing cavitary nosocomial pneumonia. Oropharyngeal anaerobes are unimportant from a therapeutic standpoint.
Duration of therapy
In general, for both hospital-acquired pneumonia (HAP) and VAP, 7 days of treatment with appropriate antibiotics/antibiotics is recommended. This duration may be shortened or lengthened depending on the clinical response of the individual. Normalization of PCT levels may provide useful corroboration of clinical judgment in deciding to stop antibiotic therapy.
Patient Consultations
Consult an infectious disease specialist to assess the microbiology of the specimens obtained from the patient, to rule out the mimics of nosocomial pneumonia, and to administer empiric or specific antimicrobial therapy.
Consult a pulmonologist to help with mechanical ventilation (often required in patients with nosocomial pneumonia).
Other consultations include the following, if indicated:
-
Rheumatologist (if the patient appears to have lupus or SLE pneumonitis)
-
Oncologist (for possible pulmonary infiltrates caused by a lymphangitic spread of a malignancy)
Diet, Activity, and Deterrence
Many patients with nosocomial pneumonia have significant nutritional deficiencies. Early (within 48 hours) enteral nutrition appears to decrease infectious complications. Parenteral nutrition does not seem to have this effect and should be considered only in patients with a contraindication to enteral replacement. [35, 36]
Beds that permit some degree of patient turning may decrease the likelihood of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) in at-risk patients. Decontamination of the mouth and gut may affect the risk of producing MDR organisms. [37]
Complications in Nosocomial Pneumonia
Failure to successfully wean the patient from the respirator (possibly because of a lack of cardiopulmonary function or a superimposed process [eg, HSV-1 pneumonitis]) is a common problem following intubation for nosocomial pneumonia.
HSV-1 pneumonitis develops in intubated patients who have unchanging or persistent pulmonary infiltrates after 2 weeks of antimicrobial therapy. These patients usually have low-grade fevers with variable degrees of leukocytosis. Demonstrating HSV-1 in samples of respiratory secretions may establish the diagnosis.
Start treatment with acyclovir in patients diagnosed with HSV-1 infection; acyclovir decreases hypoxemia and subsequently permits weaning of the patient from the respirator.
Questions & Answers
Overview
What is healthcare-associated pneumonia (HCAP)?
What is hospital-acquired pneumonia (HAP)?
Where is hospital-acquired pneumonia (HAP) most prevalent?
How is bacterial infection transmitted in hospital-acquired pneumonia (HAP)?
What is the pathogenesis of primary inhalation hospital-acquired pneumonia (HAP)?
What is the pathophysiology of aspiration hospital-acquired pneumonia (HAP)?
What factors increase the risk for developing hospital-acquired pneumonia (HAP)?
What is the pathophysiology of hematogenous hospital-acquired pneumonia (HAP)?
What causes hospital-acquired pneumonia (HAP) to develop?
Which bacteria are common etiological agents of hospital-acquired pneumonia (HAP)?
Which pathogens are less-causes of nosocomial pneumonia?
Which organisms are etiologic agents of ventilator-associated pneumonia (VAP)?
What are the risk factors for developing hospital-acquired pneumonia (HAP)?
What is the mortality rate of nosocomial pneumonia in the United States?
What is the global prevalence of nosocomial pneumonia?
What are the racial and sexual predilections of nosocomial pneumonia?
What is the age predilection of nosocomial pneumonia?
What guidelines are available for management of nosocomial pneumonia?
Which conditions may be included in the differential diagnosis for nosocomial pneumonia?
What are the most common causes of infiltrates in ventilated patients?
Which tests are performed to exclude differential diagnoses of hospital-acquired pneumonia (HAP)?
What are the IDSA/ATF recommendations for the diagnosis of hospital-acquired pneumonia (HAP)?
What are the IDSA/ATF recommendations for the diagnosis of ventilator-associated pneumonia (VAP)?
Which physical findings are characteristic of nosocomial pneumonia?
What is the role of WBC count in the evaluation of nosocomial pneumonia?
What is the role of blood culture in the evaluation of nosocomial pneumonia?
What is the role of radiography in the evaluation of nosocomial pneumonia?
What is the role of CT scanning in the evaluation of nosocomial pneumonia?
What is the role of bronchoscopy in the evaluation of nosocomial pneumonia?
What are histologic findings characteristic of nosocomial pneumonia?
When is antimicrobial therapy indicated for nosocomial pneumonia?
How is hospital-acquired pneumonia (HAP) treated?
How is ventilator-associated pneumonia (VAP) treated?
Which antibiotics are used for the treatment of MRSA nosocomial pneumonia?
In which patients is a single antibiotic with activity against P aeruginosa contraindicated?
What is the role of aminoglycosides in the treatment of ventilator-associated pneumonia (VAP)?
What is the role of inhaled antibiotic therapy for nosocomial pneumonia?
What are the optimal combination drug therapies for nosocomial pneumonia?
What are the important considerations in treatment selection for nosocomial pneumonia?
What is the duration of therapy for nosocomial pneumonia?
What is the role of nutrition in the treatment of nosocomial pneumonia?
What are complications of nosocomial pneumonia?
-
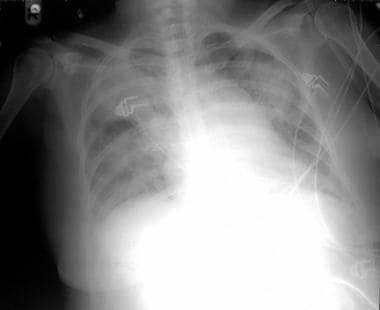
Typical chest radiograph of a patient with nosocomial pneumonia.
Tables
What would you like to print?
- Overview
- Pathophysiology
- Etiology
- Epidemiology of Nosocomial Pneumonia
- Patient Prognosis
- Differential Diagnoses of Nosocomial Pneumonia
- Diagnostic Concerns
- Physical Examination
- White Blood Cell Count and Blood Cultures
- Radiography
- Bronchoscopic Techniques
- Histologic Findings
- Other Tests
- Treatment Considerations
- Patient Consultations
- Diet, Activity, and Deterrence
- Complications in Nosocomial Pneumonia
- Questions & Answers
- Show All
- References