Practice Essentials
Percutaneous coronary intervention (PCI), also known as coronary angioplasty, is a nonsurgical technique for treating obstructive coronary artery disease, including unstable angina, acute myocardial infarction (MI), and multivessel coronary artery disease (CAD). See the image below.
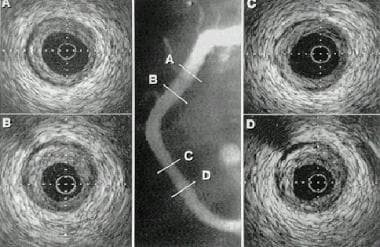 Example of intravascular ultrasonography (IVUS) image in percutaneous transluminal coronary angioplasty (PTCA).
Example of intravascular ultrasonography (IVUS) image in percutaneous transluminal coronary angioplasty (PTCA).
Indications and contraindications
Clinical indications for PCI include the following:
-
Acute ST-elevation myocardial infarction (STEMI)
-
Non–ST-elevation acute coronary syndrome (NSTE-ACS)
-
Unstable angina
-
Stable angina
-
Anginal equivalent (eg, dyspnea, arrhythmia, or dizziness or syncope)
-
High risk stress test findings
In an asymptomatic or mildly symptomatic patient, objective evidence of a moderate to large area of viable myocardium or moderate to severe ischemia on noninvasive testing is an indication for PCI. Angiographic indications include hemodynamically significant lesions in vessels serving viable myocardium (vessel diameter >1.5 mm).
Clinical contraindications for PCI include intolerance of long-term antiplatelet therapy or the presence of any significant comorbid conditions that severely limit the lifespan of the patient (this is a relative contraindication). A Heart Team approach (involving interventional cardiologists and cardiac surgeons) should be used in patients with diabetes and multivessel coronary artery disease and in patients with severe left main disease and a high Syntax score.
Relative angiographic contraindications include the following:
-
Arteries < 1.5 mm in diameter
-
Diffusely diseased saphenous vein grafts
-
Other coronary anatomy not amenable to PCI
In patients with stable angina, medical therapy is recommended as first-line therapy unless one or more of the following indications for cardiac catheterization and PCI or coronary artery bypass grafting (CABG) are present:
-
Severe symptoms
-
A change in symptom severity
-
Failed medical therapy
-
High-risk coronary anatomy
-
Worsening left ventricular (LV) dysfunction
For patients with STEMI, immediate coronary angiography with PCI is recommended (primary PCI).
For patients with NSTE-ACS, American College of Cardiology Foundation (ACCF)/American Heart Association (AHA) guidelines on the management of NSTE-ACS (updated in 2014 [1] ) recommend an early invasive strategy in most cases, with timing as follows:
-
Immediate (within 2 hours) - Patients with refractory angina, recurrent angina after initial treatment, signs/symptoms of heart failure, new/worsening mitral regurgitation, hemodynamic instability, sustained ventricular tachycardia, or ventricular fibrillation
-
Early (within 24 hours) - None of the immediate characteristics but new ST-segment depression, a GRACE risk score >140, or temporal change in troponin
-
Delayed invasive (within 25-72 hours) - None of the immediate or early characteristics but renal insufficiency (glomerular filtration rate [GFR] < 60 mL/min/1.73 m 2), left ventricular ejection fraction (LVEF) < 40%, early postinfarct angina, history of PCI within the preceding 6 months, prior CABG, GRACE risk score of 109-140, or TIMI score of 2 or higher
Ischemia-guided approach is recommended for patients with a low-risk score (TIMI 0 or 1, GRACE < 109).
Equipment
Balloon catheters for PCI have the following features:
-
A steerable guide wire precedes the balloon into the artery and permits navigation through the coronary tree
-
Inflation of the balloon compresses and axially redistributes atheromatous plaque and stretches the vessel wall
-
The balloon catheter also serves as an adjunctive device for many other interventional therapies
Atherectomy devices have the following features:
-
These devices are designed to physically remove coronary atheroma, calcium, and excess cellular material
-
Rotational or orbital atherectomy, which relies on plaque abrasion and pulverization, is used mostly for fibrotic or heavily calcified lesions that can be wired but not crossed or dilated by a balloon catheter
-
Atherectomy devices may be used to facilitate stent delivery in complex lesions
-
Directional coronary atherectomy (DCA) has been used to debulk coronary plaques
-
Laser atherectomy is not widely used at present
-
Atherectomy is typically followed by balloon dilation and stenting
Intracoronary stents have the following features:
-
Stents differ with respect to composition (eg, cobalt chromium or platinum chromium), architectural design, delivery system and the drug delivered
-
Drug-eluting stents (DESs) have demonstrated significant reductions in restenosis and target-lesion revascularization rates, with further reduction with the second-generation DESs (compared with first-generation DESs or bare-metal stents [BMSs])
-
In the United States, the commercially available DESs are second-generation models that elute everolimus and zotarolimus
-
Both stents with bioabsorbable polymer and fully bioresorbable scaffolds have been approved by the FDA and are available for commercial use in the United States
-
Stents are conventionally placed after balloon predilation, but in selected coronary lesions, direct stenting may lead to better outcomes
Other devices used for PCI include the following:
-
Thrombus aspiration is no longer recommended as a routine practice in patients undergoing primary PCI; two large randomized trials showed no reduction in the rate of death from any cause or the composite of death from any cause, rehospitalization for myocardial infarction, or stent thrombosis [2]
-
Distal embolic protection during saphenous vein graft intervention can be considered when technically feasible
See Periprocedural Care and Equipment for more detail.
