Overview
The progress in immunology, surgical techniques, and technological advancements in recent years of vascularized organ transplantation has made transplantation a routine treatment for organ dysfunction since its introduction in the 1950s. With rising numbers of patients being added to organ transplant waiting lists, the imbalance between organ supply and demand is ever increasing. The lack of suitable organs for all who could benefit leads to longer waiting times and greater morbidity and mortality. To overcome this imbalance, much attention is being directed towards increasing the available donor pool.
Donation after circulatory death (DCD) and expanded criteria donor organs that were previously discarded are now routinely utilized [1, 2] to cater to the 108,655 candidates in the United States who are currently on the waiting list. [3] However, in 2018, 4,994 organs were recovered for transplant but were ultimately discarded. Of these organs, kidneys had the highest and intestines had the least number of discards. [4] Increased utilization of these discarded organs could help narrow the expanding gap between supply and demand (Figure 1).
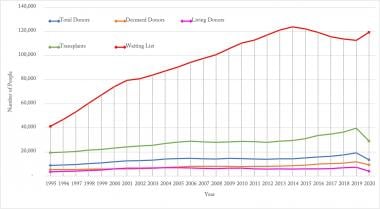 Yearly number of organ transplants, patients on waiting list, living and deceased Donors. Source of data: Organ Procurement and Transplantation Network (https://optn.transplant.hrsa.gov/).
Yearly number of organ transplants, patients on waiting list, living and deceased Donors. Source of data: Organ Procurement and Transplantation Network (https://optn.transplant.hrsa.gov/).
While much attention has been focused on identifying other sources of organs for transplant, such as stem cell-derived organs and xenografts, the mainstay of organ supply comes from deceased donor donation (DDD) (ie, cadaveric donors). Approximately 30% of all nationwide DDDs come from trauma patients. Evaluation of trauma patients as potential organ donors is critical to maximize the organ usage for transplantation. The circumstances and mechanism of death in organ donors from 1998 to 2020 are shown in Figure 2.
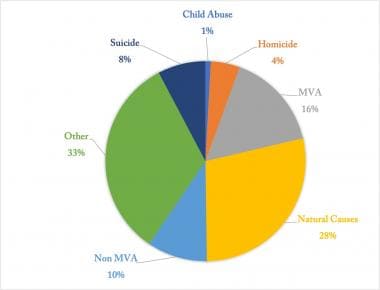 Circumstances of clinical grain death in organ donors for 1998-2020. [MVA = Motor Vehicle Accident]. Source of data: Organ Procurement and Transplantation Network (https://optn.transplant.hrsa.gov/).
Circumstances of clinical grain death in organ donors for 1998-2020. [MVA = Motor Vehicle Accident]. Source of data: Organ Procurement and Transplantation Network (https://optn.transplant.hrsa.gov/).
Between 1998 and 2020, motor vehicle accidents (MVAs) resulted in 36,791 donors, with an average of 1,363±135.5 each year. Furthermore, within donors in the age group of 18-34 years, the mechanisms of death of 16,187 were anoxic injury and of 32,242 were head trauma. Of these donors, 6,424 were related to homicide, 17,714 to MVA, 6,857 to non-MVA, and 9,050 to suicide. By contrast, the contribution of living donors on the overall donor pool with respect to organ/multiorgan transplantation performed in the United States between 1998 and 2020 is represented in Figure 3.
