Practice Essentials
Ectopic pregnancy is the result of a flaw in human reproductive physiology that allows the conceptus to implant and mature outside the endometrial cavity (see the image below), which ultimately ends in the death of the fetus. Without timely diagnosis and treatment, ectopic pregnancy can become a life-threatening situation. [1]
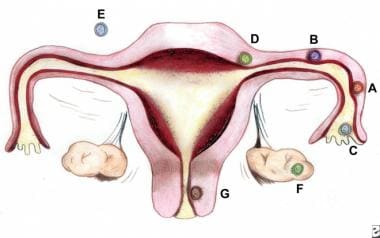 Sites and frequencies of ectopic pregnancy. By Donna M. Peretin, RN. (A) Ampullary, 80%; (B) Isthmic, 12%; (C) Fimbrial, 5%; (D) Cornual/Interstitial, 2%; (E) Abdominal, 1.4%; (F) Ovarian, 0.2%; and (G) Cervical, 0.2%.
Sites and frequencies of ectopic pregnancy. By Donna M. Peretin, RN. (A) Ampullary, 80%; (B) Isthmic, 12%; (C) Fimbrial, 5%; (D) Cornual/Interstitial, 2%; (E) Abdominal, 1.4%; (F) Ovarian, 0.2%; and (G) Cervical, 0.2%.
Signs and symptoms
The classic clinical triad of ectopic pregnancy is as follows:
-
Abdominal pain
-
Amenorrhea
-
Vaginal bleeding
Unfortunately, only about 50% of patients present with all 3 symptoms.
Patients may present with other symptoms common to early pregnancy (eg, nausea, breast fullness). The following symptoms have also been reported:
-
Painful fetal movements (in the case of advanced abdominal pregnancy)
-
Dizziness or weakness
-
Fever
-
Flulike symptoms
-
Vomiting
-
Syncope
-
Cardiac arrest
The presence of the following signs suggests a surgical emergency:
-
Abdominal rigidity
-
Involuntary guarding
-
Severe tenderness
-
Evidence of hypovolemic shock (eg, orthostatic blood pressure changes, tachycardia)
Findings on pelvic examination may include the following:
-
The uterus may be slightly enlarged and soft
-
Uterine or cervical motion tenderness may suggest peritoneal inflammation
-
An adnexal mass may be palpated but is usually difficult to differentiate from the ipsilateral ovary
-
Uterine contents may be present in the vagina, due to shedding of endometrial lining stimulated by an ectopic pregnancy
See Clinical Presentation for more detail.
Diagnosis
Serum β-HCG levels
In a normal pregnancy, the β-HCG level doubles every 48-72 hours until it reaches 10,000-20,000mIU/mL. In ectopic pregnancies, β-HCG levels usually increase less. Mean serum β-HCG levels are lower in ectopic pregnancies than in healthy pregnancies.
No single serum β-HCG level is diagnostic of an ectopic pregnancy. Serial serum β-HCG levels are necessary to differentiate between normal and abnormal pregnancies and to monitor resolution of ectopic pregnancy once therapy has been initiated.
The discriminatory zone of β-HCG (ie, the level above which an imaging scan should reliably visualize a gestational sac within the uterus in a normal intrauterine pregnancy) is as follows:
-
1500-1800 mIU/mL with transvaginal ultrasonography, but up to 2300 mIU/mL with multiple gestates [2]
-
6000-6500 mIU/mL with abdominal ultrasonography
Absence of an intrauterine pregnancy on a scan when the β-HCG level is above the discriminatory zone represents an ectopic pregnancy or a recent abortion.
Ultrasonography
Ultrasonography is probably the most important tool for diagnosing an extrauterine pregnancy.
Visualization of an intrauterine sac, with or without fetal cardiac activity, is often adequate to exclude ectopic pregnancy. [3]
Transvaginal ultrasonography, or endovaginal ultrasonography, can be used to visualize an intrauterine pregnancy by 24 days post ovulation or 38 days after the last menstrual period (about 1 week earlier than transabdominal ultrasonography). An empty uterus on endovaginal ultrasonographic images in patients with a serum β-HCG level greater than the discriminatory cut-off value is an ectopic pregnancy until proved otherwise.
Color-flow Doppler ultrasonography improves the diagnostic sensitivity and specificity of transvaginal ultrasonography, especially in cases in which a gestational sac is questionable or absent.