Technique
Intravascular ultrasonography (IVUS) and optical coherence tomography (OCT) are used in PCI for the following purposes:
-
Provision of information about atherosclerotic plaque composition and burden, the vessel wall, vessel size, degree of calcium, and degree of luminal narrowing
-
Assessment of indeterminate lesions
-
Evaluation of adequate stent deployment
Intracoronary pressure wires are used in PCI as follows:
-
Characterization of coronary lesion physiology and estimation of lesion severity
-
Comparison of pressure distal to a lesion with aortic pressure enables determination of fractional flow reserve (FFR); FFR < 0.80 during maximal hyperemia (induced via administration of adenosine) is consistent with a hemodynamically significant lesion
Antithrombotic therapy
-
Aspirin 162-325 mg is given to all patients on the day of PCI
-
Unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), or bivalirudin may be used at the time of balloon angioplasty or PCI; fondaparinux must be supplemented with UFH to prevent catheter thrombosis and therefore is less commonly used
Antiplatelet therapy
Patients receiving stents are treated with a combination of aspirin and a P2Y12 receptor inhibitor (clopidogrel, prasugrel, or ticagrelor). The minimum duration of P2Y12 receptor inhibitor therapy, as per the current ACCF/AHA guidelines, is as follows:
-
BMSs - Minimum of 4 weeks
-
DESs (for acute coronary syndrome patients) - Minimum of 12 months
-
DESs (for stable ischemic heart disease patients) - Minimum of 6 months
-
DESs (for patients who are at high risk for bleeding) - Minimum of 3 months may be reasonable
Use of proton pump inhibitors (PPIs) is appropriate in patients with multiple risk factors for gastrointestinal bleeding who require antiplatelet therapy.
Glycoprotein inhibitor therapy
-
Abciximab, tirofiban, and eptifibatide have all been shown to reduce ischemic complications in patients undergoing balloon angioplasty and coronary stenting; however, evidence supporting their use was established largely before the use of oral P2Y12 inhibitors; all glycoprotein IIb/IIIa (GPIIb/IIIa) inhibitors are associated with an increased risk of bleeding
-
Several studies have failed to show the benefit of “upstream” administration of GPIIb/IIIa inhibitors in the era of dual antiplatelet therapy (DAPT); because GPIIb/IIIa inhibitors increase the risk of bleeding, their routine use before PCI is no longer recommended
-
GPIIb/IIIa inhibitors can be used as an adjunctive therapy at the time of PCI, on an individual basis, for large thrombus burden or inadequate P2Y12 receptor antagonist loading
See Technique and Medication for more detail.
Background
Since the first human percutaneous transluminal coronary angioplasty (PTCA) procedure was performed in 1977, the use of percutaneous coronary intervention (PCI) has increased dramatically; it is now one of the most commonly performed medical interventions. Originally developed in Switzerland by Andreas Gruentzig, PCI has transformed the practice of revascularization for coronary artery disease (CAD).
Coronary angioplasty, initially used in the treatment of patients with stable angina and discrete lesions in a single coronary artery, currently has multiple indications, including unstable angina, acute myocardial infarction (AMI), and multivessel CAD. With the combination of sophisticated equipment, experienced operators, and modern drug therapy, PCI has evolved into an effective nonsurgical modality for treating patients with CAD. Ongoing technical advances are allowing more patients with chronic total occlusions (CTOs) to be successfully treated percutaneously.
Improvements in catheter technique and the development of new devices and medications have paralleled our growing understanding of cardiovascular physiology, the pathogenesis of atherosclerosis, and the response to vascular injury. Intracoronary stents and atherectomy devices have been developed to increase the success and decrease the complications of conventional balloon dilation, as well as to expand the indications for revascularization. Interventionalists now can safely treat more complex coronary lesions and restenosis.
The development of drug-eluting stents (DESs) has substantially reduced the problem of restenosis seen with bare-metal stents (BMSs). At the same time, advances in intravascular ultrasonography (IVUS), optical coherence tomography (OCT), and fractional flow reserve (FFR) evaluation have improved the understanding of coronary plaque morphology, plaque vulnerability, and coronary physiology.
Furthermore, many of these technologies are able to help identify patients who will benefit most from PCI, coronary artery bypass grafting (CABG), or medical therapy. Adjunctive pharmacologic therapies aimed at preventing acute reocclusion have also improved the safety and efficacy of PCI.
The growth of PCI has been remarkable. Stents are now used in more than 80% of PCI cases in the United States. This prominent use of stents will be sustained they result in improved outcomes. Over the past two decades, innovations in PCI have been paralleled by dramatic reductions in 30-day death, myocardial infarction (MI), and target-vessel revascularization rates. (See Unstable Angina.)
Indications
Clinical indications for PCI include the following:
-
Acute ST-elevation MI (STEMI)
-
Non–ST-elevation acute coronary syndrome (NSTE-ACS)
-
Stable angina
-
Anginal equivalent (eg, dyspnea, arrhythmia, or dizziness or syncope)
-
Asymptomatic or mildly symptomatic patient with objective evidence of a moderate-sized to large area of viable myocardium or moderate to severe ischemia on noninvasive testing
Angiographic indications include hemodynamically significant lesions in vessels serving viable myocardium (vessel diameter >1.5 mm).
Contraindications
Clinical contraindications for PCI include intolerance of chronic antiplatelet therapy and the presence of any significant comorbid conditions that severely limit patient lifespan (this is a relative contraindication). A Heart Team approach (involving interventional cardiologists and cardiac surgeons) should be used in patients with diabetes and multivessel CAD and in patients with severe left main disease and a high Syntax score.
Relative angiographic contraindications include the following:
-
Arteries < 1.5 mm in diameter
-
Diffusely diseased saphenous vein grafts
-
Other coronary anatomy not amenable to PCI
Although CABG has been considered the standard of care for patients with unprotected left main CAD (ie, patients without prior CABG or a patent graft to the left anterior descending [LAD] or left circumflex artery), PCI to improve survival is a reasonable alternative to CABG in selected stable patients who have ≥50% diameter stenosis and either of the following [3] :
-
Anatomic conditions associated with a low risk of PCI procedural complications and a high likelihood of good long-term outcome (eg, stenosis of the ostium or trunk vs distal bifurcation or trifurcation stenoses)
-
Clinical characteristics that predict a significantly higher risk of adverse surgical outcomes
In addition, the patient’s ability to tolerate and comply with dual antiplatelet therapy is a consideration in the choice of PCI rather than CABG.