Organ Distribution
To maintain listings of potential organ recipients, the Department of Health and Human Services contracts the United Network for Organ Sharing (UNOS). Local organ procurement organizations (OPOs) are authorized by the Health Care Financing Administration and UNOS to manage the procurement of organs in their region. OPOs are responsible for organizing and overseeing the following:
-
Identification of donors
-
Evaluation of potential donors
-
Confirmation of diagnosis of brain death
-
Arranging consent from family
-
Clinical management of potential donor
-
Obtaining permission for visiting transplant surgeons to remove organs at the location of the donor
-
Preservation and packaging of organs for transplant
Organ allocation is decided by a complex set of guidelines that continuously evolve. UNOS maintains the lists of potential recipients divided by organ and ABO blood type. Potential recipients can be listed under multiple blood group lists as well as in multiple regions. Priority on each organ list is based upon several factors, including proximity to the donor, severity of illness, length of time on the waiting list, and special circumstances related to particular medical conditions. Objective scoring systems have been set up for the liver (MELD/PELD; see the MELD Score and PELD Score calculators) and the lung (LAS). These objective scoring systems are based upon defined physiologic and laboratory parameters. A point scale system determines the recipient's rank on each list. Organ allocation is then decided by the recipient's points and the following additional factors:
-
Location (local, regional, national)
-
Severity of illness (except kidneys)
-
ABO blood type compatibility
-
Length of time on waiting list
-
Histocompatibility leukocyte antigen (HLA) match (kidneys only)
-
Degree of preformed antigen sensitivity (panel of reactive antibody [PRA] score, kidneys and pancreas only)
-
Other special factors (eg, pediatric patients in specific age categories, reciprocal sharing arrangements or pay back agreements, dual organ recipient, liver transplant for hepatic malignancy, acute failure of recent transplanted organ)
A study by Tsuang et al used a thoracic simulated allocation model and found that broader geographic sharing of pediatric donor lungs may increase pediatric candidate access to lung transplant. [5, 6, 7] Changes in organ allocation have attempted to reduce geographic inequity in organ availability by increasing organ sharing across larger distances. [8] Furthermore, allocation has shifted to using concentric rings of distance from the location of the donor hospital in order to assign local, regional, or national priority status.
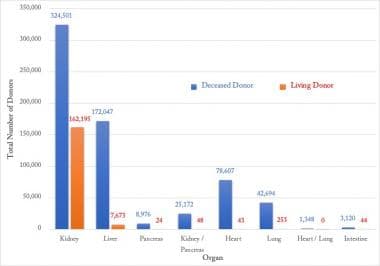
The trend in individual organ donation over the period of 1988-2020 is been depicted in Figure 4. The Organ Procurement and Transplantation Network (OPTN) along with UNOS introduced a system for kidney allocation (the Kidney Allocation System, KAS) in 2014 that expedites placement of expanded criteria donors and transplants in highly sensitized individuals, which may explain the increasing trend in kidney donors from 2015 onwards.
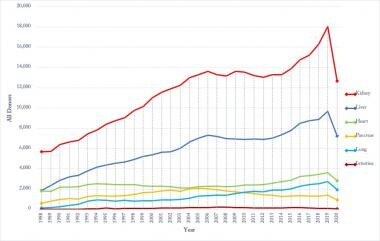 Organ specific donation changing trends for 1988-2020. Source of data: Organ Procurement and Transplantation Network (https://optn.transplant.hrsa.gov/).
Organ specific donation changing trends for 1988-2020. Source of data: Organ Procurement and Transplantation Network (https://optn.transplant.hrsa.gov/).
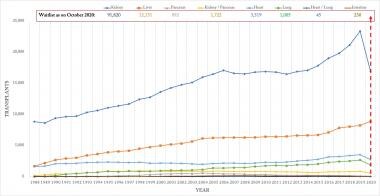
The waitlist for each organ/multi-organ as of October 2020 is depicted and compared to changing trends of organ/multi-organ transplantation from 1988 to 2020 in Figure 5.
Criteria for Organ Donors
Regional transplant centers have different sets of absolute and relative criteria for excluding potential organ donors. Early criteria were fairly strict, limiting evaluation to ideal donors aged 10-50 years with no comorbid conditions. With the increasing demand for organs, donation from an expanded donor pool has loosened restrictions considerably. Organs are harvested routinely from patients younger than 10 years and older than 50 years. Previously, such factors as hepatitis C, HIV infection, or active bacterial infection were absolute contraindications. Now, such donors are often used for specific recipients. Relatively few absolute contraindications exist, and most potential donors are reviewed on a case-by-case basis. Additional absolute and relative contraindications are assessed for donation of specific organs.