Laparoscopy
Laparoscopy remains the criterion standard for diagnosis; however, its routine use on all patients suspected of ectopic pregnancy may lead to unnecessary risks, morbidity, and costs. Moreover, laparoscopy can miss up to 4% of early ectopic pregnancies.
Laparoscopy is indicated for patients who are in pain or hemodynamically unstable.
See Workup for more detail.
Management
Therapeutic options in ectopic pregnancy are as follows:
-
Expectant management
-
Methotrexate
-
Surgery
Expectant management
Candidates for successful expectant management should be asymptomatic and have no evidence of rupture or hemodynamic instability. Candidates should demonstrate objective evidence of resolution (eg, declining β-HCG levels).
Close follow-up and patient compliance are of paramount importance, as tubal rupture may occur despite low and declining serum levels of β-HCG.
Methotrexate
Methotrexate is the standard medical treatment for unruptured ectopic pregnancy. A single-dose IM injection is the more popular regimen. The ideal candidate should have the following:
-
Hemodynamic stability
-
No severe or persisting abdominal pain
-
The ability to follow up multiple times
-
Normal baseline liver and renal function test results
Absolute contraindications to methotrexate therapy include the following:
-
Existence of an intrauterine pregnancy
-
Immunodeficiency
-
Moderate to severe anemia, leukopenia, or thrombocytopenia
-
Sensitivity to methotrexate
-
Active pulmonary or peptic ulcer disease
-
Clinically important hepatic or renal dysfunction
-
Breastfeeding
-
Evidence of tubal rupture
Surgical treatment
Laparoscopy has become the recommended surgical approach in most cases. Laparotomy is usually reserved for patients who are hemodynamically unstable or for patients with cornual ectopic pregnancies; it also is a preferred method for surgeons inexperienced in laparoscopy and in patients in whom a laparoscopic approach is difficult.
See Treatment and Medication for more detail.
Background
Ectopic pregnancy refers to the implantation of a fertilized egg in a location outside of the uterine cavity, including the fallopian tubes (approximately 97.7%), cervix, ovary, cornual region of the uterus, and abdominal cavity. Of tubal pregnancies, the ampulla is the most common site of implantation (80%), followed by the isthmus (12%), fimbria (5%), cornua (2%), and interstitia (2-3%). (See the image below.)
 Sites and frequencies of ectopic pregnancy. By Donna M. Peretin, RN. (A) Ampullary, 80%; (B) Isthmic, 12%; (C) Fimbrial, 5%; (D) Cornual/Interstitial, 2%; (E) Abdominal, 1.4%; (F) Ovarian, 0.2%; and (G) Cervical, 0.2%.
Sites and frequencies of ectopic pregnancy. By Donna M. Peretin, RN. (A) Ampullary, 80%; (B) Isthmic, 12%; (C) Fimbrial, 5%; (D) Cornual/Interstitial, 2%; (E) Abdominal, 1.4%; (F) Ovarian, 0.2%; and (G) Cervical, 0.2%.
In ectopic pregnancy (the term ectopic is derived from the Greek word ektopos, meaning out of place), the gestation grows and draws its blood supply from the site of abnormal implantation. As the gestation enlarges, it creates the potential for organ rupture, because only the uterine cavity is designed to expand and accommodate fetal development. Ectopic pregnancy can lead to massive hemorrhage, infertility, or death (see the images below). (See Etiology and Prognosis.)
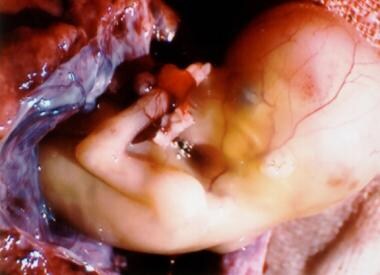 A 12-week interstitial gestation, which eventually resulted in a hysterectomy. Courtesy of Deidra Gundy, MD, Department of Obstetrics and Gynecology at Medical College of Pennsylvania and Hahnemann University (MCPHU).
A 12-week interstitial gestation, which eventually resulted in a hysterectomy. Courtesy of Deidra Gundy, MD, Department of Obstetrics and Gynecology at Medical College of Pennsylvania and Hahnemann University (MCPHU).
 A 12-week interstitial gestation, which eventually resulted in a hysterectomy. Courtesy of Deidra Gundy, MD, Department of Obstetrics and Gynecology at Medical College of Pennsylvania and Hahnemann University (MCPHU).
