Practice Essentials
In approximately 1/3 of patients with epilepsy, seizures persist despite adequate trials of several antiepileptic drugs (AEDs). Surgery can provide seizure freedom for some patients with seizures resistant to AEDs.
Criteria for surgical intervention
A candidate for epilepsy surgery must have not attained acceptable seizure control with sufficient trials of AEDs and must have a reasonable chance of benefiting from surgery. The American Academy of Neurology, the American Association of Neurological Surgeons, and the American Epilepsy Society have recommended the following practice parameters [1] :
-
Patients with disabling complex partial seizures, with or without secondarily generalized seizures, who have failed appropriate trials of first-line AEDs, should be considered for referral to an epilepsy surgery center, though criteria for failure of drug treatment have not been definitely established (level A)
-
Patients referred to an epilepsy center for the reasons stated above who meet established criteria for anteromedial temporal resection (AMTR) and who will accept the risks and benefits of this procedure, as opposed to continuing pharmacotherapy, should be offered surgical treatment (level A)
Preoperative assessment and surgical strategy
Modalities used for evaluation of seizures include the following:
-
Neuroimaging – Skull radiography, CT, MRI, positron emission tomography (PET), single-photon emission CT (SPECT), and magnetoencephalography/magnetic source imaging (MEG/MSI)
-
Electroencephalography (EEG; the single most useful test in epilepsy diagnosis)
-
Neuropsychological (neurocognitive) testing
-
Intracarotid amobarbital (Wada) test
-
Invasive intracranial monitoring (eg, intracranial EEG recording, or chronic electrocorticography [ECoG])
The surgical strategy may be either definitive or palliative, as follows:
-
Definitive – The aim is to produce complete, or at least 70-90%, improvement in seizures; in general, these procedures physically remove seizure-producing cortex from the brain
-
Palliative – The aim is to decrease seizure frequency (seizure freedom is rare); these procedures usually disrupt pathways involved in seizure production and propagation or attempt to disrupt seizures with the use of electrical stimulation
Surgical techniques
The following 4 procedures may be considered for the surgical treatment of epilepsy:
-
Anteromedial temporal resection (AMTR) – This is the most commonly performed procedure, with the clearest indications and best results
-
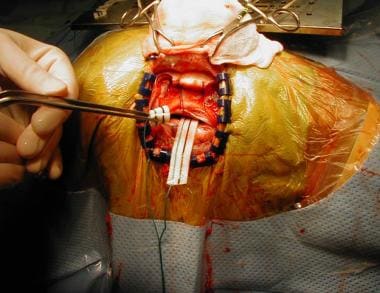
Corpus callosotomy – The aim of this procedure is to disrupt 1 or more major central nervous system (CNS) pathways used in seizure generalization, thus reducing the frequency and severity of primary or secondary generalized seizures (see the image below)
-
Multiple subpial transection (MST) – The aim of this nonresective procedure is to abolish epileptiform discharges and correlative seizures from epileptogenic cortex by disrupting intracortical synchronization and thereby (theoretically) reducing or eliminating the epileptogenic potential of the seizure focus
-
Functional hemispherectomy – In this procedure, the cortex is disconnected from all subcortical structures, and the interhemispheric commissures are divided, but the brain remains in place
Postoperative complications that may occur include the following:
-
AMTR – Hemiparesis, visual field deficit, infections, cranial nerve palsy, fever, verbal deficits and memory problems
-
Corpus callosotomy – Hydrocephalus, aseptic meningitis, bleeding from the superior sagittal sinus, frontal lobe edema, venous infarction, and air embolism
-
Functional hemispherectomy – Hemogenic meningitis, ventriculitis, cerebrospinal fluid leakage, and hydrocephalus; less commonly, stroke, infection, coma, and postoperative hemorrhage
Overview
Background
Epilepsy is one of the most common neurologic diseases, with a worldwide prevalence of approximately 0.8%. In developing countries, antiepileptic drugs (AEDs) are the mainstay of treatment. Nevertheless, many patients with epilepsy never receive treatment that leaves them seizure free. Epilepsy surgery is underutilized in developed, and especially in developing countries, whether because of a lack of resources or because many physicians do not recognize that a treatable syndrome exists.
This article addresses some of the most common surgical procedures performed to treat epilepsy and explains the syndromes for which they are most useful. The type of evaluation required to prepare a patient for epilepsy surgery is discussed. Common procedures and imaging modalities used to aid in the diagnosis and localization of epilepsy before epilepsy surgery are also discussed. Advanced diagnostic techniques—in particular, intracranial electrode placement—are covered in some technical detail.
The following 4 operative procedures are presented as models on which a preliminary understanding of the various surgical strategies can be gained:
-
Anteromedial temporal resection (AMTR) - This is the most frequently performed operation for a common and well-described disorder—namely, medial temporal lobe epilepsy. It serves as a model for other focal resections.
-
Corpus callosotomy - This is the only surgical procedure that is applicable to generalized epilepsy syndromes.
-
Functional hemispherectomy (hemispherotomy)
-
Multiple subpial transection (MST) - This has limited applications and is being used less in recent years.
For patient education resources, see the Brain and Nervous System Center, as well as Epilepsy.
Reasons for considering surgical intervention
Although intracranial surgery involves inherent risks, these risks do not equal the risks of uncontrolled seizures. The morbidity and mortality of seizures include the following:
-
Accidental injury
-
Cognitive decline
-
Sudden unexplained death in epilepsy (SUDEP)
-
Psychological, social, and vocational impairment
Accidental injuries commonly include fractures, burns, dental injuries, lacerations, and head injuries. Cognitive decline and memory loss over time has been demonstrated to occur in patients with certain epilepsy syndromes who have recurrent convulsive seizures or episodes of status epilepticus.
Mortality rates for patients with nonconvulsive and convulsive seizures far exceed those for age-matched controls. Among patients with poorly controlled epilepsy, SUDEP can reach a rate of 1 death per 500 patients per year. Risk factors are poorly controlled seizures and low serum antiepileptic drug (AED) levels.
Both depression and anxiety are very common among patients with medically refractory epilepsy. Intractable epilepsy prevents driving, and reduces fertility and marriage rates. Vocational issues include inability to be employed or, if employed, underemployment.
The above factors clearly suggest that for certain forms of epilepsy, continued medical therapy after failure to control seizures with aggressive trials of AEDs is not optimal treatment. In several retrospective trials and 2 prospective, randomized, controlled trials for a well-defined syndrome with a known favorable surgical outcome (ie, mesial temporal lobe epilepsy), the efficacy of surgery significantly exceeds that of continued treatment with AEDs and the morbidity and mortality associated with surgical treatment has been demonstrated to be less than that associated with the disorder. [2, 3, 1]
In addition, surgery yields a better quality of life and reduced depression and anxiety as soon as 3 months after AMTR, compared with continued medical therapy. [3] This improved quality of life is specifically related to the occurrence of complete seizure freedom in both the medical and surgical study groups.
Criteria for surgical intervention
A candidate for epilepsy surgery must have not attained acceptable seizure control with sufficient trials of AEDs and must have a reasonable chance of benefiting from surgery. Adequate AED trials must be considered within the context of the patient’s circumstances and form of epilepsy. The precise numbers and types of AEDs that should be tried before surgery is recommended are unknown for the various epilepsy syndromes.
However, the American Academy of Neurology, the American Association of Neurological Surgeons, and the American Epilepsy Society have recommended the following practice parameters [1] :
-
Patients with disabling complex partial seizures, with or without secondarily generalized seizures, who have failed appropriate trials of first-line antiepileptic drugs, should be considered for referral to an epilepsy surgery center, although criteria for failure of drug treatment have not been definitely established (level A rating).
-
Patients referred to an epilepsy center for the reasons stated above who meet established criteria for an anteromedial temporal lobe resection and who will accept the risks and benefits of this procedure, as opposed to continuing pharmacotherapy, should be offered surgical treatment (level A rating).
In 1996, Engel emphasized that the strategy of trying all combinations of drugs is not an acceptable approach to patients with syndromes for which the chances of benefiting from surgical therapy are known to be excellent. [4] For example, with the advent of 9 completely new AEDs since 1993, an estimated 300 years would be required to try all medications in all combinations.
Far more important, subsequent studies of patients with new-onset seizures have shown that only 64% have seizure freedom by the time they try their third AED. [5] Thus, after 3 different AEDs have failed to control seizures, more than 35% of patients continue to have seizures. Therefore, the decision to proceed with surgery must take into consideration both the chance of seizure freedom with additional AED trials and the adverse long-term effects of uncontrolled seizures.
Strategy for surgical planning
Preoperative evaluation for epilepsy has changed substantially in the past few decades, most notably since the advent of long-term video-electroencephalographic (EEG) monitoring in the late 1970s, advanced neuroimaging, and subspecialty epilepsy centers. The preoperative evaluation requires input from many members of an integrated team, which includes neurologists, neurophysiologists, neuropsychologists, social workers, radiologists, nurses, and epilepsy neurosurgeons.
Key aspects of the evaluation include the patient’s history and physical examination findings, social circumstances, seizure syndrome and severity, and diagnostic testing. A surgical plan is usually developed at a multidisciplinary team conference. This allows open discussion among multiple experts so that the surgical approach is unique and is tailored to the individual’s personal needs and epilepsy syndrome.
When all preoperative information points to a unifying location and theory regarding focal seizure onset (also referred to as concordant data), then the patient may proceed directly to resective surgery. When data are inadequate to define a resective strategy, then diagnostic intracranial electrodes may be considered to further define the syndrome or site of seizure onset before any resective surgery.