The OPTN has amended policies for organ donation and has established criteria to evaluate eligible deaths for organ donation, which are described as follows [9] :
-
General contraindications:
- Age older than 75 years
- Body weight less than 5 kg or a body mass index (BMI) greater than 50 kg/m2
- Previous malignant neoplasms with active metastasis. History of melanoma, except non-melanoma skin cancers such as basal cell and squamous cell cancer, and primary CNS tumors without evident metastasis
- Hematologic malignancies, which includes leukemia, Hodgkin’s disease, lymphoma, and multiple myeloma
- Active fungal, parasitic, viral, or bacterial meningitis or encephalitis
- Aplastic anemia, agranulocytosis
- No discernable cause of death
-
Kidney contraindications:
- Age older than 70 years
- Age 50-69 years with history of type 1 diabetes for more than 20 years
- Polycystic kidney disease
- Glomerulosclerosis greater than or equal to 20% by kidney biopsy
- Terminal serum creatinine greater than 4.0 mg/dL
- Chronic renal failure
- No urine output for 24 hours or longer
-
Liver contraindications:
- Cirrhosis
- Terminal total bilirubin greater than or equal to 4 mg/dL
- Portal hypertension
- Macrosteatosis greater than or equal to 50% or fibrosis greater than or equal to stage II
- Fulminant hepatic failure
- Terminal aspartate aminotransferase (AST)/alanine aminotransferase (ALT) levels greater than 700 U/l
-
Heart contraindications:
- Age older than 60 years
- Age 45 years or older with a history of 10 or more years of hypertension or 10 or more years of type 1 diabetes
- History of coronary artery bypass graft (CABG)
- History of coronary stent/intervention
- Current or past medical history of myocardial infarction (MI)
- Severe vessel diagnosis as supported by cardiac catheterization (more than 50% or more than 2-vessel disease)
- Acute myocarditis or endocarditis or both
- Heart failure due to cardiomyopathy
- Internal defibrillator or pacemaker
- Moderate to severe single valve or 2-valve disease documented by echocardiogram or cardiac catheterization, or previous valve repair
- Serial echocardiographic results showing severe global hypokinesis
- Myxoma
- Congenital defects
-
Lung contraindications:
- Age older than 65 years
- Diagnosis of chronic obstructive pulmonary disease (COPD)
- Terminal PaO2/FiO2 less than 250 mmHg
- Asthma (with daily prescription)
- Asthma as the cause of death
- Pulmonary fibrosis
- Previous lobectomy
- Multiple blebs documented on CT scan
- Pneumonia as indicated on CT scan, x-ray, bronchoscopy, or cultures
- Bilateral severe pulmonary contusions as per CT
Brain and Cardiac Death
Determination of brain death
Donation after brain death (DBD) is the mainstay of deceased organ donation. The diagnosis of brain death is based principally on clinical examination, but diagnostic tests often are used for confirmation. To assist in raising the suspicion of clinical brain death for at-risk patients, several clinical indicators augment a periodic neurologic examination, as follows:
-
Early indicators of brain death:
Hemodynamic lability
Heart rate instability
Decreased bronchial secretions
To complete the documentation of clinical brain death, the physician must demonstrate the following:
-
Correction of potentially reversible causes of coma:
Hypothermia
Sedating medications
Metabolic disturbances
Endocrine disturbances
Hypoxia or hypercarbia
-
Absence of brainstem reflexes (eg, cornea, pupillary light, oculovestibular, gag, oculocephalic)
-
Lack of respiratory effort (apnea test, ie, absence of respiratory movement after disconnection from respirator for sufficient duration to have pCO2 rise to >50-60 mm Hg)
To confirm the diagnosis of clinical brain death, several additional diagnostic modalities may be employed. The confirmatory test can be repeated after an interval of 2-24 hours so that observer error can be avoided and persistence of the clinical state can be documented. Diagnostic tests include electroencephalogram (EEG), isotopic flow study, and transcranial Doppler.
Donation after cardiac death
Under some circumstances, the family of a trauma patient may wish to withdraw care from a critically injured patient who is unlikely to make a meaningful recovery. Although these patients may not meet criteria for brain death, the family may wish for donation. In these cases, procuring organs from the non–heart-beating donor is possible. Exact guideline protocols are established regionally or at individual institutions but involve the withdrawal of mechanical support followed by rapid organ procurement after the clinical pronouncement of death by noting cessation of cardiac activity. The manner in which this occurs is subject to ethical and practical consideration.