A 12-week interstitial gestation, which eventually resulted in a hysterectomy. Courtesy of Deidra Gundy, MD, Department of Obstetrics and Gynecology at Medical College of Pennsylvania and Hahnemann University (MCPHU).
In 1970, the Centers for Disease Control and Prevention (CDC) began to record statistics regarding ectopic pregnancy, reporting 17,800 cases. By 1992, the number of ectopic pregnancies had increased to 108,800. Concurrently, however, the case-fatality rate decreased from 35.5 deaths per 10,000 cases in 1970 to 2.6 per 10,000 cases in 1992. (See Epidemiology.)
The increased incidence of ectopic pregnancy has been partially attributed to improved ability in making an earlier diagnosis. Ectopic pregnancies that previously would have resulted in tubal abortion or complete, spontaneous reabsorption and remained clinically undiagnosed are now detected. (See Presentation, DDx, and Workup.)
In the 1980s and 1990s, medical therapy for ectopic pregnancy was implemented; it has now replaced surgical therapy in many cases. [4, 5, 6] As the ability to diagnose ectopic pregnancy improves, physicians will be able to intervene sooner, preventing life-threatening sequelae and extensive tubal damage, as well as, it is hoped, preserving future fertility. (See Treatment and Medication.)
Implantation sites
The faulty implantation that occurs in ectopic pregnancy occurs because of a defect in the anatomy or normal function of either the fallopian tube (as can result from surgical or infectious scarring), the ovary (as can occur in women undergoing fertility treatments), or the uterus (as in cases of bicornuate uterus or cesarean delivery scar). Reflecting this, most ectopic pregnancies are located in the fallopian tube; the most common site is the ampullary portion of the tube, where over 80% of ectopic pregnancies occur. (See Etiology.)
Nontubal ectopic pregnancies are a rare occurrence, with abdominal pregnancies accounting for 1.4% of ectopic pregnancies and ovarian and cervical sites accounting for 0.2% each. Some ectopic pregnancies implant in the cervix (< 1%), in previous cesarean delivery scars, [7] or in a rudimentary uterine horn; although these may be technically in the uterus, they are not considered normal intrauterine pregnancies. [8]
About 80% of ectopic pregnancies are found on the same side as the corpus luteum (the old, ruptured follicle), when present. [9] In the absence of modern prenatal care, abdominal pregnancies can present at an advanced stage (>28 wk) and have the potential for catastrophic rupture and bleeding. [10]
Etiology
An ectopic pregnancy requires the occurrence of 2 events: fertilization of the ovum and abnormal implantation. Many risk factors affect both events; for example, a history of major tubal infection decreases fertility and increases abnormal implantation.
Multiple factors contribute to the relative risk of ectopic pregnancy. In theory, anything that hampers or delays the migration of the fertilized ovum (blastocyst) to the endometrial cavity can predispose a woman to ectopic gestation. The following risk factors have been linked to ectopic pregnancy:
-
Tubal damage - Which can be the result of infections such as pelvic inflammatory disease (PID) or salpingitis (whether documented or not) or can result from abdominal surgery or tubal ligation or from maternal in utero diethylstilbestrol (DES) exposure
-
History of previous ectopic pregnancy
-
Smoking - A risk factor in about one third of ectopic pregnancies; smoking may contribute to decreased tubal motility by damage to the ciliated cells in the fallopian tubes
-
Altered tubal motility - As mentioned, this can result from smoking, but it can also occur as the result of hormonal contraception; progesterone-only contraception and progesterone intrauterine devices (IUDs) have been associated with an increased risk of ectopic pregnancy
-
History of multiple sexual partners [11]
-
Maternal age - Although this is not an independent risk factor [11]
The most logical explanation for the increasing frequency of ectopic pregnancy is previous pelvic infection; however, most patients presenting with an ectopic pregnancy have no identifiable risk factor. [13]
A 2009 literature review found 56 reported cases of ectopic pregnancy (by definition), dating back to 1937, after hysterectomy. [14]
Pelvic inflammatory disease
The most common cause of PID is an antecedent infection caused by Chlamydia trachomatis. Patients with chlamydial infection have a range of clinical presentations, from asymptomatic cervicitis to salpingitis and florid PID. More than 50% of women who have been infected are unaware of the exposure.
Other organisms that cause PID, such as Neisseria gonorrhoeae, also increase the risk of ectopic pregnancy, and a history of salpingitis increases the risk of ectopic pregnancy 4-fold. The incidence of tubal damage increases after successive episodes of PID (ie, 13% after 1 episode, 35% after 2 episodes, 75% after 3 episodes).