Although PCI is generally an acceptable alternative revascularization strategy compared to CABG, several studies have found a higher rate of repeat revascularization in patients who underwent PCI. [4, 5, 6]
Outcomes
In the focused update on appropriate use criteria published in 2012, [7] the use of coronary revascularization for patients with ACS and combinations of significant symptoms or ischemia was generally viewed favorably. However, the use of revascularization for asymptomatic patients or patients with low-risk findings on noninvasive testing and minimal medical therapy was viewed probably as unnecessary.
Same-day discharge after PCI
Rao et al examined the safety of same-day discharge in 107,018 low-risk patients 65 years or older who underwent elective PCI at 903 sites. [8] Only 1.25% of patients were discharged on the same day, and there was significant variation across facilities. Patients who were discharged on the same day had shorter procedures with less multivessel intervention. Notably, there were no significant differences between same-day discharge and overnight-stay patients with regard to mortality or rehospitalization rate either at 2 days or at 30 days.
Two meta-analyses that compared same-day discharge after elective PCI with overnight admission, including both radial and femoral approaches, showed no evidence for harm. [9, 10] Same-day discharge seems reasonable in carefully selected patients undergoing largely elective PCI.
PCI vs medical therapy: stable angina
Early trials demonstrated the advantages of PCI over medical therapy for symptomatic angina in single-vessel and multivessel CAD, with amelioration of symptoms, reduction of the need to take antianginal medications, improvement in exercise duration, and maintenance of survival rates comparable to those of medical therapy. [11, 12, 13]
There have been limited trials of coronary stenting versus medical therapy in patients with stable angina. Most of the data are derived from studies of balloon angioplasty vs medical therapy (eg, the RITA-II [Randomized Intervention in the Treatment of Angina] [14] and AVERT [Atorvastatin Versus Revascularization Treatment] [15] trials) or studies involving minimally symptomatic patients (eg, the COURAGE [Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation] [16] trial).
The RITA-II study, in which 1018 patients with stable angina were randomized to balloon angioplasty or medical therapy, demonstrated that balloon angioplasty results in better control of ischemic symptoms and greater improvement in exercise capacity than medical therapy does, though balloon angioplasty was associated with an increased incidence of the combined endpoint of death and myocardial infarction. [14]
In this study, death or definite myocardial infarction occurred in 6.3% of the balloon angioplasty patients and in 3.3% of the medical patients; only 44% of the deaths were actually due to heart disease. [14] Angina improved in both groups, but a 16.5% absolute excess of grade 2 or worse angina was noted in the medical group 3 months after randomization.
In the medical group, 23% of patients required revascularization during follow-up. [14] In the angioplasty group, 7.9% of patients required bypass surgery during follow-up, compared with 5.8% in the medically treated group. It is important to emphasize that although the patients in RITA-II were asymptomatic or mildly symptomatic, most had severe anatomic CAD: 62% had multivessel CAD, and 34% had important disease of the proximal LAD artery.
In the AVERT trial, 13% of the medically treated group and 21% of the angioplasty group had ischemic events at 18 months, suggesting that in low-risk patients with stable CAD, aggressive lipid-lowering therapy may reduce ischemic events as effectively as balloon angioplasty does. [15] A total of 341 patients with stable CAD symptoms, normal left ventricular (LV) function, and class I or II angina were assigned randomly to balloon angioplasty or atorvastatin therapy.
On the basis of the limited data available from randomized trials comparing medical therapy with balloon angioplasty, it seems appropriate to consider medical therapy for initial management of most patients with Canadian Cardiovascular Society Classification class I and II symptoms and to reserve percutaneous or surgical revascularization for patients with more severe symptoms and ischemia.
The COURAGE trial demonstrated that in patients with minimal, stable angina symptoms and coronary artery stenosis, medical therapy alone may be an appropriate strategy if such therapy can control the angina symptoms. [16] The trial randomized the addition of PCI to intensive pharmacologic therapy, with the endpoints of death from any cause and nonfatal MI during a median follow-up period of 4.6 years.
It is important to emphasize that all patients in the COURAGE study underwent coronary angiography. Inclusion criteria included the presence of a 70% or greater lesion in one or more proximal epicardial arteries, American College of Cardiology (ACC)/American Heart Association (AHA) class I or II indications for PCI, and objective evidence of myocardial ischemia on stress testing. [16] For both primary endpoints, there was no statistically significant difference between patients who received PCI with medical therapy and those who received only medical therapy.
The COURAGE trial has been heavily criticized on several grounds, including the following:
-
All patients underwent coronary angiography before enrollment
-
Only one in 12 patients who were screened were actually enrolled
-
At the time of enrollment, most patients were either asymptomatic or had minimal symptoms
Teo et al found that in older patients with stable CAD, optimal medical treatment without PCI remains an appropriate initial management strategy. [16] Analysis of 904 patients aged 65 years or older showed that, during a median 4.6-year follow-up, clinical outcome was no better or worse in patients randomized to optimal medical treatment plus PCI than in patients who received optimal medical treatment alone.
Compared with 1381 patients younger than 65 years with CAD, older patients had similar success in achieving treatment targets and similar rates of myocardial infarction, stroke, and major cardiac events, though the death rate was two to three times higher in the older patients. [16] It should be kept in mind that the analysis was done from patients enrolled in the COURAGE trial and thus must be interpreted in terms of the limitations outlined above.
Overall, medical therapy is recommended as first-line therapy in patients with stable angina unless one or more of the following indications for cardiac catheterization and PCI or CABG are present:
-
Severe symptoms
-
A change in symptom severity
-
Failed medical therapy
-
High-risk coronary anatomy or noninvasive findings
-
Worsening LV dysfunction
PCI vs surgical revascularization: stable angina
Two prospective clinical trials evaluated balloon angioplasty against surgery for revascularization of isolated LAD artery disease. [17, 18]
Using a combined endpoint (cardiac death, myocardial infarction, or refractory angina necessitating revascularization by surgery), the MASS (Medicine, Angioplasty, or Surgery Study) trial showed that after 3 years of follow-up, endpoint events occurred in 24% of angioplasty patients, 17% of medical patients, and 3% of surgical patients. [17] However, overall survival rates were similar in the three groups.