The concept that epilepsy consists of a focus that can be removed has evolved into a more unified theory in which the neural network, environment, genetic predisposition, and epileptogenic substrate all must be considered during the evaluation of the patient with epilepsy, if surgery is to be effective. [6, 7]
Relevant Anatomy
The brain is composed of 3 main structural divisions: the cerebrum, the brainstem, and the cerebellum. At the base of the brain is the brainstem, which extends from the upper cervical spinal cord to the diencephalon of the cerebrum. The brainstem is divided into the medulla, pons, and midbrain. Posterior to the brainstem lies the cerebellum.
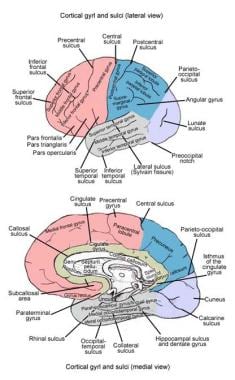
The cerebrum is the largest component of the brain. It is divided into right and left hemispheres. The left and right cerebral hemispheres are separated by the longitudinal cerebral fissure. The principal connection between the 2 hemispheres is the corpus callosum. Each cortical hemisphere can be divided into 4 lobes: frontal, temporal, parietal, and occipital. The frontal lobe can be distinguished from the temporal lobe by the lateral sulcus (Sylvian fissure). The frontal lobe can be distinguished from the parietal lobe by the central sulcus (Rolandic fissure). The parieto-occipital fissure, which is visible on the medial aspect of the hemisphere, divides the parietal and occipital lobes. Within the lateral sulcus is another cortical surface referred to as the insula.
The frontal lobe can then be further divided into the superior, middle, and inferior frontal gyri, which are divided by the superior and inferior frontal sulci, respectively. Similarly, the temporal lobe is divided into the superior, middle, and inferior temporal gyri, which are separated by the superior and inferior temporal sulci. Within the parietal lobe, the superior temporal sulcus is capped by the angular gyrus. Just above this, the lateral sulcus is capped by the supramarginal gyrus. Just below the angular gyrus, the lateral occipital gyrus caps the inferior temporal sulcus. See the image below.
For more information about the relevant anatomy, see Brain Anatomy. See also Arteries to the Brain and Meninges, Central Nervous System Anatomy, and Facial Nerve Anatomy.
Indications
Anteromedial temporal resection
Indications for AMTR include the following:
-
Complex partial seizures with semiology typical of mesial temporal lobe epilepsy
-
Magnetic resonance imaging (MRI) evidence of unilateral hippocampal atrophy and increased T2-weighted signal (at some centers, volumetric measurements of the hippocampus are routinely obtained)
-
Unilateral temporal lobe hypometabolism on positron emission tomography (PET) scans if MRI findings are nonlesional in nature
-
EEG confirmation that seizures begin over the temporal area ipsilateral to the hippocampal atrophy or PET scan evidence of hypometabolism
Although AMTR for mesial temporal lobe epilepsy has now been shown to be more effective than continued medication, studies to date have included patients who have had epilepsy that has been refractory to medical treatment for a prolonged period. Most patients who are finally referred for AMTR have had epilepsy for approximately 20 years.
Currently, however, because continued complex partial and generalized tonic-clonic seizures have such negative effects on vocational, educational, social, cognitive, and psychological areas and because these seizures can cause injury and even sudden death, most epileptologists believe that AMTR should be offered to good surgical candidates earlier.
An issue that remains unclear is how much time must elapse, or how many AEDs must be found to be inadequate, before surgery is recommended. For this reason, the US National Institutes of Health sponsored a large prospective study, the Early Randomized Surgery for Epilepsy Trial (ERSET). ERSET was designed to compare AMTR against 2 additional years of aggressive AED management. The results of ERSET should help address this issue.
Corpus callosotomy
Aside from the requirement that the patient must experience medically refractory seizures, indications for corpus callosotomy have not been clearly defined. Moreover, in contrast with AMTR for complex partial seizures, there are no clear and consistent indicators that help identify patients likely to benefit from corpus callosotomy.
Overall, corpus callosotomy seems to lessen the frequency of primarily and secondarily generalized seizures (ie, tonic, clonic, tonic-clonic, and atonic seizures). Callosotomy significantly improves atonic seizures, but having atonic seizures does not guarantee that a patient will benefit from surgery. Complex partial seizures can be ameliorated somewhat, but the results are far more capricious.
Some epileptologists believe that people with epilepsy who have mental handicaps should not be considered for callosotomy, because it seldom renders patients seizure free and because these patients may benefit less than patients of normal intelligence. The presence or absence of mental handicaps is not a reliable predictor of outcome. The authors have personally observed gratifying results from callosotomy in patients with mental handicaps.
The goals of corpus callosotomy differ from those of resective surgery, in which a seizure-free outcome is more likely and expectations are higher. The usual aim of callosotomy is to reduce seizure frequency and associated morbidity. The additional goals of social or vocational rehabilitation, applicable to resective surgery, are often not realistic expectations after callosotomy.
Multiple subpial transections
The most effective surgical treatment of partial (focal) seizures has been removal of the seizure-producing cortex from the brain. However, this procedure cannot be performed if the seizure-producing cortex also serves an indispensable function, such as speech. Consequently, MST is the only acceptable surgical treatment of a focus within such cortex.
In 1989, Morrell et al reported on the use of MST to treat seizures from chronic (Rasmussen) encephalitis that affected the speech-dominant hemisphere. [8] The authors applied MST to speech and motor cortex and resected or disconnected the remainder of the hemisphere. Their results in these cases were not good, and they have since abandoned this indication.
In 1997, Patil et al reported on the application of MST to bilateral seizure foci in very difficult cases otherwise not considered for surgical intervention. [9]
MST has been used as surgical treatment of Landau-Kleffner syndrome (LKS), a form of acquired aphasia that is associated with epileptiform spiking (but not necessarily seizures) in the central neocortex.
Functional hemispherectomy
Functional hemispherectomy (often referred to as hemispherotomy in its current refined forms) is highly effective for treating epilepsy in well-selected candidates with catastrophic epilepsy. Candidates are persons who have injury and seizures limited to 1 hemisphere of the brain. Their seizures occur frequently enough to interfere with cognition and impair quality of life. The goal of surgery is to isolate the affected brain from the healthier hemisphere and thus allow the latter to function without the burden of seizures or interictal discharges. [10]
Most patients upon whom hemispherectomy has been performed have been children, though some previous reports include adults. [11] Etiologies of the catastrophic epilepsies leading to hemispherotomy include Rasmussen encephalitis, other encephalitides, prenatal ischemia (often with porencephaly), Sturge-Weber syndrome, cortical dysplasia, hemiconvulsion-hemiplegia-epilepsy (HHE) syndrome, hemimegalencephaly, malignancy, and tuberous sclerosis.
Preferentially, the pathology is limited to a single hemisphere of the brain; recovery after surgical treatment depends on the remaining hemisphere’s ability to take over cognitive and language functions that may have previously been within the domain of the injured hemisphere.
Delaying surgery can lead to a decline in cognitive ability; accumulating evidence suggests that seizures themselves may delay cognitive development. [12, 10] The timing of surgery depends upon the severity of the seizures, the natural history of the illness affecting the patient, and the response to antiepileptic drug treatments.
When the syndrome presents early in life, many centers recommend proceeding with surgery rapidly to avoid loss of cognitive abilities due to an epileptic encephalopathy, which may impair learning and cognition. [13, 14, 15] When it presents later in life, the timing of surgery can be more controversial. However, there is evidence that patients with late-onset syndromes can respond to surgery with improvements in neurologic function as well, suggesting that plasticity after hemispherectomy is not limited to early childhood. [16]
Classification and Preoperative Diagnosis of Epilepsy
A preoperative diagnosis is made after a classification of the patient’s seizure types and specific epilepsy syndrome. The International League Against Epilepsy (ILAE) recognizes approximately 10 types of recurrent seizures and approximately 40 forms of epilepsy syndromes. Both classification schemes are based on the idea that seizures and epilepsies naturally fall into 2 major groups, based on the site of seizure onset in the brain: (1) partial (focal, localization-related) and (2) generalized. [17, 18]
The signs and symptoms (semiology) experienced by the patient and the EEG pattern recorded at ictal onset determine the seizure diagnosis. This process begins by recording a careful history. For example, an event that is initiated with a blank stare and arrest of motion and then progresses to the development of automatisms (ie, automatic repetitive semipurposeful movements) is likely a complex partial seizure.
For initial AED therapy, a presumptive diagnosis based on the history may suffice. Even under the best circumstances, however, a diagnosis based solely on the history can be incorrect. Therefore, when surgical therapy is being considered for intractable epilepsy, the most accurate way to determine the epilepsy syndrome diagnosis and brain location of seizure origin is to use long-term video-EEG (VEEG) monitoring.
The following is a simplification of the international classification of epileptic seizures:
-
Partial seizures (seizures that begin locally) - These include (1) simple partial seizures (consciousness not impaired; SPS), (2) complex partial seizures (consciousness impaired; CPS), and (3) partial seizures that secondarily progress to generalized tonic-clonic seizures (SGTCS).
-
Generalized seizures (seizures that arise diffusely) - These include absence seizures, atypical absence seizures, clonic seizures, tonic seizures, tonic-clonic seizures, myoclonic seizures, and atonic seizures.