Effective vaccination against Chlamydia trachomatis is under investigation. Once clinically available, it should have a dramatic impact on the frequency of ectopic pregnancy, as well as on the overall health of the female reproductive system.
History of previous ectopic pregnancy
After 1 ectopic pregnancy, a patient incurs a 7- to 13-fold increase in the likelihood of another ectopic pregnancy. Overall, a patient with a previous ectopic pregnancy has a 50-80% chance of having a subsequent intrauterine gestation and a 10-25% chance of a future tubal pregnancy.
History of tubal surgery and conception after tubal ligation
Previous tubal surgery has been demonstrated to increase the risk of developing ectopic pregnancy. The increase depends on the degree of damage and the extent of anatomic alteration. Surgeries carrying higher risk of subsequent ectopic pregnancy include salpingostomy, neosalpingostomy, fimbrioplasty, tubal reanastomosis, and lysis of peritubal or periovarian adhesions.
Conception after previous tubal ligation also increases a women's risk of having an ectopic pregnancy; 35-50% of patients who conceive after a tubal ligation are reported to experience an ectopic pregnancy. Failure after bipolar tubal cautery is more likely to result in ectopic pregnancy than is occlusion using suture, rings, or clips. This failure is attributed to fistula formation that allows sperm passage. In one study, 33% of pregnancies occurring after tubal ligation were ectopic; those who underwent electrocautery and women younger than 35 years were at higher risk. [15]
Ectopic pregnancies following tubal sterilizations usually occur 2 or more years after sterilization rather than immediately after. In the first year, only about 6% of sterilization failures result in ectopic pregnancy.
Smoking
Cigarette smoking has been shown to be a risk factor for ectopic pregnancy development. Studies have demonstrated an elevated risk ranging from 1.6 to 3.5 times that of nonsmokers. A dose-response effect has also been suggested.
Based on laboratory studies in humans and animals, researchers have postulated several mechanisms by which cigarette smoking might play a role in ectopic pregnancies. These mechanisms include one or more of the following: delayed ovulation, altered tubal and uterine motility, and altered immunity. To date, however, no study has supported a specific mechanism by which cigarette smoking affects the occurrence of ectopic pregnancy.
Use of oral contraceptives or an intrauterine device
All contraceptive methods lead to an overall lower risk of pregnancy and therefore to an overall lower risk of ectopic pregnancy. However, among cases of contraceptive failure, women at increased risk of ectopic pregnancy compared with pregnant controls included those using progestin-only oral contraceptives, progestin-only implants, or IUDs and those with a history of tubal ligation. [16]
The presence of an inert, copper-containing or progesterone IUD traditionally has been thought to be a risk factor for ectopic pregnancy. Data from the Contraceptive CHOICE Project demonstrated a relative risk of 3.16 for ectopic pregnancy in women not using any form of contraception as compared with women using the progesterone IUD. [17] Nevertheless, if a woman ultimately conceives with an IUD in place, it is more likely to be an ectopic pregnancy. [18] The incidence of ectopic pregnancy in IUD users is 1 in 1000 over a 5-year period. [17]
Emergency contraception (levonorgestrel, or Plan B) does not appear to lead to a higher-than-expected rate of ectopic pregnancy. [19]
Use of fertility drugs or assisted reproductive technology
Ovulation induction with clomiphene citrate or injectable gonadotropin therapy has been linked to a 4-fold increase in the risk of ectopic pregnancy in a case-control study. This finding suggests that multiple eggs and high hormone levels may be significant factors.