Countries like the United States and Europe have been using donation after cardiac death (DCD) organs for many years. Canada, on the other hand, has only more recently begun to use DCD organs. Previously, the country had been almost exclusively using brain death organs for donation. In 2006, the Canadian Council for Organ Donation and Transplantation released DCD recommendations, and, by 2009, DCD donations had occurred in 30 Ontario hospitals. The establishment of a DCD program has led to a significant increase in deceased donation activity in Ontario. The province now has one of the highest organ donation rates in Canada. [10]
DCD donations can potentially increase the number of donor organs, but consent must occur before death. A multidisciplinary body consisting of representatives from the American Thoracic Society, the Society of Critical Care Medicine, the International Society for Heart and Lung Transplantation, the Association of Organ Procurement Organizations, and the United Network of Organ Sharing issued an ethics and policy statement that discussed such issues as the consent process, interventions, how death is determined, and end-of-life care. [11]
Consent for Organ Donation
The Uniform Anatomical Gift Act of 1968 requires explicit consent for organ donation. This act was revised in 1987 and then again in 2006 to better reflect current practices and encourage organ donation. [12, 13] In its current form, this act reaffirms that if a donor has gifted organs, their wishes should be honored and the family need not give additional consent. It also expanded the list of people able to consent for organ or tissue donation if a potential donor has not registered their wishes before death.
The Uniform Anatomical Gift Act requires that hospitals notify organ procurement organizations of patients who have died or are near death. In 2003 the Organ Donation Breakthrough Collaborative was formalized by the US Department of Health and Human Services in response to the organ shortage. The goal of this collaborative is to achieve donation rates of 75% or greater where possible.
Evaluation of the Potential Organ Donor
Evaluation of the potential donor continues after the determination of brain death with both general and organ-specific testing. The exact set of laboratory and diagnostic tests used varies from center to center, but an outline is presented, as follows:
-
General screening
Basic laboratory values (eg, complete blood cell [CBC] count, electrolytes, arterial blood gas [ABG], serum glucose, blood urea nitrogen [BUN], creatinine, creatine phosphokinase [CPK], isoenzymes)
ABO blood typing
HLA typing
Chest radiograph
Blood cultures (×2, aerobic, anerobic from two different venipunctures sites)
Sputum Gram stain, culture, and sensitivities
Urinalysis, culture, and sensitivities
HIV, hepatitis B and C virus assessment by serologic and/or nucleic acid testing (NAT)
-
Heart donor
12-Lead ECG
Chest radiograph
Echocardiogram
Cardiac catheterization (male >40 y or female >45 y, or younger if other cardiovascular risk factors present)
Creatine kinase (CK), isoenzyme of CK with muscle and brain subunits (CK-MB), and troponin levels
-
Lung donor
ABG on 100% FiO2 and 5 cm/H2/PEEP (with vent settings documented), within 2 hours prior to the offer
Chest radiograph 3 hours prior to the offer
Bronchoscopy
Sputum Gram stain and description of sputum
Lung laterality
-
Pancreas donor
Record any family history of diabetes (type I or II)
Hemoglobin A1C level
Insulin use, dose, and duration
Serum amylase and lipase levels
-
Liver donor
Liver function tests (LFTs)
Liver biopsy - For patients with BMI of > 34, age older than 70 years (>60 years if diabetic), past medical history suggestive of liver disease, significant history of alcohol abuse, radiographic studies suggestive of fatty liver infiltration, or positive hepatitis serologies
International normalized ratio (INR), prothrombin time (PT), activated partial thromboplastin time (aPTT)
Pre-procurement CT scan
-
Kidney donor
Anatomical description, including the number of renal vessels, ureters and approximate length, and injuries
Biopsy results, if available
Electrolytes, BUN, creatinine
Kidney laterality
-
Coronavirus disease 2019 (COVID-19) screening [14]
NAT, including polymerase chain reaction (PCR) for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), performed 3 days prior to organ procurement and just prior to organ donation for DDD; living donors should be counseled on and encouraged to use preventive strategies as much as possible, particularly in the 14 days prior to donation, to avoid infection
For DDD, testing by NAT for SARS-CoV-2 should be obtained at least once from upper (nasopharyngeal, mid-nasal-turbinate, oropharyngeal or saliva, tracheal aspirate) or lower respiratory tract samples (deep lavage, bronchoalveolar lavage)
For living donor donation, testing by NAT for SARS-CoV-2 should be at least once from upper (nasopharyngeal, mid-nasal-turbinate, oropharyngeal or saliva) as close to the time of donation as possible
OPTN policy requires storage of blood for all deceased donors, which could be used retrospectively to look for positive donor serology if needed
Management of the Potential Organ Donor
Treatment of the trauma patient continues in the manner deemed optimal for the injuries sustained until the determination of brain death. After this determination, treatment is directed at maintenance of organ function, while determination of intent to donate organs or familial consent for organ donation is sought, or until mechanical support is withdrawn.