The other trial evaluated balloon angioplasty against bypass surgery with an internal thoracic (mammary) artery graft to the LAD artery and also reported no difference in survival during follow-up. [18] Although 94% of angioplasty patients and 95% of bypass patients were free of limiting symptoms, the former required more antianginal drugs. At 2.5 years’ follow-up, 86% of surgery patients were free from late events, compared with 43% of angioplasty patients. This difference was primarily due to restenosis necessitating a second revascularization procedure.
It is important to emphasizing that balloon angioplasty, rather than stent placement, was used in both of these trials; with the almost exclusive use of stenting in the current era, restenosis rates are now lower.
Five large (N > 300) randomized trials comparing balloon angioplasty with bypass surgery in patients with multivessel CAD all showed that in appropriately selected patients, the rates of death or of MI were similar, regardless of which treatment was employed. [19, 20, 21, 22, 23] However, more of the angioplasty-treated patients required a second revascularization procedure. Three of these studies are summarized in Table 1 below.
Table 1. Comparison of Surgical Therapy and Coronary Angioplasty (Open Table in a new window)
Endpoint |
Pocock et al* |
Pocock et al† |
BARI Study‡ |
|||
CABG (N=358) |
PTCA (N=374) |
CABG (N=1303) |
PTCA (N=1336) |
CABG (N=914) |
PTCA (N=915) |
|
Death (%) |
0.3 |
1.9 |
2.8 |
3.1 |
10.7 |
13.7 |
Death or MI |
4.5 |
7.2 |
8.5 |
8.1 |
11.7 |
10.9 |
Repeat CABG |
1.4 |
16.0§ |
0.8 |
18.3§ |
0.7 |
20.5§ |
Repeat CABG or PTCA |
3.6 |
30.5§ |
3.2 |
34.5§ |
8.0 |
54.0§ |
More than mild angina |
6.5 |
14.6§ |
12.1 |
17.8§ |
... |
... |
*Meta-analysis of results of 3 trials at 1 year. Patients with single-vessel disease were studied. [23] †Meta-analysis of results of 3 trials at 1 year. Patients with multivessel disease were studied. [23] ‡Reported results are for 5-year follow-up. Patients with multivessel disease were studied. [22] § P < .05. BARI = Bypass Angioplasty Revascularization Investigation; CABG = coronary artery bypass grafting; MI = myocardial infarction; PTCA = percutaneous transluminal coronary angioplasty. |
||||||
In the BARI (Bypass Angioplasty Revascularization Investigation) study, 5-year survival was 86.3% for those assigned to angioplasty versus 89.3% for those assigned to surgery, and 5-year freedom from Q-wave MI was 78.7% for the former and 80.4% for the latter. [22] However, after 5 years of follow-up, 54% of those assigned to angioplasty required an additional revascularization procedure, compared with only 8% of those assigned to surgery.
Similarly, the ERACI (Argentine Randomized Trial of Percutaneous Transluminal Coronary Angioplasty Versus Coronary Artery Bypass Surgery in Multivessel Disease) study showed that freedom from combined cardiac events at 3 years was significantly better for bypass surgery than for angioplasty (77% vs 47%), though the groups did not differ in terms of overall and cardiac mortality or frequency of MI. [24] Bypass patients were more often free of angina (79% vs 57%) and had fewer additional revascularization procedures (6% vs 37%).
In most patient subgroups with multivessel CAD, long-term mortality after CABG is comparable to that after PCI; therefore, the choice of treatment should depend on patient preference. In a collaborative analysis of individual patient data from 10 randomized trials, Hlatky et al found CABG to be a superior option for patients with diabetes and patients aged 65 years or older because mortality was lower in these subgroups. [25, 26]
Bare-metal stents vs CABG
The major limitations of balloon angioplasty were acute vessel closure and restenosis. Early studies with intracoronary stents showed that these devices were highly effective for treating or preventing acute or threatened vessel closure and thereby avoiding emergency bypass surgery.
Two randomized trials, BENESTENT (Belgian Netherlands Stent) [27] and STRESS (Stent Restenosis Study), [28] demonstrated that coronary stenting of de novo lesions in native vessels reduced angiographic restenosis by approximately 30% as compared with conventional balloon angioplasty. Stenting produces a larger lumen diameter than conventional balloon angioplasty both immediately after the procedure (acute gain) and at follow-up (net gain), resulting in less restenosis.
The use of BMSs was compared to bypass surgery for the treatment of multivessel CAD in the ARTS (Arterial Revascularization Therapies Study) trial. [29] After 1 year of follow-up, no difference was noted between the groups in the rate of death, stroke, or MI. Event-free survival was better in the surgery group than in the stent group (87.8% vs 73.8%), and only 3.5% in the surgery group required a second revascularization procedure, compared with 16.8% in the stent group.
The SoS (Stent or Surgery) trial compared BMSs with CABG and reported a 2-year target vessel revascularization rate of 21% in stent patients, compared with 6% in CABG patients. [30] Death and MI rates were similar in the two groups. However, the SoS trial had a higher noncardiac death rate in the PCI arm, thought to be attributed to a type II error that may have affected the study results.
The SoS trial and the ARTS study demonstrate the safety of PCI treatment in multivessel disease. Cardiac mortality risk is low, and the rates of repeat target vessel revascularization are less than half of those seen with balloon angioplasty. [31]
According to the New York Cardiac Registry, as with the prior trials, patients who received PCI as the initial therapy had a higher incidence of target vessel revascularization (35.1%) than those who received CABG (4.9%). [32] The registry identified 59,314 patients with multivessel disease who either underwent CABG (n = 37,212) or had PCI with bare-metal stents (n = 22,102), with reported endpoints of repeat revascularization and survival rates within 3 years.