-
Unclassified seizures
An epilepsy syndrome diagnosis combines the seizure type with its associated MRI, physical examination, genetic, and other features. For example, if a seizure (1) has correlative EEG epileptiform patterns (interictal spikes or sharp waves) and ictal discharges over the right temporal lobe, (2) occurs in a patient who had a febrile seizure as a child but no family history of epilepsy, and (3) is associated with ipsilateral atrophy and increased signal of the hippocampus on an MRI, it is likely a complex partial seizure of right mesial temporal lobe epilepsy.
The greatest value of a syndrome diagnosis is to provide a prognosis. In the above example, if the patient is right-handed with normal intelligence, he or she has excellent odds of becoming seizure free after right-sided AMTR.
A simplification of the previous international classification of the epilepsy syndromes has been proposed. [19, 18]
Focal (partial, localization-related) syndromes include the following:
-
Idiopathic (some are hereditary) - Benign childhood epilepsy with centrotemporal spikes, childhood epilepsy with occipital paroxysms, autosomal dominant nocturnal frontal lobe epilepsy, and familial temporal lobe epilepsies
-
Symptomatic (to known cause or lesion) - Temporal lobe epilepsies (mesial, lateral), frontal lobe epilepsies (several locations), parietal lobe epilepsies, occipital lobe epilepsies, and Rasmussen encephalitis
Generalized syndromes include the following:
-
Idiopathic (most are hereditary) - Benign neonatal familial convulsions, childhood absence epilepsy, juvenile absence epilepsy, juvenile myoclonic epilepsy, generalized epilepsy and febrile seizures plus, and various progressive myoclonic epilepsies
-
Symptomatic (or probably symptomatic) - West syndrome (infantile spasms) and Lennox-Gastaut syndrome
Mixed syndromes include continuous spike waves in slow sleep and acquired epileptic aphasia (Landau-Kleffner syndrome [LKS]). Special situations is also a category.
Structural and Metabolic Brain Imaging
Because seizures may result from cortical lesions or malformations, neuroimaging can often help identify and localize this damage and, therefore, the site of seizure onset. Imaging modalities include the following:
-
Skull radiography
-
Computed tomography (CT) scanning
-
MRI
-
PET
-
Single-photon emission tomography (SPECT)
-
Magnetoencephalography/magnetic source imaging (MEG/MSI)
Routine skull films are of little value in this setting. Routine CT scanning has largely been replaced by MRI because the latter provides superior images. The single exception to this generalization is that CT scanning demonstrates intraparenchymal calcium and acute bleeding better than MRI; this may be helpful in distinguishing certain types of tumors or central nervous system (CNS) syndromes (eg, tuberous sclerosis).
Brain MRI is unquestionably the best structural imaging study. Every surgical evaluation should include a complete brain MRI study with special thin-cut coronal magnified views perpendicular to the axis of the temporal horn. These views can demonstrate mesial temporal sclerosis.
Unlike MRI or CT scanning, PET scanning demonstrates brain glucose metabolism rather than structure. The typical finding from an interictal scan is hypometabolism in the region of the epileptic focus; if the scan is obtained during a seizure, the typical finding is hypermetabolism from the focus.
SPECT scanning helps visualize blood flow through the brain and, therefore, has been evaluated as another method for localizing the epileptic focus. Interictal SPECT scans are less accurate than ictal scans. However, ictal scans are problematic because the tracer must be injected within the initial seconds of seizure onset. This requires that the radionucleotide be available on the monitoring ward 24 hours per day along with personnel licensed (under state law) to administer intravenous injections.
Subtraction ictal-interictal SPECT co-registered to MRI (SISCOM) is a newer modality that is more accurate than either ictal or interictal SPECT scanning. Scans are obtained during an interictal period (at least 48 h apart, to accommodate radionucleotide washout) and within seconds of seizure onset. They are then subtracted from one another with specialized computer software. This method gives a better indication of the cortical area of ictal onset. The subtracted scan can then be co-registered onto the patient’s MRI to provide support for the location of the focus.
MEG/MSI is a noninvasive imaging technique that is based on the brain’s ability to produce small magnetic dipoles with neuronal discharges. Synchronous firing of large groups of neurons, as occurs in an interictal epileptiform discharge, creates a miniscule magnetic dipole that can be sensed with sophisticated imaging equipment and complicated computer analysis. This map of the epileptiform discharge can be useful for diagnostic purposes and for planning preoperative intracranial electrode placement.
Electroencephalographic Evaluation
EEG is the single most useful test in epilepsy diagnosis. An essential mistake is to place too much value on an isolated individual interictal recording. The assumption that a normal finding from the interictal scalp electrode EEG precludes the diagnosis of epilepsy is erroneous. The presence or absence of epileptiform discharges is highly variable, has no relationship to seizure frequency (for most epilepsies), and may be affected by AEDs. Thus, EEGs may have to be repeated several times before epileptiform discharges are observed.
By definition, epileptiform discharges are interictal patterns that include spikes, spike-and-slow-wave complexes, sharp waves, and sharp-and-slow-wave complexes. The reader should refer to the second international glossary of EEG terms for precise definitions. [20]
When the waking scalp EEG fails to demonstrate evidence of epilepsy but the diagnosis is still suspected, a sleep EEG is recommended. Epileptiform discharges commonly activate during non–rapid eye movement sleep in some epilepsies. On fewer occasions than in the past, sphenoidal and additional extracranial electrodes are used to help reveal epileptiform (interictal) and ictal discharges. [21]
Although the standard scalp EEG is helpful in making a diagnosis of epilepsy, it is not usually used when the physician makes major surgical decisions. This is because the distribution of interictal EEG discharges may not correctly localize epileptic foci. This error occurs for several reasons. First, discharges can be multifocal, although a single focus can be the origin of all seizures.
Furthermore, because the EEG consists of volume-conducted potentials that originate over a relatively large area of cortical gray matter, some discharges can shift apparent location within or between hemispheres, and others may appear widely or even diffusely over the scalp. In addition, to obtain the most accurate data possible, recording sufficient numbers of the patient’s typical seizures is important.
Like interictal discharges, ictal discharges vary somewhat. Also, more than 1 seizure focus or psychogenic or physiologic nonepileptic seizure may be found when numerous episodes are recorded. [22, 23] The latter may greatly affect a decision to proceed with surgery. Therefore, all surgical candidates should undergo long-term VEEG monitoring before surgery to record several typical seizures.
Features of the scalp EEG ictal discharge, other than just location, can be helpful in the preoperative evaluation. In 1 study, for example, the authors reported that the frequency of the initial ictal discharge in the scalp EEG correlated with the degree of hippocampal pathology in temporal lobe epilepsy. [24]
Combining EEG with functional MRI in patients with focal epilepsy may help localize the epileptic foci. [25]
Neuropsychological Testing
Neuropsychological testing, also known as neurocognitive testing, is the process of empirically testing an individual’s functioning across a variety of cognitive domains, including attention, concentration, language, visuospatial skills, verbal and visual memory, and executive abilities (ie, problem solving, organization, strategic planning), as well as personality and emotional functioning.
Neuropsychologists use a variety of standardized tests to assess all areas of cognitive functioning. An individual’s performance on these standardized tests is then analyzed against normative data, and their performance is compared with that of their peers and with what is expected on the basis of their estimated premorbid level of cognitive functioning. Patterns of cognitive strengths and weaknesses then provide information about brain-related deficits in cognitive functioning.
The idea that performance on specific neuropsychological tests is subserved by various areas of the brain is well established. For example, a right-handed individual’s performance on verbal memory tests (eg, story memory, verbal paired associates learning, and list-learning tasks) is largely subserved by left mesial temporal structures. [26] Similarly, a right-handed individual’s performance on visual memory tests (eg, figural memory and complex figure memory tasks) is largely subserved by right mesial temporal structures.
Thus, an individual’s cognitive strengths and weaknesses on neuropsychological testing can indicate areas of brain dysfunction, which provides additional information that can help localize an epileptogenic focus. [27] In addition, an individual’s cognitive strengths can provide information about possible cognitive risks involved in undergoing a neurosurgical resection to treat medically intractable epilepsy because a risk of increased memory deficits after resection of mesial temporal structures exists. [28]
Routinely, all epilepsy surgical candidates undergo extensive neuropsychological testing. Neuropsychological testing is tailored so as to best assess the type of epilepsy with which each individual presents, often focusing heavily on the area of cognitive functioning that likely has been most affected by a hypothesized epileptogenic focus. As a result, such testing often varies considerably from one patient to another.
Neuropsychological testing can also be used in nonoperative or postoperative epilepsy patients to assess their level of cognitive functioning and thereby assist with vocational and cognitive rehabilitation in the context of their neurologic disorder.
Intracarotid Amobarbital (Wada) Test
The intracarotid amobarbital test was developed by Jun Wada as a means of preoperatively determining which hemisphere contains language function. Although it is still used for this purpose, functional MRI for language now provides a noninvasive and more accurate way of lateralizing and localizing language functioning. The primary use of the Wada test is to assess language lateralization and the ability of the contralateral mesial temporal structures to support memory postoperatively when AMTR is being considered to treat medically intractable epilepsy.
The Wada test is conducted by individually cannulating each internal carotid artery. After contrast arteriography verifies that blood flows to the corresponding hemisphere and not to the brainstem or the contralateral side, a dose of sodium amobarbital (sufficient to impede hemispheric function) is injected. If the drug produces a contralateral hemiparesis, the function of that hemisphere is assumed to be minimized.