One study demonstrated that infertility patients with luteal phase defects have a statistically higher ectopic pregnancy rate than do patients whose infertility is caused by anovulation. In addition, the risk of ectopic pregnancy and heterotopic pregnancy (ie, pregnancies occurring simultaneously in different body sites) dramatically increases when a patient has used assisted reproductive techniques—such as in vitro fertilization (IVF) or gamete intrafallopian transfer (GIFT)—to conceive. [20]
In a study of 3000 clinical pregnancies achieved through in vitro fertilization, the ectopic pregnancy rate was 4.5%, which is more than double the background incidence. Furthermore, studies have demonstrated that up to 1% of pregnancies achieved through IVF or GIFT can result in a heterotopic gestation, compared with an incidence of 1 in 30,000 pregnancies for spontaneous conceptions. [21]
In a retrospective (2006-2014) cohort study of 8120 assisted reproduction technology cycles, Rombauts et al found that endometrial combined thickness (ECT) measured prior to embryo transfer was associated with ectopic pregnancy. [22] The investigators reported that, following IVF, there was a 4-fold increased risk of ectopic pregnancy in women with an ECT of up to 9 mm compared with women with an ECT of at least 12 mm. They noted that increased ECT is a marker for increased fundus-to-cervix uterine peristalsis, which may be a reason for the increased risk for placenta praevia but a decreased risk for ectopic pregnancy. [22]
Increasing age
The highest rate of ectopic pregnancy occurs in women aged 35-44 years. A 3- to 4-fold increase in the risk of developing an ectopic pregnancy exists compared with women aged 15-24 years. One proposed explanation suggests that aging may result in a progressive loss of myoelectrical activity in the fallopian tube; myoelectrical activity is responsible for tubal motility.
Salpingitis isthmica nodosum
Salpingitis isthmica nodosum is defined as the microscopic presence of tubal epithelium in the myosalpinx or beneath the tubal serosa. These pockets of epithelium protrude through the tube, similar to small diverticula. Studies of serial histopathologic sections of the fallopian tube have revealed that approximately 50% of patients treated with salpingectomy for ectopic pregnancy have evidence of salpingitis isthmica nodosum. The etiology of salpingitis isthmica nodosum is unclear, but proposed mechanisms include postinflammatory and congenital changes, as well as acquired tubal changes, such as those observed with endometriosis. [23]
DES exposure
Before 1971, several million women were exposed in utero to DES, which was given to their mothers to prevent pregnancy complications. In utero exposure of women to DES is associated with a high lifetime risk of a broad spectrum of adverse health outcomes, including infertility, spontaneous abortion, and ectopic pregnancy. [24]
Other
Other risk factors associated with increased incidence of ectopic pregnancy include anatomic abnormalities of the uterus such as a T-shaped or bicornuate uterus, fibroids or other uterine tumors, previous abdominal surgery, failure with progestin-only contraception, and ruptured appendix. [13]
Epidemiology
United States statistics
The incidence of ectopic pregnancy is reported most commonly as the number of ectopic pregnancies per 1000 conceptions. Since 1970, when the reported rate in the United States was 4.5 cases per 1000 pregnancies, the frequency of ectopic pregnancy has increased 6-fold, with ectopic pregnancies now accounting for approximately 1-2% of all pregnancies. Consequently, the prevalence is estimated at 1 in 40 pregnancies, or approximately 25 cases per 1000 pregnancies. These statistics are based on data from the US Centers for Disease Control and Prevention (CDC), which used hospitalizations for ectopic pregnancy to determine the total number of ectopic pregnancies.
Looking at raw data, 17,800 hospitalizations for ectopic pregnancies were reported in 1970. This number rose to 88,000 in 1989 [25] but fell to 30,000 in 1998. An estimated 108,800 ectopic pregnancies in 1992 resulted in 58,200 hospitalizations, with an estimated cost of $1.1 billion.
Changes in the management of ectopic pregnancy, however, have made it difficult to reliably monitor incidence (and therefore mortality rates). [26] A review of hospital discharges in California found a rate of 15 cases per 1,000 in 1991, declining to a rate of 9.3 cases per 1,000 in 2000, [27] but a review of electronic medical records (inpatient and outpatient) from a large health maintenance organization (HMO) in northern California found a stable rate of 20.7 cases per 1,000 reported pregnancies from 1997-2000. [28] This suggests that the incidence of ectopic pregnancy in the United States remained steady at about 2% in the 1990s, despite the shift to outpatient treatment.
The above data raise the question of whether the number of ectopic pregnancies is declining or whether many ectopic pregnancies are now being treated in ambulatory surgical centers or are even being addressed with medical therapy, without admission. Some authors believe the latter is true, but truly accurate statistics are lacking.
Diagnoses of ectopic pregnancy in US emergency departments (ED) may be on the rise. From 2006 to 2013, the overall ratio of ED visits with an ectopic pregnancy diagnosis increased from 11.0 per 1000 live births to 13.7 per 1000 live births. [29]
Approximately 85-90% of ectopic pregnancies occur in multigravid women. In the United States, rates are nearly twice as high for women of other races compared with white women.