Using unadjusted survival curves, the registry demonstrated that for patients who had two-vessel disease without LAD artery involvement, PCI offered a small survival benefit. [32] For patients who had two-vessel disease with proximal LAD artery involvement, the two procedures had similar mortalities (91.4% for CABG and 91.2% for PCI). The registry reported a statistically significant survival benefit of CABG over PCI in patients who had three-vessel disease with proximal LAD artery involvement.
Drug-eluting stents vs CABG
In the ARTS II trial, a registry comparing the use of sirolimus-eluting stents (SESs) with the PTCA and CABG arms of the ARTS I trial, SESs were associated with an 8% major cardiovascular event (MACE) rate (vs 13% for CABG in ARTS I) and an 8.5% target vessel revascularization rate (vs 4% for CABG and 21% for PTCA in ARTS I). The 1-year MACE rate was 10.5% for SES patients. [33]
The New York Cardiac Registry found that patients who underwent PCI with a DES had a higher rate of target vessel revascularization than those who underwent CABG (30.6% vs 5.2%). [32] They analyzed 17,400 patients who either received a DES (n = 9963) or underwent CABG (n = 7437) and observed them for 18 months. Unadjusted survival curves did not demonstrate a statistical significance in survival for two- or three-vessel disease.
Nevertheless, when adjustments were made for several factors (ie, age; sex; ejection fraction; hemodynamic state; history or no history of MI before the procedure; the presence or absence of cerebrovascular disease, peripheral arterial disease, congestive heart failure, chronic obstructive pulmonary disease [COPD], diabetes, and renal failure; and involvement of the proximal LAD artery), CABG had a statistically significant 18-month survival benefit over PCI with a DES. [32]
The SYNTAX (Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery) study was a large randomized controlled trial that enrolled 1800 patients with multi-vessel CAD to receive either a paclitaxel-eluting stent or CABG. [34]
At 5 years, the major adverse cardiac and cerebrovascular events (MACCE)—a composite of death, stroke, MI, or repeat revascularization—was significantly higher in patients with PCI than in those with CABG (37.5% vs 24.2%). [34] PCI, as opposed to CABG, resulted in significantly higher rates of all-cause death (14.6% vs 9.2%), MI (9.2 vs 4.0%), and repeat revascularization (25.4% vs 12.6%); however, the rate of stroke was similar.
In this trial, the extent of CAD was assessed by using a SYNTAX score that was based on location, severity, and degree of stenosis. [34] In patients with a low (0-22) SYNTAX score, PCI and CABG resulted in similar rates of MACCE (33.3% vs 26.8%) but PCI was associated with significantly more repeat revascularization (25.4% vs 12.6%). In patients with intermediate (23-32) or high (≥33) SYNTAX scores, CABG demonstrated clear superiority, with lower rates of MACCE, all-cause death, MI, and repeat revascularization.
In conclusion, the SYNTAX trial suggested that in patients with multivessel CAD, survival rates with CABG and PCI are comparable in patients with relatively uncomplicated and lesser degrees of CAD. [34] However, in patients with complex and diffuse CAD, CABG appears to be preferable. One caveat to be remembered is that the SYNTAX trial used first-generation paclitaxel-eluting stents. These stents have a higher rate of restenosis than the currently used second-generation DESs.
In summary, in deciding between PCI and CABG in patients with complex multivessel CAD, a Heart Team approach is recommended.
Diabetics with multivessel coronary artery disease
Patients with diabetes mellitus appear to constitute an exception to the general findings that balloon angioplasty and bypass surgery yield essentially equivalent results in patients with multivessel disease.
Among diabetic patients in the BARI trial, 5-year survival was 65.5% in those treated by balloon angioplasty and 80.6% for those treated with bypass surgery. [22] The improved survival with surgery was due to reduced cardiac mortality (5.8% vs 20.6%) and was confined to those receiving at least one internal thoracic artery graft. Better survival among diabetic patients with multivessel disease treated with bypass surgery rather than angioplasty was also observed in a large retrospective study.
The BARI 2D (Bypass Angioplasty Revascularization Investigation 2 Diabetes) trial randomized 2364 men and women with type 2 diabetes mellitus, documented CAD, stable symptoms, and myocardial ischemia treated with optimal medical therapy to an initial strategy of either coronary revascularization or watchful waiting with the option of subsequent revascularization. [35] At 5 years, rates of survival or the composite endpoint of cardiovascular death, MI, and stroke did not differ significantly between the groups.
A substudy of the BARI 2D trial reported that the coronary revascularization strategy improved outcomes at the 3-year follow-up, with patients experiencing a lower rate of worsening angina, new angina, and subsequent coronary revascularizations, as well as a higher rate of angina-free status. [36]
The FREEDOM (Future Revascularization Evaluation in Patients with Diabetes Mellitus Optimal Management of Multivessel Disease) trial randomly assigned 1900 patients with diabetes and multivessel CAD to either PCI with a DES or CABG. At 5 years, the rate of primary outcome—a composite of death, nonfatal MI, or nonfatal stroke—was lower in the CABG group (18.7%) than in the DES group (26.6%). CABG also had lower rates of death (10.9% vs 16.3% for PCI) and MI (6.0% vs 13.9% for PCI) but higher rates of stroke (5.2% vs 2.4% for PCI). [37]
A meta-analysis of 3131 patients from eight randomized, controlled trials (including SYNTAX and FREEDOM) that compared CABG with PCI in patients with diabetes suggested that all-cause mortality was lower with CABG than with PCI. [38]
In summary, in deciding between PCI and CABG in patients with diabetes mellitus and complex multivessel CAD, a Heart Team approach is recommended. CABG is generally recommended in preference to PCI, provided that the patient is a good candidate for surgery, there is extensive CAD(eg, three-vessel CAD or complex two-vessel CAD involving the proximal LAD artery), and the LAD artery can be anastomosed with a left internal mammary artery (LIMA) graft, CABG is generally recommended in preference to PCI.