Language lateralization is determined by conducting a comprehensive screen of various components of language, including expressive language, receptive language, naming, repetition, and complex syntactical comprehension. If an individual is able to engage in all aspects of language while hemiparetic, language function is assumed to not be represented within that hemisphere.
Assessing a variety of aspects of language is important because some individuals possess a bilateral representation of language—that is, some aspects of language are subserved by one hemisphere, whereas other aspects of language are subserved by the contralateral hemisphere.
Memory functioning is assessed by presenting the patient with visual and auditory items (often using multiple sensory modalities to maximize the chance of encoding) while the hemisphere is anesthetized. After recovery of hemispheric function, which is confirmed by return of gross and fine motor function, as well as language if possible, the patient is then tested for free, cued, and recognition recall of the items with which he or she was presented during anesthetization.
If the patient can accurately recall 75% of the items presented during anesthetization, this provides evidence that the contralateral hemisphere should be able to support memory after AMTR of the hemisphere that was anesthetized. If the patient cannot accurately recall a sufficient number of items, this provides evidence that the patient may experience significant memory difficulties postoperatively.
Importantly, patients should undergo neuropsychological testing before undergoing a Wada test because neuropsychological testing provides important information about their baseline level of memory functioning and more accurately informs the results of the Wada test.
The deficiencies of this evaluation for memory function directly relate to the multiple problems of targeting a drug effect to specific brain structures via cerebral blood flow. Injection of a drug into the internal carotid artery does not assure drug effect in the basal temporal area in general or the hippocampal region specifically (an area that subserve memory function). This is due to variations in the direct blood supply to the hippocampus and inequalities in delivery when the drug is injected into the bloodstream.
Invasive Intracranial Monitoring
Intracranial EEG recording, also known as chronic electrocorticography (ECoG), is an invasive procedure that is performed when noninvasive preoperative evaluation has not led to definitive localization of a seizure syndrome and surgical plan. The preoperative team, which includes a neurosurgeon, a neurologist, a neuropsychologist, a social worker, and a neuroradiologist, considers all previous accumulated data to determine an appropriate strategy for placement of intracranial electrodes.
The following are examples of situations in which invasive intracranial monitoring may be required:
-
Seizures are lateralized but not localized (eg, a left-sided, widespread frontal-temporal onset).
-
Seizures are localized but not lateralized (eg, ictal EEG patterns that appear maximally over both temporal lobes).
-
Seizures are neither localized nor lateralized (eg, stereotyped complex partial seizures with diffuse ictal changes or initial changes obscured by artifact).
-
Seizure localization is discordant with other data (eg, EEG ictal scalp data are discordant with neuroimaging [MRI, PET, SPECT/SISCOM, MEG] or neuropsychological data).
-
The relation of seizure onset to functional tissue must be determined (eg, seizures with early involvement of language or motor function).
-
The relation of seizure onset to lesion must be determined (eg, dual pathology or multiple intracranial lesions).
-
Seizures are clinically suspected but VEEG is inadequate for defining them (eg, simple partial seizures with no detectable scalp EEG ictal discharge or suspected epileptic seizures with unusual semiology that suggests psychogenic seizures [pseudo-pseudoseizures]). [29]
Invasive intracranial monitoring is a diagnostic procedure that is designed to identify the site of ictal onset of seizures. Intracranial electrodes are reserved for the most difficult of cases; therefore, one risk is that the study will end up with a nondiagnostic result. The onus is on the surgeon to question where electrodes should be placed on the basis of the information available preoperatively and to consider what other alternative diagnosis should be included or excluded to obtain the best possible electrode placement.
Depth, strip, and grid electrodes are implantable intracranial devices used to record the ECoG over an lnger period and to stimulate the cortex to determine function. Any combination of these electrodes may be employed as necessary to answer the specific questions posed by the case history and presentation specific to a given patient, depending on the needs of the patient, experience of the monitoring team, and resources available for use.
Depth electrodes are multicontact, thin, tubular, rigid or semirigid electrodes that penetrate the brain to allow recording from deep structures. Strip electrodes are linear arrays of 2-16 disk electrodes embedded in a strip of Silastic, [30] (or they can be tubular, like depth electrodes). [31] Grid electrodes are parallel rows of electrodes that can be configured in standard or custom designs according to the surgeon’s preferences and the manufacturer’s capabilities. Grid and strip electrodes are designed to be in direct contact with brain neocortex.
In most cases, electrodes are placed in the subdural space, although they may occasionally be used in the epidural space. All of these electrode types are constructed from biologically inert materials (eg, Silastic, stainless steel, platinum). Platinum electrodes are more easily seen on fluoroscopic images than are stainless steel electrodes and are compatible with MRI so that postoperative diagnostic and localizing neuroimaging studies can be obtained.
Placement of strip electrodes
Strip electrodes are used most often to lateralize the side of seizure onset in frontal and temporal lobe epilepsy, but they may also be used to obtain survey studies over all cortical surfaces of the brain. They are usually implanted while the patient is under general anesthesia, according to the preoperative plan created by the epilepsy monitoring team.
Electrodes can be directed safely over long distances within the calvaria by surfing them over the brain with a gentle fluid pulse. Fluoroscopy is used to confirm placement before closure of the wound. The electrode wires are tunneled under the skin with a 13-gauge passing needle designed for that purpose (Ad-Tech Medical; Racine, Wis); to decrease the risk of infection, they exit the skin several inches away from the burr-hole incision.
Cerebrospinal fluid (CSF) may leak from around the electrode wires during the first 3 days after implantation. This leakage can be minimized if the scalp exit sites for the electrode tails are directed superiorly toward the vertex of the skull. The suggested risk of infection from a CSF leak differs from center to center; however, completely sealing the skin with a foreign body in place is difficult, and CSF leaks are not uncommon. Because of this, the dressing is changed as often as needed.
Some authors suggest placing a cable-retaining suture in the scalp, [32, 33] both to attempt to secure the electrode and to decrease CSF leakage. The lead author has not found this to be helpful in decreasing CSF leakage, and all electrode companies manufacture quick-release connectors designed to break apart easily if tugged.
Patients are monitored with electrodes in place in the monitoring unit for 2-7 days to record their typical seizures. Subdural strip electrodes are removed through the skin without an open surgical procedure by placing gentle traction on the electrodes with the patient under conscious sedation or general anesthesia.
Although strip electrodes can be inserted epidurally, [34, 35] this practice is not advisable for routine cases because the exposure is limited to the lateral convexities of the brain. The epidural space in the temporal fossa does not allow the electrode to be advanced medially enough to record from the parahippocampal gyrus, and electrodes cannot be placed over mesial frontal lobe cortex.
In most exploratory investigations, these locations should be sampled. However, epidural placement may be the most reasonable option when recording from a patient with a prior craniotomy because scarring may obliterate the subdural space.
Placement of grid electrodes
Arrays of electrodes more than 1 column wide are considered intracranial grids (see the image below). Practically speaking, electrode arrays that are 2-3 contacts wide cannot be easily passed for any substantial distance through a burr hole and require a craniotomy for placement.
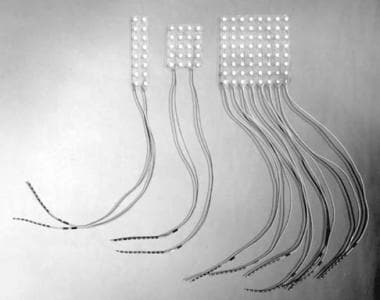 Examples of various grid electrodes available for specific needs. These range in size and number of contacts.
Examples of various grid electrodes available for specific needs. These range in size and number of contacts.
Once the decision to proceed with a craniotomy is made, grid arrays of 5-8 rows (20-64 contacts) are usually used to maximize coverage over the craniotomy site. The craniotomy site is determined based on data gathered during the preoperative evaluation; usually, a large craniotomy is performed to accommodate up to an 8 × 8-cm grid.
Prophylactic antibiotics and dexamethasone are routinely administered. Mannitol is not used unless necessary, because the putative space created by a fluid shift could adversely contribute to hematoma formation after closure.
Once placed, the grid is sutured to the dura to prevent motion. Often, 1 or more strip electrodes are added to sample adjacent areas or lobes (eg, the interhemispheric fissure or basal temporal lobe). The electrode tails of grids are tunneled in much the same way as strip electrodes (ie, toward the vertex) to prevent CSF leakage.
At some centers, the bone flap is frozen under sterile conditions until the patient returns to the operating room (OR) for grid removal. However, the author prefers to leave the bone flap in place to decrease the risk of hematoma formation at the craniotomy site.
After recovery in the postanesthesia care unit, the patient is transferred to the VEEG monitoring suite, where the patient is hooked up on the day of surgery and a formal head dressing is placed. Acute nursing care is provided in the monitoring suite for the first 24 hours after craniotomy, similar to the level practiced in a neurologic step-down unit. The grid is removed when sufficient data have been obtained to determine the site of ictal onset or, alternatively, to determine that more recording is not likely to lead to satisfactory localization.
If resective surgery is planned, the relationship of the grid to the underlying cortex must stay unchanged while the craniotomy is reopened. The dura is opened, with the grid-stabilizing sutures left intact and all relations between electrode contacts and unique underlying cortical topography (eg, blood vessels) left undisturbed. Once these relations have been documented and the surgeon has extrapolated the mapped data to the underlying cortex, the grid is removed and discarded.