International statistics
The increase in incidence of ectopic pregnancy in the 1970s in the United States was also mirrored in Africa, although data there tend to be hospital based rather than derived from nationwide surveys, with estimates in the range of 1.1-4.6%. [30]
The United Kingdom estimated the incidence of ectopic pregnancy at about 11.1 per 1,000 reported pregnancies from 1997 to 2005, compared with 9.6 per 1,000 from 1991 to 1993. [31]
Racial- and age-related demographics
In the United States from 1991 to 1999, ectopic pregnancy was the cause of 8% of all pregnancy-related deaths among black women, compared with 4% among white women. [32]
Any woman with functioning ovaries can potentially have an ectopic pregnancy, which includes women from the age of menarche until menopause. Women older than 40 years were found to have an adjusted odds ratio of 2.9 for ectopic pregnancy. [13]
Prognosis
Ectopic pregnancy presents a major health problem for women of childbearing age. It is the result of a flaw in human reproductive physiology that allows the conceptus to implant and mature outside the endometrial cavity, which ultimately ends in the death of the fetus. Without timely diagnosis and treatment, ectopic pregnancy can become a life-threatening situation. [1]
The evidence in the literature reporting on the treatment of ectopic pregnancy with subsequent reproductive outcome is limited mostly to observational data and a few randomized trials comparing treatment options.
Assessment of successful treatment and future reproductive outcome with various treatment options is often skewed by selection bias. For example, comparing a patient who was managed expectantly with a patient who received methotrexate or with a patient who had a laparoscopic salpingectomy is difficult.
A patient with spotting, no abdominal pain, and a low initial beta–human chorionic gonadotropin (β-HCG) level that is falling may be managed expectantly, whereas a patient who presents with hemodynamic instability, an acute abdomen, and high initial β-HCG levels must be managed surgically. These 2 patients probably represent different degrees of tubal damage; thus, comparing the future reproductive outcomes of the 2 cases would be flawed.
Salpingostomy, salpingectomy, and tubal surgery
Data in the literature have failed to demonstrate substantial and consistent benefit from either salpingostomy or salpingectomy with regard to improving future reproductive outcome. However, despite the risk of persistent ectopic pregnancy, some studies have shown salpingostomy to improve reproductive outcome in patients with contralateral tubal damage. Yao and Tulandi concluded from a literature review that laparoscopic salpingostomy had a reproductive performance that was equal to or slightly better than salpingectomy; however, slightly higher recurrent ectopic pregnancy rates were noted in the salpingostomy group. [33]
In reporting on 10 years of surgical experience in Paris, Dubuisson et al concluded that, for selected patients who desire future fertility, using salpingectomy, which is simpler and avoids the risk of persistent ectopic pregnancy, is possible and can result in a comparable fertility rate to tubal conservation surgery. [34] Future fertility rates were no different with either surgical approach when the contralateral tube was either normal or scarred but patent.
Clausen reviewed literature from the previous 40 years and concluded that only a small number of investigators have suggested, indirectly, that conservative tubal surgery increases the rate of subsequent intrauterine pregnancy. He also concluded that the more recent studies may reflect an improvement in surgical technique. [35]
In an earlier study, Maymon et al, after reviewing 20 years of ectopic pregnancy treatment, concluded that conservative tubal surgery provided no greater risk of recurrent ectopic pregnancy than the more radical salpingectomy. [36]
The modern pelvic surgeon has been led to believe that the treatment of choice for unruptured ectopic pregnancy is salpingostomy, sparing the affected fallopian tube and thereby improving future reproductive outcome.
However, if the treating surgeon has neither the laparoscopic skill nor the instrumentation necessary to atraumatically remove the trophoblastic tissue via linear salpingostomy, then salpingectomy by laparoscopy or laparotomy is not the wrong surgical choice. Leaving a scarred, charred fallopian tube behind after removing the ectopic pregnancy but requiring extensive cautery to control bleeding does not preserve reproductive outcome.
Fertility following surgery
Previous history of infertility has been found to be the most significant factor affecting postsurgical fertility.
Parker and Bistis concluded that when the contralateral fallopian tube is normal, the subsequent fertility rate is independent of the type of surgery. [37] Similarly, a prospective study of 88 patients by Ory et al indicated that the surgical method had no effect on subsequent fertility in women with an intact contralateral tube. [38]
Several other studies reported that the status of the contralateral tube, the presence of adhesions, and the presence of other risk factors, such as endometriosis, have a more significant impact on future fertility than does the choice of surgical procedure.