PCI in NSTE-ACS (unstable angina and non-STEMI)
The management of patients with non-STEMI (NSTEMI) and unstable angina (called NSTE-ACS in the 2014 ACC/AHA guideline update to reflect the similarity between the two groups) has changed considerably over the past 15 years. [1] Several trials have helped provide a better understanding of risk stratification, selection of initial management strategy, and appropriate use of adjunctive medical therapy and revascularization, thereby leading to improved outcomes.
In general, two pathways have emerged for the treatment of NSTE-ACS. The early invasive strategy, with a diagnostic coronary angiogram for risk stratification, allows rapid definitive evaluation and affords the option for early revascularization to prevent ACS complications and facilitate early discharge.
In contrast, the ischemia-guided strategy (previously termed conservative strategy) recommends invasive evaluation only if patients have failed medical therapy, have objective evidence of ischemia on stress test, or have high prognostic risk (ie, high Thrombolysis in Myocardial Infarction [TIMI] or Global Registry of Acute Coronary Events [GRACE] scores). This is based on the premise that medical therapy alone can stabilize some patients, thus obviating costly and possibly unnecessary invasive procedures.
In terms of outcome data, several studies have assessed the use of an ischemia-guided strategy against the use of an early invasive strategy of revascularization
The VANQWISH (Veterans Affairs Non–Q-Wave Infarction Strategies in Hospital) trial compared an invasive strategy with conservative medical treatment in patients with non–Q-wave MI and found that the rates of death or nonfatal MI were higher in the invasive strategy group than in the conservative strategy group before hospital discharge, at 1 month, and at 1 year. [39]
Criticisms of this study include the exclusion of patients at very high risk; the lack of current aggressive medical therapies; a high rate of crossover to angiography in the conservative arm; a higher surgical mortality than expected in view of with contemporary standards; and the observation that most of the complications at 30 days occurred in patients who underwent CABG, with very few occurring in those who underwent balloon angioplasty. [39]
In contrast to the VANQWISH trial, four randomized studies found that an early invasive approach in patients with ACS was associated with improved outcomes.
The TIMI IIIb study showed less ischemia, shorter hospital stays, fewer readmissions, and fewer symptoms in patients treated by an early invasive approach. [40]
The FRISC (Fragmin and Fast Revascularization during Instability in Coronary Artery Disease) II trial prospectively randomized 2457 patients to receive either early invasive treatment with intracoronary stenting or noninvasive treatment and found that at 6 months, the composite endpoint of death or MI was higher in the latter arm than in the former. [41] Additionally, symptoms of angina and hospital readmissions were twice as common in the noninvasive arm as in the invasive arm.
The RITA-III study reported improved outcomes with early invasive therapy in 1810 patients at 5 years’ follow-up. [42] There was a statistically significant difference favoring an interventional strategy over conservative therapy with respect to all-cause mortality (15.1% vs 12.1%) and the rate of cardiac death or MI (15.9% vs 12.2%).
Data from the TACTICS-TIMI (Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy–Thrombolysis in Myocardial Infarction) 18 trial showed that the primary endpoint of death, MI, or rehospitalization at 6 months occurred in 19.4% of the conservative group and 15.9% of the invasive group, with death or MI occurring in 9.5% and 7.3%, respectively. [43]
In this study, patients who had a positive troponin test result, those who had ST-segment changes, those who were older than 65 years, and, especially, those who were women with elevated brain natriuretic peptide (BNP) and C-reactive protein (CRP) levels derived particular benefit from an early invasive strategy. [43]
The ICTUS (Early Invasive versus Selectively Invasive Management for Acute Coronary Syndromes) trial, which compared an early invasive strategy (angiography and revascularization within 48 hours) with a selective invasive strategy (medical stabilization with angiography and revascularization in refractory cases) in 1200 Dutch patients, demonstrated no statistical difference in mortality or the composite endpoint (death, nonfatal MI, or rehospitalization for anginal symptoms within 1 year). [44]
At 3 years’ follow-up, the ICTUS trial documented a trend toward significance favoring the selective invasive strategy for the combined endpoints (30% early invasive vs 26% selective invasive) but reported no differences in all-cause mortality and cardiac death. Overall, the weight of evidence has favored early invasive therapy over the ischemia-guided strategy, with one collaborative meta-analysis of randomized trials showing an 18% relative reduction in death or MI. [45] The invasive arm was also associated with less angina and fewer hospitalizations.
In a meta-analysis of patient-level data from FRISC, ICTUS, and RITA trials, 14.7% of patients treated according to the early invasive strategy had cardiovascular death or nonfatal MI, versus 17.9% in the selective invasive group. [46] Absolute risk reduction of cardiovascular death and nonfatal MI was 2-3.8 % in the low-to-intermediate group and 11.1% in the highest-risk patient.
With respect to the timing of the invasive strategy, some studies have demonstrated the benefit of early angiography, [47] particularly in high-risk patients (GRACE >140). A more delayed strategy is reasonable in low-to-intermediate risk patients. Two meta-analyses showed that whereas the early invasive approach yields no survival benefit or reduction in recurrent MI or major bleeding rates, it also poses no early hazard and has the advantages of less recurrent ischemia and a shorter hospital stay. [48, 49]
The 2014 ACC Foundation/AHA guidelines for management of unstable angina/NSTEMI recommend the use of an early invasive strategy or ischemia-guided strategy in patients with NSTE-ACS. An ischemia-guided approach is recommended for patients with a low-risk score (TIMI 0 or 1, GRACE < 109). Other patients will benefit from an early invasive strategy stratified by timing as follows:
-
Immediate (within 2 hours) - Patients with refractory or recurrent angina with initial treatment, signs/symptoms of heart failure, new/worsening mitral regurgitation, hemodynamic instability, sustained ventricular tachycardia, or ventricular fibrillation
-
Early (within 24 hours) - None of the immediate characteristics but new ST-segment depression, a GRACE risk score > 140, or temporal change in troponin
-
Delayed invasive (within 25-72 hours) - None of the immediate or early characteristics but renal insufficiency (Glomerular filtration rate [GFR] < 60 mL/min/1.73 m 2), left ventricular ejection fraction (LVEF) < 40%, early postinfarct angina, history of PCI within the past 6 months, prior CABG, GRACE risk score of 109-140, or TIMI score of 2 or higher
PCI in acute ST-elevation myocardial infarction (STEMI)
The recognition that intracoronary thrombosis from a ruptured plaque is the primary cause of vessel occlusion in STEMI and that prompt restoration of vessel patency provides significant clinical benefit has led to the development of two main reperfusion strategies.