The donor is managed with intensive care unit (ICU)–level care as the evaluation proceeds. Care is directed at preservation of the donor's hemodynamic state, protection of the donor organs, and avoidance or treatment of complications that are observed in the brain-dead donor. In addition to common problems observed in patients who are critically ill, many pathologic states are observed frequently in the patient who is brain dead. As the time from brain death to organ procurement increases, so does the number and severity of complications. Common complications in patients who are brain dead are hypotension (incidence, 81%), diabetes insipidus (65%), disseminated intravascular coagulation (28%), cardiac arrhythmias (25%), pulmonary edema (18%), and metabolic acidosis (11%). [15]
In particular, donors who are brain dead are vulnerable to the effects of diabetes insipidus, cardiac arrhythmia, and endocrine dysfunction. Diabetes insipidus and its resultant sequelae (ie, hypovolemia, hypernatremia, hypokalemia, hyposmolarity) are managed with vasopressin or desmopressin acetate (DDAVP). This treatment has been shown to delay asystole following brain death from 2 days to 3 weeks. Brain death is associated with disruption of the hypothalamic-pituitary axis. Donors can display adrenal insufficiency, lack of glycemic control, and hypothyroidism. Empiric steroids often are used, and management of other conditions follows clinical presentation.
Previously, ultrarapid progression from declaration of brain death to procurement was advised. Currently, there is a shift toward greater optimization of donor physiology prior to procurement. This also enables more precise coordination with recipient institutions and lessens cold-ischemia time.
Guidelines for donor management are prepared by each OPO. Specific areas of management are as follows:
-
Patient monitoring
- Lines - Central venous and radial artery lines, often pulmonary arterial catheters
- Vitals - Systolic blood pressure, mean arterial pressure, central venous pressure, cardiac output, heart rate
- Temperature - Monitor every 1 hour and use cooling or warming blankets to maintain temperature at 97.7-99.5°F/36.5-37.5°C
- pH - 7.35-7.45; monitor ABG every 4 hours and correct acidosis or alkalosis accordingly with relevant medications, considering nephrotoxicity
-
Blood pressure and ventilation
- Measure blood pressure every hour to ensure mean arterial pressure (MAP) of 60-100 mm Hg; central venous pressure (CVP) of 4-10 mm Hg
- In hypovolemia, maintain systolic blood pressure greater than 90 mm Hg with minimal inotropic support (eg, levothyroxine, Levophed, Neosynephrine, dopamine, vasopressin, dobutamine, and milrinone)
- Target urine output is greater than or equal to 0.5 mL/kg/h
- Goal PaO2 > 100 mm Hg on FiO2 40%
- History of hypertension: MAP goals are 70-110 mm Hg
- Manage hypertension (systolic blood pressure of >150 mm Hg) using clevidipine, nicardipine, nitroprusside, esmolol, labetalol, and/or nitroglycerin
-
Fluid balance
- Adjust intravenous (IV) fluid to maintain CVP of 4-10 mm Hg and urine output of >0.5 mL/kg/h
- Treat diabetes insipidus. Suspect diabetes insipidus if urine output is >4 mL/kg/h for 2 hours with urinary specific gravity of < 1.006; serum Na+ is >150 mEq/L; serum osmolality is >310 mmol/L; urine osmolality is < 300 mmol/kg. Treat with vasopressin gtt 0.2-3 U/h or 0.03 U/min. Titrate to maintain urine output of at least 1 mL/kg/h. Discontinue 2 hours prior to procurement time
- If vasopressin is not available, use DDAVP 1-2 µg IVP every 8 to 12 hours
- If Na+ is >150 mEq/L, administer free water bolus per nasogastric tube, 5 mL/kg every hour (after cross clamping for 30 minutes and return to suctioning); repeat until half of the free water deficit is administered. Record input and output
-
Miscellaneous
- Electrolytes - Correct electrolyte abnormalities as observed (Na+:135-145 mEq/L; K+: 3.5-4.5 mEq/L; corrected serum Ca2+: 8.5-10.5 mg/dL); elevated sodium is associated with adverse outcomes in liver transplantation