According to Rulin, salpingectomy should be the treatment of choice in women with intact contralateral tubes, because conservative treatment provides no additional benefit and incurs the additional costs and morbidity associated with persistent ectopic pregnancy and recurrent ectopic pregnancy in the already damaged tube. [39]
Future fertility rates have been found to be similar in patients who are treated surgically by laparoscopy or laparotomy. Salpingectomy by laparotomy carries a subsequent intrauterine pregnancy rate of 25-70%, compared with laparoscopic salpingectomy rates of 50-60%. Very similar rates exist for laparoscopic salpingostomy versus laparotomy. The rate of persistent ectopic pregnancy between the 2 groups is also similar, ranging from 5-20%.
A slightly higher recurrent ectopic pregnancy rate exists in patients treated by laparotomy (7-28%), regardless of conservative or radical approach, when compared with laparoscopy (6-16%). This surprising finding is believed to be secondary to increased adhesion formation in the group treated by laparotomy.
Comparison of medical and surgical treatment of small, intact extrauterine pregnancies also revealed similar success and subsequent spontaneous pregnancy rates in a prospective, randomized trial. [40]
A study by Xu et al found that in women undergoing 51,268 fresh in vitro fertilization-intracytoplasmic sperm injection (IVF-ICSI) cycles, previous ectopic pregnancy has no effect on IVF-ICSI outcomes. The study also found that women with a prior history of ectopic pregnancy have a higher recurrence risk of ectopic pregnancy after IVF in comparison with women with no history of ectopic pregnancy. [41]
Methotrexate versus surgery
The success rates after methotrexate are comparable with laparoscopic salpingostomy, assuming that the previously mentioned selection criteria are observed. The average success rates using the multiple-dosage regimen are in the range of 91-95%, as demonstrated by multiple investigators. One study of 77 patients desiring subsequent pregnancy showed intrauterine pregnancies in 64% of these patients and recurrent ectopic pregnancy in 11% of them. Other studies have demonstrated similar results, with intrauterine pregnancy rates ranging from 20-80%.
The average success rates for the single-dosage methotrexate regimen are reported to be from 88-94%. In a study by Stovall and Ling, 113 patients (94%) were treated successfully, 4 (3.3%) of whom needed a second dose. [40] No adverse effects were encountered. Furthermore, 87.2% of these patients achieved a subsequent intrauterine pregnancy, whereas 12.8% experienced a subsequent ectopic pregnancy. [40] Other studies have reported similar results, with some mild adverse effects and lower reproductive outcomes.
A meta-analysis that included data from 26 trials demonstrated a success rate of 88.1% with the single-dose methotrexate regimen and a success rate of 92.7% with the multiple-dose regimen. [42] A small, randomized clinical trial also demonstrated the single-dose regimen to have a slightly higher failure rate. [43] A hybrid protocol, involving 2 equal doses of methotrexate (50 mg/m2) given on days 1 and 4 without the use of leucovorin, has been shown to be an effective and convenient alternative to the existing regimens. [44]
Complications
Complications of ectopic pregnancy can be secondary to misdiagnosis, late diagnosis, or treatment approach. Failure to make the prompt and correct diagnosis of ectopic pregnancy can result in tubal or uterine rupture (depending on the location of the pregnancy), which in turn can lead to massive hemorrhage, shock, disseminated intravascular coagulopathy (DIC), and death. Ectopic pregnancy is the leading cause of maternal death in the first trimester, accounting for 9-13% of all pregnancy-related deaths. In the United States, an estimated 30-40 women die each year from ectopic pregnancy.
Any time a surgical approach is chosen as the treatment of choice, consider the complications attributable to the surgery, whether it is laparotomy or laparoscopy. These include bleeding, infection, and damage to surrounding organs, such as the bowel, bladder, and ureters, and to the major vessels nearby. Infertility may also result secondary to loss of reproductive organs after surgery. Also consider the risks and complications secondary to anesthesia. Make the patient aware of these complications, and obtain the appropriate written consents.
Mortality
In the United States, ectopic pregnancy is estimated to occur in 1-2% of all pregnancies and accounts for 3-4% of all pregnancy-related deaths. [45] It is the leading cause of pregnancy-related mortality during the first trimester in the United States. In a review of deaths from ectopic pregnancy in Michigan, 44% of the women who died were either found dead at home or were dead on arrival at the emergency department. [46]
Virtually all ectopic pregnancies are considered nonviable and are at risk of eventual rupture and resulting hemorrhage. In addition to the immediate morbidity caused by ectopic pregnancy, the woman's future ability to reproduce may be adversely affected as well. However, patients who are diagnosed with ectopic pregnancy before rupture have a low mortality rate and also have a chance at preserved fertility.