Thrombolytic therapies, such as front-loaded tissue plasminogen activator (t-PA), reteplase (r-PA), and tenecteplase (TNK), open approximately 60-80% of infarct-related vessels within 90 minutes, but only 50% of these vessels will have normal (TIMI grade 3) flow. In addition, 10% of vessels opened by thrombolysis either become reoccluded or are the source for recurrent symptoms of angina. Also, patients older than 75 years, who have the most to gain from reperfusion, have unacceptably high rates of intracerebral hemorrhage with thrombolysis.
Because of these limitations, several randomized trials have evaluated mechanical revascularization with primary angioplasty in the setting of STEMI. The advantage of this approach is that the artery can be opened more frequently (>95%), and the underlying plaque rupture can be treated.
An analysis of 23 trials confirmed the superiority of primary angioplasty to thrombolytic therapy in terms of adverse events and mortality reduction, both in the short term and in the long term. Overall, primary PCI was associated with significant reductions in death, recurrent MI, reinfarction, and the combined endpoint of death, MI, and stroke. [50]
Subsequent studies showed the importance of rapid reperfusion. Rathore et al, in a prospective cohort study of 43,801 patients enrolled in the ACC National Cardiovascular Data Registry in 2005-2006, found that any delay in primary PCI after a patient with STEMI arrives at the hospital is associated with higher mortality. [51]
In this study, longer door-to-balloon times were associated with a higher adjusted risk of in-hospital mortality, in a continuous nonlinear fashion (30 min = 3%, 60 min = 3.5%, 90 min = 4.3%, 120 min = 5.6%, 150 min = 7%, 180 min = 8.4%). [51] A reduction in door-to-balloon time from 90 minutes to 60 minutes was associated with a 0.8% reduction in mortality, and a reduction from 60 minutes to 30 minutes was associated with a 0.5% reduction in mortality.
Brodie et al, analyzing the CADILLAC (Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications) trial and the HORIZONS-AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) trial, found that a door-to-balloon time of less than 90 minutes was associated with a lower mortality in patients with STEMI; however, the benefit was primarily noted in patients who presented with less than 90 minutes of symptoms. [52]
In this study, a door-to-balloon time shorter than 90 minutes was associated with similar relative risk reductions in high-risk and low-risk patients, though the absolute benefit was greatest in high-risk patients. [52]
The salient recommendations from the 2013 update of the ACCF/AHA STEMI guidelines, which were written in collaboration with the PCI guideline writing group, are as follows [2] :
-
Emergency medical services should transport patients directly to a PCI-capable hospital for primary PCI, with an ideal goal of a first medical contact (FMC)-to-device time of 90 minutes or less
-
Non–PCI-capable hospitals should immediately transfer patients to a PCI-capable hospital, with an FMC-to-device goal of 120 minutes or less; the concept of door-in-door-out time is discussed, and whereas no specific time frame is set, it is emphasized that a time of ≤30 minutes (associated with lower in-hospital mortality), is achieved in only 11% of patients; factors to improve (shorten) treatment time for PCI-treated patients include use of prehospital electrocardiography (ECG) to diagnose STEMI, emergency physician activation of the PCI team, use of a central paging system to activate the PCI team, and establishing a goal of having the PCI team arrive in the catheterization laboratory within 20 minutes of being paged
-
Primary PCI is indicated (class I) in patients with ischemic symptoms < 12 hours and contraindications to thrombolytic therapy (irrespective of the time delay from FMC), patients with cardiogenic shock, and patients with acute severe heart failure (irrespective of the time delay from MI onset); primary PCI is reasonable (class IIa) in patients with ongoing ischemia 12-24 hours after symptom onset
When thrombolytic therapy is used as the primary reperfusion strategy in a non–PCI-capable facility, the goal remains administration of such therapy within 30 minutes of hospital arrival. Whereas a great deal of research has been devoted to comparing primary PCI, facilitated PCI, and thrombolytic strategies, the guidelines emphasize that “the appropriate and timely use of some form of reperfusion therapy is likely more important than the choice of therapy.”
The use of thrombolytic therapy followed by referral for intentional PCI (facilitated PCI) has not been shown to be superior to primary PCI and may actually worsen outcomes, with increased risk of stroke and bleeding (ASSENT 4). However, urgent transfer to a PCI-capable hospital for coronary angiography and possible “rescue PCI” is reasonable for STEMI patients with failed reperfusion or reocclusion after thrombolytic therapy. [2] Indeed, the term facilitated PCI is now considered obsolete.
The recommended strategy for thrombolysis is a full dose of a thrombolytic, aspirin, clopidogrel, and immediate transfer to a PCI-capable facility.
On the basis of the OAT (Occluded Artery Trial) data, delayed PCI of a totally occluded infarct artery more than 24 hours after STEMI should generally not be performed in most asymptomatic patients. [53]
PCI of a noninfarct artery at the time of PCI in patients without hemodynamic compromise is classified as a “class III – harm” recommendation and should not be performed.
Trials are planned that will assess the risks and benefits of complete revascularization at the time of STEMI. The treatment of non–infarct-related artery in STEMI and cardiogenic shock remains a controversial area, with some evidence of benefit for revascularization. [54]
Current STEMI guidelines recommend the use of a GPIIb/IIIa inhibitor (class IIa abciximab, tirofiban or eptifibatide) at the time of primary PCI in selected patients who are receiving unfractionated heparin (those who have a large thrombus burden or inadequate P2Y12 receptor antagonist loading). Routine use of GPIIb/IIIa inhibitors with bivalirudin is not recommended and may be considered as an adjunctive or “bailout” strategy in selected cases.