Resective surgery is performed, with the neurophysiologist or pathologist present in the OR as necessary. If resection is not performed at the time of grid removal (eg, because of hemorrhage, edema, patient preference, or insufficient data), then pertinent landmarks may be documented with digital photography or frameless stereotaxy for reoperation at a later date.
Higher complication rates for intracranial grid electrode placement have been associated with an increased number of electrode contacts, increased length of the monitoring period, placement of burr holes in addition to the craniotomy, and multiple cable exit sites. [36, 37] At the authors’ institution, AEDs are stopped on the morning of surgery (except for benzodiazepines and barbiturates, which are given in reduced doses). VEEG monitoring continues until the epilepsy team believes that adequate ictal data have been obtained (usually within 2-8 days).
Placement of depth electrodes
Depth electrodes are used most commonly for recording from the hippocampus and amygdala. The approach preferred by the authors is to place electrodes via the occipital, parasagittal route. [38, 39] This trajectory allows simultaneous implantation into the amygdala and anterior and posterior hippocampus using a single multicontact electrode (see the image below).
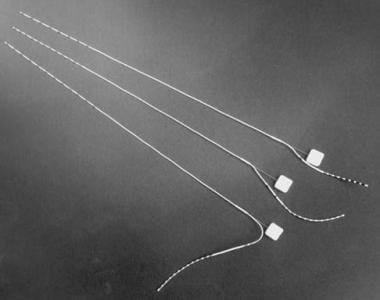 Examples of 3 depth electrodes with varying numbers of contacts. Note that stylus is in place and is removed once electrode has been inserted.
Examples of 3 depth electrodes with varying numbers of contacts. Note that stylus is in place and is removed once electrode has been inserted.
Placement is performed with either a frameless system or a stereotactic frame with adequate clearance at the back of the head. MRI is used with both frame-based and frameless stereotactic placement to allow direct visualization of the target and the trajectory. [31, 40, 41, 42, 43]
Indications for depth electrode placement are expanding as neuroimaging becomes more sophisticated and more complex epilepsy syndromes are identified. This is particularly true among the malformations of cortical development, particularly when the dysplastic lesion is subcortical. Depth electrode recordings into hypothalamic hamartomas and periventricular nodular heterotopia have shown ictal onsets beginning within the lesions, which subsequently spread to produce clinical seizures.
Depth electrodes are most often used in conjunction with other subdural strip or grid electrodes so that multiple brain areas are sampled simultaneously to avoid false localization based on insufficient data collection. Specific intracranial EEG ictal discharge frequencies, locations, and patterns can suggest, preoperatively, certain types of histopathologic findings. [44]
Completion of intracranial monitoring
At most epilepsy centers, rooms with hard-wired EEG and video telemetry instruments are used only for long-term monitoring, not for general medical or surgical patients. Patients may be taken directly from the recovery room to one of these monitoring rooms or may stay in the intensive care unit (ICU) for the first night of monitoring. Antiepileptic drugs are tapered or withdrawn, and VEEG recordings are begun on the day of electrode implantation.
The relative accuracy of ictal electrographic activity versus interictal activity remains somewhat controversial. In general, interictal spikes are usually more diffuse than the ictal onset zone, and bilateral interictal spikes can occur in patients who ultimately do well after a unilateral resection, and therefore, do not preclude a good surgical outcome.
Obtaining ictal recordings is usually preferable in order to confirm the significance of interictal abnormalities, in that ictal recordings are considered more accurate than interictal data. A possible exception to this general guideline might be when recording from a focal cortical dysplasia; distinctive interictal epileptiform patterns have been identified that may provide enough data to guide a resection based solely on interictal data.
In monitoring a lesion with intracranial electrodes, seizure outcome is best when both the lesion and the ictal onset zone are completely resected; outcome is compromised when either the lesion or the ictal onset zone is incompletely resected. [45, 46] In the case of nonlesional epilepsy, seizure freedom is more difficult to achieve, even in cases in which the ictal onset zone has been well studied with intracranial electrodes.
The number of seizures required to consider an intracranial study complete depends on the specific issues involved with treating a particular patient. In general, an arbitrary number of 3 typical clinical seizures has been considered the minimum number to be captured; however, exceptions to this rule abound. [47]
For example, a patient with a posterior temporal or parietal lesion and scalp EEG localization to the anterior temporal lobe that is delayed in comparison with the clinical onset might be considered for an intracranial study to confirm the clinical suspicion that seizures are falsely localized to the anterior temporal lobe. In such a case, 1-2 seizure onsets with intracranial EEG ictal onset directly over the lesion might yield sufficient data to allow the surgeon to proceed to surgical resection.
On the other hand, if a patient has nonlesional epilepsy and bilateral ictal onset over both temporal areas on scalp EEG monitoring, many more than 3 seizures would have to be recorded in order to exclude bilateral temporal onset or establish a predominant side of onset.
The type of ictal onset recorded may influence the number of seizures required. Fewer seizures must be recorded in patients who have identical ictal onset patterns over the exact same electrode contacts in every seizure. More seizures must be recorded in patients with multifocal ictal onsets over different electrodes from one seizure to another.
Initial ictal changes at the beginning of a seizure are more important than late changes and the propagation patterns of the seizure. Seizures that occur seconds to minutes after a previous seizure may be disregarded as being potentially misleading. [47, 48]
When intracranial monitoring is complete, most subdural strip and depth electrodes can be removed percutaneously in the OR after administration of a conscious sedation protocol or general anesthesia. Because of concern over contracting virally transmitted disease (eg, AIDS, Creutzfeldt-Jakob disease, hepatitis), recording electrodes are commonly discarded after a single use.
New computer-assisted prediction paradigms are being created to analyze ictal onset and changes in the background electrical state, interictal spike frequency, confluence analysis, and chaos theory in order to predict seizure occurrence minutes to hours before ictal onset. Intracranial monitoring will probably have to adapt in order to accommodate these new technologies in the near future. [49, 50, 51, 52, 53, 54, 55]
Repeat intracranial monitoring
The following are reasons why a second intracranial study might be considered [56, 57, 47, 58, 59, 60] :
-
Strip electrode survey study for lateralization and localization to a lobe, with a planned return at a later date for definition of the ictal onset zone and cortical mapping as necessary
-
Reimplantation of a second grid because of failed localization secondary to sampling error (seizures may occur on the margin of the grid, be diffuse, or show variable propagation that makes seizure localization uncertain.)
-
Recurrent seizures after a previous intracranial study and resection
A repeat intracranial study is typically performed months to years after the first intracranial study, either to give the wound time to heal or because seizures recur at some variable time after resective surgery. Often, an interim preoperative evaluation, which may include VEEG monitoring, ictal SPECT scanning, MRI, or MEG, is performed to reexplore the suspected cause of a person’s epilepsy and prepare a more effective intracranial study. Secondary grid implantations can be quite troublesome because dural adhesions are the rule rather than the exception.
Therefore, when planning a second intracranial study, the surgeon should anticipate a difficult entry and should use many of the strategies used for reoperation in other craniotomies, including enlarging the bone flap until pristine dura is encountered and opening the dura away from the previous operative site and away from functional cortex. If adhesions are encountered, an operating microscope should be reserved and used early in the dissection.
The authors routinely plan to spend 1-3 hours under the operating microscope when scheduling a reoperation for epilepsy. Even with tedious dissection, adhesions can limit the distal passage of electrodes and limit the effectiveness of a repeat intracranial study.
Several authors have suggested an alternative to delayed reoperation, [61, 62, 63] advocating immediate reimplantation during the same hospitalization if the findings from the first grid were not diagnostic. Doyle refers to this as a 3-stage procedure, and Lee describes the same technique as a double grid.
This technique has certain advantages over delayed return for implantation of a second grid. First, adhesions do not obscure the subdural space, which can limit grid reimplantation. Second, cortical injury can be avoided because adhesions do not need to be dissected from functional tissue. Third, intracranial EEG changes can be compared with the previous study while the subtleties of the previous intracranial ECoG are still fresh in the minds of the evaluating team.
Other authors, however, believe that placing a grid again immediately after a first operation is likely to alter the network of epileptogenesis and that resultant seizures with second grid implantation might not best represent the patient’s typical seizures.
Doyle advocates performing a limited resection of the ictal onset zone seen with the first grid, on the grounds that a partial resection of the epileptogenic region may help identify which additional areas are still contributing to seizure onset once the major site of ictal origin has been removed. Favorable seizure control was achieved with no apparent increase in surgical morbidity in 42 3-stage procedures compared with 369 traditional grid protocols [63] and in 18 double procedures compared with 165 routine intracranial procedures. [62]
Documentation of intracranial study
Each intracranial study is unique to the patient for whom it is designed. Even routine intracranial studies have subtle variations in electrode placement based on the patient’s anatomy. Some sort of documentation of the intracranial study is advisable for a number of reasons, including confirmation of the accuracy of placement, communication with the neurophysiology team, and correlation with gyral anatomy or intracranial lesions. Several documentation options, ranging from simple to complicated, are available.
When intracranial strip electrodes are used, the most vital part of the documentation of the study begins in the OR. As each electrode is inserted, the operating surgeon describes the identifying characteristics of that electrode (eg, length, color coding, scalp exit site) and its intracranial position to an assistant, usually an OR nurse, who documents this information directly in the operative record or chart notes so that no confusion is encountered when the electrodes are eventually connected.
Most EEG technologists appreciate a line diagram handwritten by the surgeon in the chart. This simple step can eliminate many potential sources of human error, particularly with extensive intracranial surveys, and facilitates communication between all members of the team.