From 1970 to 1989, the US mortality rate for ectopic pregnancies dropped from 35.5 deaths to 3.8 deaths per 10,000 ectopic pregnancies. [25] If the overall incidence of ectopic pregnancy remained stable in the 1990s, then the mortality rate dropped to 3.19 deaths per 10,000 ectopic pregnancies by 1999. [47]
Surveillance data for pregnancy-related deaths in the United States from 1991-1999 showed that ectopic pregnancy was the cause of 5.6% of 4200 maternal deaths. Of these deaths, 93% occurred via hemorrhage. [32]
During 1999–2008, the ectopic pregnancy mortality rate in the United States was 0.6 deaths per 100,000 live births. The CDC reported a higher rate in Florida, 2.5 deaths per 100,000 live births during 2009-2010. The 11 ectopic pregnancy deaths in Florida during 2009-2010 contrasted with the total number of deaths (14) identified in national statistics for 2007. There was a high prevalence of illicit drug use among the women who died in Florida. [45]
The mortality rate reported in African hospital-based studies varied from 50-860 deaths per 10,000 ectopic pregnancies; these were almost certainly underestimates resulting from underreporting of maternal deaths and misclassification of ectopic pregnancies as induced abortions. [30]
Using data from 1997 to 2002, the World Health Organization (WHO) estimated that ectopic pregnancy was the cause of 4.9% of pregnancy-related deaths in the industrialized world. [48] Ectopic pregnancy caused 26% of maternal deaths in early pregnancy in the United Kingdom from 2003-2005, second only to venous thromboembolism, despite a relatively low mortality rate of 0.035 per 10,000 estimated ectopic pregnancies. [31]
Patient Education
Advise patients receiving methotrexate therapy to avoid alcoholic beverages, vitamins containing folic acid, nonsteroidal anti-inflammatory drugs (NSAIDs), and sexual intercourse, until advised otherwise. A signed written consent demonstrating the patient's comprehension of the course of treatment must be obtained.
Provide an information pamphlet to all patients receiving methotrexate; the pamphlet should include a list of adverse effects, a schedule of follow-up visits, and a method of contacting the physician or the hospital in case of emergency, as well as the need to return to the emergency department for concerning symptoms.
Patients with risk factors for ectopic pregnancy should be educated regarding their risk of having an ectopic pregnancy. Women who are being discharged with a pregnancy of unknown location should be educated regarding the possibility of ectopic pregnancy and their need for urgent follow-up.
Patients undergoing assisted reproduction technology should be educated regarding their risk of heterotopic pregnancy.
For patient education information, see the Pregnancy Center and the Women's Health Center, as well as Ectopic Pregnancy, Bleeding During Pregnancy, Vaginal Bleeding, Birth Control Overview, and Birth Control Methods.
-
Sites and frequencies of ectopic pregnancy. By Donna M. Peretin, RN. (A) Ampullary, 80%; (B) Isthmic, 12%; (C) Fimbrial, 5%; (D) Cornual/Interstitial, 2%; (E) Abdominal, 1.4%; (F) Ovarian, 0.2%; and (G) Cervical, 0.2%.
-
Laparoscopic picture of an unruptured right ampullary tubal pregnancy; bleeding out of the fimbriated end has resulted in hemoperitoneum.
-
A 12-week interstitial gestation, which eventually resulted in a hysterectomy. Courtesy of Deidra Gundy, MD, Department of Obstetrics and Gynecology at Medical College of Pennsylvania and Hahnemann University (MCPHU).
-
A 12-week interstitial gestation, which eventually resulted in a hysterectomy. Courtesy of Deidra Gundy, MD, Department of Obstetrics and Gynecology at Medical College of Pennsylvania and Hahnemann University (MCPHU).
-
An endovaginal sonogram reveals an intrauterine pregnancy at approximately 6 weeks. A yolk sac (ys), gestational sac (gs), and fetal pole (fp) are depicted.
-
Linear incision being made at the antimesenteric side of the ampullary portion of the fallopian tube.
-
Laparoscopic picture of an ampullary ectopic pregnancy protruding out after a linear salpingostomy was performed.
-
Schematic of a tubal gestation being teased out after linear salpingostomy.