- Steroids - Administer 1 g IV Solu-Medrol over 10-15 minutes; methylprednisolone is an anti-inflammatory immunosuppressant that stabilizes leukocyte membranes and is useful in organ donation to maintain hemodynamic stability
- Levothyroxine (T4) - Consider in cases of refractory hypotension, arrhythmia, or cardiac dysfunction. Donor thyroid replacement has been shown to reduce 30-day mortality in heart transplant recipients [16]
- Correct coagulopathy and transfuse blood to maintain hematocrit (HCT) of 27 or greater
- Administer empiric cefazolin or equivalent
Organ Procurement
The organ procurement procedure requires careful coordination of several surgical teams. Commonly, teams are sent from each of the institutions of the designated recipients to the location of the donor. Separate teams for heart, lung, and abdominal organs participate. The recovery operation is conducted in defined steps to minimize the warm ischemic time of removed organs, and the organs are removed in the order of their susceptibility to warm ischemic damage. Because of the short time available for transplanting the preserved organs, particularly for heart and lungs, the preparation of the potential recipients and transplant teams also must be coordinated. The recipient operation often commences prior to the actual arrival of the organ at the recipient institution.
Prior to the operation, the donor must be adequately volume resuscitated and prepared for surgery. The management of intracerebral edema prior to brain death and the resulting diabetes insipidus often causes a hypovolemic state that must be corrected before harvesting organs. The donor is volume resuscitated and brought to the operating room, where appropriate positioning, monitoring, and ventilation are ensured prior to incision. Following this preparation, and when each team is in attendance, the procedure can begin. The outline of the surgical procedure is summarized below.
Steps in organ procurement
An incision from the suprasternal notch to the pubis is made; then, the chest is opened via median sternotomy, and the abdominal cavity is entered.
The thoracic and abdominal cavities are examined by respective teams for evidence of occult pathology and gross suitability of organs for transplant.
The small bowel is retracted, the right colon is mobilized, the posterior peritoneum is incised, and the duodenum and pancreas are reflected, allowing exposure of the inferior vena cava (IVC) and aorta.
Attachments of the heart, lung, liver, kidneys, and pancreas are incised; the organs are readied for removal.
The aortic arch, abdominal aorta, and portal vein or tributaries are cannulated for infusion of the preservative solution.
The aortic arch and supra celiac abdominal aorta are cross-clamped.
A cold preservative solution (typically University of Wisconsin solution) is infused through the inferior aortic, portal, and cardiac cannula, with drainage of effluent via the IVC. The thoracic and abdominal cavities are packed with ice.
The organs are removed to the back table for initial preparation and packaging for transport.
-
Yearly number of organ transplants, patients on waiting list, living and deceased Donors. Source of data: Organ Procurement and Transplantation Network (https://optn.transplant.hrsa.gov/).
-
Circumstances of clinical grain death in organ donors for 1998-2020. [MVA = Motor Vehicle Accident]. Source of data: Organ Procurement and Transplantation Network (https://optn.transplant.hrsa.gov/).
-
Comparison of donor type to organ/multi-organ transplantation for 1988-2020. Source of data: Organ Procurement and Transplantation Network (https://optn.transplant.hrsa.gov/).
-
Organ specific donation changing trends for 1988-2020. Source of data: Organ Procurement and Transplantation Network (https://optn.transplant.hrsa.gov/).
-
Waitlist of organ/multi-organs as on October 2020 and the changing trends in transplantation for 1988-2020. Source of data: Organ Procurement and Transplantation Network (https://optn.transplant.hrsa.gov/).