Intracoronary abciximab administration, in comparison with the intravenous (IV) standard route, can improve short-term clinical outcomes in patients with STEMI undergoing primary PCI.
A pooled analysis of individual data of 1198 patients enrolled in five trials showed that intracoronary abciximab administration, as compared with IV abciximab, significantly reduced the risk of the composite of death and reinfarction and death. After correction for baseline differences, there were no significant differences in target vessel revascularization or the risk of reinfarction. [55]
However, most of the evidence for these drugs was obtained in the era before early dual antiplatelet therapy (DAPT). A later randomized trial using bivalirudin and either prasugrel or clopidogrel in 452 patients with an anterior STEMI reported an improvement in infarct size (17.9% vs 15.1%) with intracoronary abciximab use. [56]
Stone et al studied the safety and efficacy of DESs and BMSs in 3006 patients with STEMI who underwent primary PCI. [19] Patients were assigned in a 3:1 ratio to receive paclitaxel-eluting stents or otherwise identical BMSs. The paclitaxel-eluting stents significantly reduced angiographic evidence of restenosis and recurrent ischemia necessitating repeat revascularization at 12-month follow-up. The rates of death and stent thrombosis were similar for the two groups.
The STEMI guidelines recommend 1 year of P2Y12 inhibitor therapy for patients who receive a BMS or a DES with clopidogrel, prasugrel, or ticagrelor.
No-reflow
From a procedural perspective, because primary PCI involves a thrombotic plaque, there is a potential for thrombotic complications including no-reflow and distal embolization. In these patients, there is some evidence that stenting plus GPIIb/IIIa inhibition will improve outcomes, as well as reduce target vessel revascularization and MI rates.
An analysis of 291,380 patients with AMI who underwent PCI of native coronary artery stenoses showed that no-reflow developed in 2.3%. Risk factors included older age, STEMI, prolonged interval from symptom onset to admission, and cardiogenic shock. [57] Angiographic factors associated with no-reflow included longer lesion length, class C lesions, bifurcation lesions, and impaired preprocedural TIMI flow. No-reflow was associated with greater in-hospital mortality. The authors concluded that no-reflow, though uncommon, is associated with adverse clinical outcomes.
Of interest has been the recognition that failure of complete reperfusion based on myocardial blush grade or incomplete ST-segment resolution (~50 % of patients with primary PCI) is associated with poorer outcomes despite normal epicardial flow. Efforts to reduce distal embolization using several strategies have been developed. Despite early promise from mechanical aspiration devices, intracoronary GPIIb/IIIa inhibitor use, and stent-based exclusion (Mesh Guard), none of these approaches has been proved to offer definitive benefit.
Society of Interventional Radiology Guidelines
Thrombotic and Bleeding Risk Clinical Practice Guidelines (2019)
The Society of Interventional Radiology released recommendations on the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions in August 2019. [120, 121]
A multidisciplinary team (cardiology, hematology, or vascular or internal medicine) approach is recommended for planning optimal periprocedural management in patients at high risk for thromboembolic or bleeding events.
Screening coagulation laboratory testing is not routinely recommended for patients with minimal bleeding risk factors who are undergoing procedures with low bleeding risk, but it may be considered for patients receiving warfarin or unfractionated heparin (UFH) or for those with an inherently higher risk of bleeding. Suggested laboratory value thresholds are as follows:
-
Correct the international normalized ratio (INR) to within a range of 2.0 to 3.0 or less
-
Consider platelet transfusion if the platelet count is below 20 × 109/L
-
For low bleeding risk procedures requiring arterial access, the recommended INR threshold is less than 1.8 for femoral access and below 2.2 for radial access
Obtain appropriate preprocedural coagulation testing for patients undergoing procedures with high bleeding risk. Activated partial thromboplastin time is no longer recommended. Suggested laboratory value thresholds are as follows:
-
Correct the INR to within a range of 1.5 to 1.8 or less
-
Consider platelet transfusion if the platelet count is below 50 × 109/L
In patients with chronic liver disease, judicious use of transfusion of plasma and platelets is recommended owing to the potential for increased portal pressure and transfusion-related adverse events. For patients with chronic liver disease undergoing an invasive procedure, consider adjusting the INR threshold higher and the platelet count threshold lower than in the general population to minimize unnecessary transfusions. It may be useful to measure the fibrinogen level; if it is low, replace with cryoprecipitate.
The guidelines also include a table with management recommendations for nearly two dozen specific anticoagulant and antiplatelet agents.
For more information, please go to Venous Thromboembolism (VTE), Deep Venous Thrombosis (DVT), International Normalized Ratio (INR) Targets: Venous Thromboembolism, and Perioperative Anticoagulation Management.
For more Clinical Practice Guidelines, please go to Guidelines.
-
Percutaneous transluminal coronary angioplasty (PTCA). Rotational atherectomy catheter (Rotablator) is designed for removal of plaque from coronary arteries. This device has diamond-studded burr at its tip, rotates at about 160,000 rpm, and is particularly well suited for ablation of calcific or fibrotic plaque material.
-
Percutaneous transluminal coronary angioplasty (PTCA). TRISTAR stent.
-
Percutaneous transluminal coronary angioplasty (PTCA). NIR stent.
-
Percutaneous transluminal coronary angioplasty (PTCA). Wallstent.
-
Example of intravascular ultrasonography (IVUS) image in percutaneous transluminal coronary angioplasty (PTCA).
-
Mechanism of restenosis following percutaneous transluminal coronary angioplasty (PTCA).
-
Fractional flow ratio (FFR). Pressure wire is advanced across left anterior descending (LAD) artery stenosis, and intracoronary adenosine is given. FFR ratio is recorded at baseline and then after adenosine push is given. Here, LAD lesion and FFR post adenosine are shown.