Another simple way of documenting the operative technique is for the monitoring team to use an anatomic brain diagram and transparencies of the grid montage to create a mockup of the surgery. [64] The image created is compared with fluoroscopic images taken at surgery so that a relatively accurate rendition of the electrode placement is available within minutes on the day of the procedure. These images can be quite helpful in interpreting seizure onset and propagation during EEG monitoring.
In more complicated cases, a member of the monitoring team is present in the OR to take digital photographs of the exposed cortex before and after grid placement. Digital photography helps identify the relationship of a grid to the sylvian fissure and is one of the best methods of documenting the fine anatomy of the brain, including sulcal and arteriolar anatomy that cannot be seen with advanced imaging techniques.
More sophisticated imaging techniques have been developed and are being used with increasing frequency as advanced imaging software becomes more available. Cranial MRI with a head coil can be performed safely in patients with intracranial grids and strip electrodes in place if a few safety considerations are kept in mind.
Most electrode manufacturers endorse platinum electrodes as MRI compatible, although stainless steel electrodes have also been used without apparent patient injury. Because each epilepsy center has different procedures, it is advisable to check the recommendations of the particular electrode manufacturer before obtaining an MRI. In all cases, current loops can theoretically be created within a magnetic field if the electrode tails are allowed to contact one another. Therefore, all electrode tails should be isolated before performing MRI.
Most MRI workstations or software packages allow 3-dimensional reconstruction of images, which are often more useful than traditional MRIs or CT scans. [65, 66, 67] In addition, most frameless stereotactic navigation systems allow for image reconstructions of an MRI that can be merged with preoperative anatomic, functional, or angiographic imaging to create an accurate rendition of the grid in relation to relevant operative anatomy. [33, 67]
Cortical mapping
Often, in addition to defining the location of the epileptogenic cortex, the surgeon must determine its relationship to functional cortex. This requires mapping the cortex underlying an implanted grid electrode. [68, 69] The technique is similar to that employed in acute settings in the OR and requires a testing protocol appropriate to the cortical region being investigated.
Cortical stimulation is performed using commercially available constant-current generators. Cortical mapping is performed by selecting 2 adjacent electrodes (1-cm intervals) because bipolar stimulation provides more precise control of current flow. Bipolar pulses at 50 Hz are used for language, motor, and sensory mapping.
Extraoperative cortical mapping has several advantages over acute intraoperative mapping. Functional mapping may be performed in multiple sessions if necessary. For example, if a seizure that impairs function is generated during mapping, the patient may be allowed to recover for several hours (or days) until proceeding with further mapping. Advanced paradigms may be performed over hours or days that would not be possible in the acute intraoperative setting.
Once mapping is completed, the patient, family, and surgeon have time to discuss the potential risks and benefits of surgery and to make decisions to accept or reject a functional loss that might be associated with surgery.
During brain stimulation, brain mapping is performed by a neuropsychologist or physician, who may test language or motor function. A clinical neurophysiologist reviews the ECoG during stimulation to ensure that any disruption of neurological function is due to the stimulation and not an afterdischarge. Afterdischarge potentials are repetitive spike discharges or electrical seizures directly provoked by electrical stimulation and may limit the ability to map the brain or may lead to a seizure.
The amount of current needed to produce an effect varies from patient to patient and between cortical regions. Enough current should be used to produce a reliable effect without causing afterdischarge. Occasionally, pain can result from current spread to the dura or a nearby cortical vessel. In such cases, a particular contact may not be suitable for mapping.
Surgeons are often encouraged to be present for intracranial extraoperative mapping, particularly early in their careers. Some of the slight variations in mapping technique and interpretation have subtle ramifications for the surgeon and are different from those that a neurologist or neuropsychologist might appreciate. On some occasions, mapped cortical regions vary from what one would expect from classic anatomic studies, particularly in areas of cortical malformations. [70]
On other occasions, mapping different pairs of electrodes in a specific region (eg, motor or language areas) might allow the surgeon to appreciate the orientation of a crucial region relative to the orientation of the grid or to adjacent contacts. Finally, subtle errors in naming or language may be present extraoperatively, when the patient is off AEDs, and these errors may change when the patient is reloaded with AEDs. [71] Such subtleties of extraoperative mapping can be useful to the surgeon if observed personally before a resection.
Implanted grid arrays are excellent tools for identification of the position of sensorimotor cortex through somatosensory evoked potentials (SSEPs). Allison and colleagues have reviewed the rationale and technique, [71, 72, 73, 74] and others have discussed the clinical experience with subdural grids for this purpose. [68, 69, 75, 76, 77]
SSEPs can be used during acute recording in the OR, using subdural strip electrodes to identify primary motor cortex. The strip electrode must be positioned to traverse motor and sensory cortex, and it may have to be repositioned several times during intraoperative recording to optimize the signal. Therefore, if SSEPs are planned during extraoperative monitoring, using a subdural grid to increase the surface coverage by the electrode array is advantageous in order to optimize location of the motor cortex (see images below).
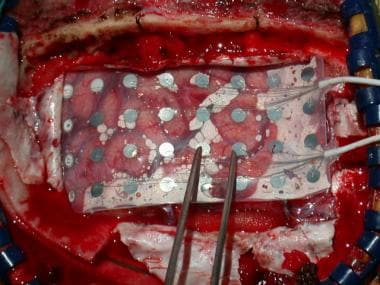 Craniotomy showing intracranial grid in place. Grid lies over left primary motor and sensory cortex, near vertex of skull. Patient is positioned laterally, and face is at right. Intracranial study was performed to document seizure onsets in patient who had previous oligodendroglioma removed. Seizures began just posterior to leg motor cortex, in primary sensory cortex.
Craniotomy showing intracranial grid in place. Grid lies over left primary motor and sensory cortex, near vertex of skull. Patient is positioned laterally, and face is at right. Intracranial study was performed to document seizure onsets in patient who had previous oligodendroglioma removed. Seizures began just posterior to leg motor cortex, in primary sensory cortex.
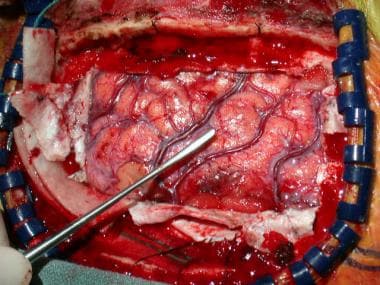 Primary motor cortex is located with use of extraoperative somatosensory evoked potentials and intraoperative cortical stimulation, as well as grid previously described (see above). Penfield instrument in field is positioned over primary motor cortex.
Primary motor cortex is located with use of extraoperative somatosensory evoked potentials and intraoperative cortical stimulation, as well as grid previously described (see above). Penfield instrument in field is positioned over primary motor cortex.
Sometimes, extraoperative mapping indicates that the ictal onset zone is close to or overlies critical motor or speech areas. On such occasions, using the advantages of awake operative language mapping may be helpful at the time of grid removal and resection of the epileptic focus. Although extraoperative mapping has many advantages, the accuracy of its spatial resolution is limited to 1 cm, namely, the distance between 2 electrode pairs.
On occasion, awake intraoperative mapping helps confirm which electrode is the contact that directly overlies cortical function. This can be particularly important when the 2 electrodes span a sulcus; in such cases, awake mapping may allow the surgeon to determine which gyrus is involved in function and which gyrus is not. [75] In such cases, the epileptic zone may be resected up to the pial margin without disturbing function, as long as the vascular structures within the pia are preserved.
Complications of intracranial monitoring
In published series, infection rates from all types of intracranial electrodes range from 0-12%. The morbidity of the procedure depends on the type of electrode implantation; intracranial strip electrodes have the lowest morbidity, and intracranial grid placement has the highest morbidity. [30, 31, 32, 36, 37, 78, 68, 38, 41, 79, 80, 81, 82, 83, 84, 85]
The most common cause of morbidity in subdural strip placement is infection. One randomized study found a 0.85% rate of infection when preoperative antibiotics were given, compared with a 3% infection rate when no antibiotics were given. [83, 85] No difference in infection rates was noted between patients who received antibiotics for the duration of strip electrode implantation and those who received only a single preoperative dose; therefore, at the authors’ institution, a single dose of antibiotics is given immediately before strip or grid implantation.
Other complications of intracranial strip placement include cortical contusion, cerebral edema, brain abscess, subdural empyema and subdural hemorrhage, placement of electrodes into the brain parenchyma, accidental extraction of electrodes, and superficial wound infection. Many of these complications are minor and cause no long-term problems; permanent neurologic deficits are seen in less than 1% of patients who undergo intracranial strip electrode implantation.
Complications of grid implantation include infection, transient neurologic deficit, hematoma, cerebral edema with increased intracranial pressure, and infarction. Transient neurologic deficits can occur secondary to edema or hematoma associated with the grid. In most cases, if neurologic compromise is evident, the grid should be removed immediately.
Cerebral edema is more likely to occur with an increase in the duration of the monitoring session or with a greater number of intracranial electrodes. [36, 37] Some authors have been concerned that pediatric patients are more likely to develop increased intracranial pressure because, theoretically, less space is available to accommodate the mass of an intracranial grid [86] ; however, others have not found this to be a concern. [36, 87, 46, 88, 89]
Complications from depth electrodes include intraparenchymal hemorrhage, subarachnoid hemorrhage, arterial spasm, and misplacement of the electrode. The rate of permanent neurologic deficit from occipital depth electrode placement has been reported at less than 1%. [90, 91]
The risk of hitting the brainstem or posterior cerebral artery with occipitally inserted depth electrodes may be decreased by (1) targeting tip placement in the lateral amygdala and lateral hippocampus, (2) making sure the occipital burr hole is not too medial, and (3) confirming the trajectory with an image guidance system before electrode placement.
Alternatively, one can attempt to place the depth electrode into the lateral ventricle rather than into the hippocampus. To do so, a rigid guide cannula is placed into the ventricle (verified by identifying CSF flow from the cannula), and the depth electrode is placed into the ventricle. The electrode then lies in the temporal horn adjacent to the hippocampus, with its tip entering the targeted amygdala.
Ictal recording from the ventricle usually conducts signal well, with only occasional failure. [44] The signal obtained amplifies (1) both hippocampal and parahippocampal ictal onsets and (2) intraparenchymal amygdala onset. The risk of subarachnoid hemorrhage may be minimized if depth electrodes are placed under direct visualization through a burr hole rather than using a closed twist-drill technique, which helps avoid draining veins during placement, and by using image-guided stereotaxy and contrast-enhanced MRI to avoid surface veins at the entry point.
Some surgeons prefer orthogonal placement of depth electrodes, which has been facilitated by frameless stereotactic techniques. Several systems allow placement of orthogonal depth electrodes via twist drill or burr holes in the temporal fossa. Stereotaxy is required to avoid vascular injury to vessels of the middle cerebral artery and to gain accurate placement into the amygdala and hippocampus. Electrodes must be secured at the skin to ensure migration of the electrodes does not occur as the medial trajectory of these electrodes is towards the brainstem.
Formulation of Appropriate Surgical Strategy
Surgery may be considered as either definitive or palliative. Definitive surgery carries a significant chance of producing complete, or at least 70-90%, improvement in seizures. The goal of palliative procedures is to decrease seizure frequency, but rarely results in seizure freedom.
In general, definitive surgeries physically remove seizure-producing cortex from the brain. Examples are resections of small seizure-producing tumors, vascular abnormalities, cortical malformations, or lesions such as mesial temporal sclerosis. Palliative surgeries usually disrupt pathways involved in seizure production and propagation or attempt to disrupt seizures with the use of electrical stimulation; thus, the potential for continued seizures always remains.
General approach to epileptogenic lesions
Tumors that cause epilepsy are frequently low-grade astrocytomas, oligodendrogliomas, or gangliogliomas. Commonly, they are well circumscribed, and excellent outcomes can be achieved with a lesionectomy that includes the immediate surrounding abnormal cortex. [92]
However, there is a temptation to rush to surgery, on the assumption that the tumor must be causing the seizures. The more cautious epilepsy surgeon may consider the possibility of alternative diagnoses, such as dual pathology (eg, the presence of hippocampal atrophy and tumor in the same patient), or look for evidence for concomitant cortical dysplasia, depending on the suspected tumor pathology.
The preoperative evaluation in the setting of tumor pathology is to determine that the EEG findings and the tumor are not discordant, which can occur approximately 10-15% of the time. In such cases, the lesion may not be solely responsible for the epilepsy, and a more extensive resection may be required to control the epilepsy.
When the tumor and the lesion are concordant, the most common mistake in treating these tumors is to remove only the gross tumor and not the immediate surrounding tissue, thus leaving epileptogenic tissue in place and resulting in clinical seizure resumption. If seizures continue after tumor removal, it may be assumed that the tumor was not completely removed, regardless of whether MRI suggests a clean resection.
Small vascular abnormalities, such as cavernous hemangiomas surrounded by hemosiderin, can be extremely epileptogenic. Removal of the vascular abnormality and surrounding hemosiderin-stained cortex may be all that is necessary for an excellent seizure outcome in approximately 80% of patients, provided that the preoperative evaluation is concordant. [93]
Large vascular abnormalities (eg, high-flow arteriovenous malformations) are commonly associated with seizures. These abnormalities, unlike the smaller vascular lesions, do not always exhibit a clear relation between the structural lesion and the epileptogenic cortex, and simple lesionectomy often fails to stop the seizures.
Because of the technical difficulties involved, implanting a large grid electrode over these lesions for the purpose of mapping is usually not feasible. Therefore, treatment of such lesions should seldom be approached purely as epilepsy surgery. One successful approach is to stage surgical procedures; the vascular abnormality is removed with the intent of further investigation and possible surgery if seizures remain.
Epileptogenic congenital malformations of cortical development, such as cortical dysplasias, heterotopia, schizencephalic clefts, and the various forms of phakomatoses, are very difficult to treat surgically and usually necessitate more extensive evaluations and tailored resections based on data from implanted grid electrodes. [70] Seizure outcome after resection of such malformations is variable and directly relates to the complexity of the lesion.
Traumatic encephalomalacia is treatable surgically, with varied results. The difficulty with these lesions is that cortical damage may extend much further than the area of obvious anatomic damage or that multiple regions of the brain may be affected simultaneously.
Controversial issues in definitive surgery
With regard to performing an acute ECoG, some surgeons believe that epileptiform discharges recorded during surgery indicate which part of the brain is epileptogenic; therefore, these surgeons use the ECoG to define boundaries for cortical resections. [94] This approach is referred to as a tailored resection, in that no 2 operations are identical. Because anesthetics affect the reliability of acute ECoG recordings and prevent the surgeon from mapping cortical function (eg, language), tailored resections are performed best with the patient under local anesthesia.
Other surgeons believe that an acute interictal ECoG is not reliable enough to determine surgical boundaries and can be inaccurate; these surgeons proceed with a standardized operative approach, wherein tissue important for generating seizures is removed without considering the ECoG for some surgical procedures.
If there is a question that must be answered with ECoG, then the patient receives an intracranial study, long-term monitoring with intracranial electrodes is performed to capture ictal ECoG, and a tailored resection is done on the basis of intracranial ictal recording rather than short-term, less accurate, interictal data.
For AMTR, no scientific data support the belief that ECoG-guided resections produce better results than standard procedures do. A more rational view is that the surgical approach should be suited to the syndrome.
There are specific syndromes for which a standardized operation is very effective. The best example is complex partial seizures of mesial temporal lobe epilepsy with hippocampal sclerosis. Such patients have excellent seizure and language outcomes after standard surgical procedures done with general anesthesia. Likewise, excellent results can be expected from the removal of a well-circumscribed capillary hemangioma from a patient with complex partial seizures.
In contrast, patients with epilepsy secondary to other etiologies in other brain regions may need extensive cortical mapping and an ictal ECoG. In such cases, implantation of a large subdural grid electrode for long-term monitoring (with an ECoG) and mapping is preferred because it yields results superior to those obtained by performing an acute interictal ECoG.
Preparation
Anesthesia
The anesthetic technique for a routine anteromedial temporal resection (AMTR) is general anesthesia. Anesthesia is maintained with consideration of the need for an electrocorticogram (ECoG), depending on the particular details of the case. [95]
Because patients undergoing callosotomy with narcotic anesthesia are often slow to arouse immediately after surgery and are therefore difficult to evaluate neurologically, the authors have abandoned narcotic anesthesia in favor of inhalation agents in these patients. The authors induce anesthesia with an appropriate amount of propofol, followed by an inhalational agent of choice.
Positioning
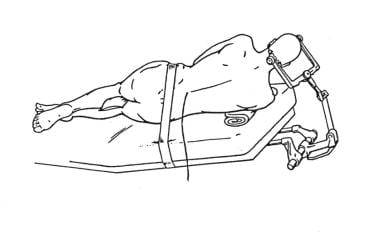
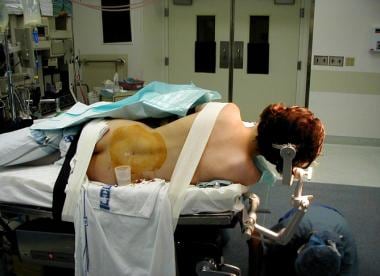
For AMTR, the patient is placed supine on an alternating air mattress, with the head held in the Mayfield 3-pin holder. The sagittal midline and the axial axis of the head should be at angles of 20° and 30°, respectively, to horizontal. Positioned properly, the zygoma is the highest point of the head (see the image below).
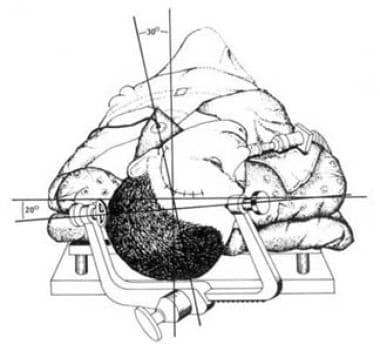 Method for positioning patient for temporal lobectomy. Head is angled so that malar prominence is highest portion of patient's head.
Method for positioning patient for temporal lobectomy. Head is angled so that malar prominence is highest portion of patient's head.
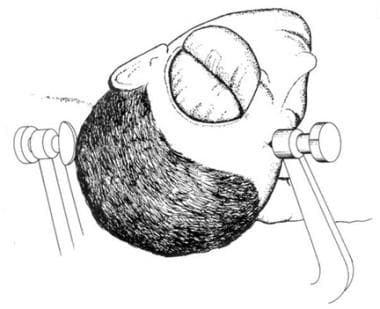
Corpus callosotomy is performed from the vertex of the head. The supine position favored by most surgeons necessitates frontal lobe retraction. The authors’ practice is to place the patient in the right lateral decubitus position, with the head positioned so that the left hemisphere is superior and the right hemisphere inferior. The Mayfield head holder is applied, and the neck is secured in a neutral position. The operating table is tilted at a head-up incline of approximately 15° (see the image below).
In this position, the left hemisphere is supported out of the operative field by the falx cerebri, and the dependent right hemisphere is gently pulled away from the midline by gravity as cerebrospinal fluid (CSF) is removed. With CSF drainage and a partial pressure of carbon dioxide (PCO2) of 30 mm Hg, brain retraction should be minimal.
Complication Prevention
Early studies of corpus callosotomy reported a 50% rate of hydrocephalus and aseptic meningitis, with several deaths. This high rate may be due partly to opening ependyma into the ventricles, and it can be minimized by entering only the cavum septum pellucidum. Other potentially serious operative complications include excessive bleeding from the superior sagittal sinus, frontal lobe cerebral edema, and venous infarction from sacrificing major bridging veins.
The most dangerous complication of corpus callosotomy is air embolism, which is most likely to occur from a tear in the superior sagittal sinus during the initial stage of craniotomy. In addition, bleeding from the sagittal sinus can be extensive, with significant blood volume loss accumulating rapidly. This is especially critical in children. Therefore, a recommended policy for pediatric cases is not to begin the operation without ensuring that transfusable blood is available in the operating room (OR).
Complications from functional hemispherectomy can be formidable: patients may experience hemogenic meningitis, ventriculitis, CSF leakage, hydrocephalus, or, less commonly, stroke, infection, coma, or postoperative hemorrhage. [15] The procedure must be performed in centers with experienced surgeons with advanced equipment available for intraoperative and postoperative support of the patient and management of any complications that may arise.
Other Preoperative Considerations
In patients scheduled to undergo AMTR, valproic acid (which can be associated with bleeding disorders) should be discontinued at least 3 weeks preoperatively and, if possible, replaced with another medication. On the morning of surgery, the patient receives the usual medication dosage with a few sips of water.
A 1-g dose of cephalosporin may be administered intravenously (IV) 1 hour before the incision is made; no further antibiotics are administered. Adults are given dexamethasone 10 mg IV 1 hour before surgery (the pediatric dose is adjusted appropriately). IV steroids are continued postoperatively at 4 mg every 6 hours for the first 24 hours, then discontinued.
All patients being considered for corpus callosotomy should have an MRI performed to uncover any structural lesion that could help identify an etiology for epilepsy. In addition, a good MRI allows the surgeon to visualize preoperatively the anatomic relationships to the corpus callosum. For example, coronal images can alert the surgeon to a singular or so-called simian pericallosal artery, which is a worthwhile point to know before dissection.
Long-term VEEG monitoring is essential for an epilepsy diagnosis. If, after a well-conceived workup, the patient has tonic, clonic, tonic-clonic, or atonic seizures and is not a candidate for a focal resection of a clearly identifiable epileptogenic region, then corpus callosotomy should be considered.
The usefulness of neuropsychological testing on every patient considered for callosotomy is debatable. If such testing is needed for long-term postoperative planning or if specific issues need to be addressed (eg, memory integrity), then specific testing should be performed. Routine extensive neuropsychological testing is not required to determine a patient’s candidacy for surgery.
The usefulness of routine Wada testing is questionable. If a special situation requires speech lateralization, then the test should be performed (eg, if specifically concerned about mixed cerebral dominance).
In 1990, Sass et al reported that patients with mixed cerebral dominance for handedness and language (ie, a right-handed person with right-hemisphere language dominance) are at risk for postcallosotomy language impairments. [96] This complication appears to involve primarily speech and writing and spares verbal comprehension. However, more data are needed for a full understanding of postoperative language deficits.
Technique
Overview
Anteromedial temporal resection (AMTR) is the most commonly performed surgery for epilepsy. It has the clearest indications and best results. Thus, AMTR is used as a criterion standard against which other procedures can be judged and strategies extrapolated.
Corpus callosotomy for epilepsy was first reported in 1940, when Van Wagenen and Herren described 10 patients who underwent the operation between February and May and whose cases were followed until July 1939. [97] The rationale was based solely on Van Wagenen’s observation that, as a tumor that involved the corpus callosum grew, the patient’s generalized seizures became less common and less severe, with increased preservation of consciousness.
Results in this first group of patients were encouraging enough to warrant further evaluation of the surgery. Van Wagenen subsequently reported 14 additional patients in whom surgery occasionally included sectioning the anterior or fornix commissure.
Interest in callosotomy remained dormant until the 1960s, when Bogen and colleagues published a series of articles on the clinical and neuropsychological outcome of the procedure. In 1970, Luessenhop et al described a group of patients treated with corpus callosotomy as an alternative to hemispherectomy. [98] In the mid 1970s, Wilson et al reported the Dartmouth series of callosotomies. [99] Since then, a renewed interest in epilepsy surgery has prompted a reevaluation of the clinical usefulness of callosotomy.
According to published data, the aim of this operation is to disrupt one or more major central nervous system (CNS) pathways used in seizure generalization. The rationale assumes disruption of this pathway decreases the frequency and severity of either primary or secondary generalized seizures. As a result, callosal sectioning has been applied to almost all seizure disorders.
Multiple subpial transection (MST) is a nonresective surgical technique for abolishing epileptiform discharges and correlative seizures from epileptogenic cortex. The rationale for MST rests on the observation that all partial epileptic seizures originate from a restricted region of cortex, which is considered (for lack of a better term) the focus. The exact mechanisms responsible for the generation of focal seizures are not completely understood but are believed to involve abnormally discharging neurons and abnormal interneuronal synchrony.
Whether epileptogenic neurons fire in action potential bursts solely because of abnormal interneuronal synchrony or because of intrinsic abnormalities (or a combination of both) is unknown. However, recordings from chronic animal models and human cortex have demonstrated that seizures are associated with periods of increased interneuronal synchrony within cortex as well as between cortex and subcortical nuclei.
Theoretically, if this intracortical synchronization can be disrupted, the focus’ epileptogenic potential can be reduced or eliminated. The rationale for placing parallel slices through cortex is to permanently disrupt side-to-side intracortical synchronizing neural networks.
Because the neocortex is organized in functional columnar units, right-angle cuts to the pial surface should not disrupt cortex-subcortical input-output interactions. Therefore, the MST technique theoretically is ideal for treating epileptogenesis while preserving intrinsic cortical function.
In 1995, Sugiyama et al reported on MST-induced histologic changes and the effects of MST on interneuronal cortical spread in acutely kindled rats. [100] They showed that the transection severed horizontal fibers but left vertical fibers and neuronal cell bodies preserved. Cortical hyperactivity across the transected zone was reduced, and afterdischarge propagation was also inhibited. These results suggest that MST interrupts not only neuronal synchronization but also excitatory interneuronal conduction.
In 1994, Tanaka et al reported on MST effects on propagation in a cat kainic acid model. [101] They showed that MST suppressed epileptic activity of the cortical surface. However, residual hypermetabolic areas (defined by carbon 14–labeled deoxyglucose autoradiography) were observed in the deep layer of the cortex and caudate nucleus and in the contralateral sensorimotor cortex. These results suggest that seizure propagation uses side-to-side connections more than cortical subcortical connections in neocortical epilepsy.
In a functional hemispherectomy, cortex is disconnected from all subcortical structures, and the interhemispheric commissures are divided, but the brain remains in place. Excellent postoperative seizure control and good quality of life can be achieved among children treated with hemispherectomy.
Anteromedial Temporal Resection
For the opening, minimal hair is shaved—just enough to allow the surgeon to make a standard question-mark curvilinear frontal temporal incision (see the image below). The temporalis muscle is incised directly beneath the skin and reflected with the scalp. The bone flap should be based low in the middle fossa, extending just above the sphenoid wing but within the confines of the temporalis muscle fan. A rongeur or high-speed drill is used to enlarge the bone opening towards the middle fossa floor and anteriorly into the sphenoid wing for several centimeters.
The dura is opened by a cruciate incision to optimize temporal tip exposure and should extend approximately 1 cm above the sylvian fissure (see the image below). The remainder of the operation should proceed with the surgeon using the operating microscope.
 After muscle is retracted and small craniotomy performed, dura is opened to expose temporal tip and small portion of suprasylvian cortex.
After muscle is retracted and small craniotomy performed, dura is opened to expose temporal tip and small portion of suprasylvian cortex.
A distance of 3.5-4 cm from the temporal tip is measured along the middle temporal gyrus posteriorly from the sphenoid wing. This marks the posterior limit of the proposed lobectomy, regardless of speech laterality or side of operation. An incision is made in the sulcus between the superior and middle temporal gyri and is carried mesially until the temporal ventricular horn is entered. A small cotton pledget is placed into the ventricle to maintain orientation.
With the help of an irrigating bipolar coagulator and suction device, the middle and inferior temporal gyri are removed as a single surgical specimen. At some centers, the superior temporal gyrus is removed; at others, it is not. If it is removed, one must be careful to maintain an intact pia along the sylvian fissure and basal temporal regions. The superior temporal gyrus is removed to the level of the ventricle, and then the amygdala is partially removed.
The remainder of the inferior temporal structures are removed piecemeal mesially until the parahippocampal gyrus is encountered. At this point, only the mesial-temporal structures remain, and the ependymal surface of the hippocampus should be easily identified (see the image below).
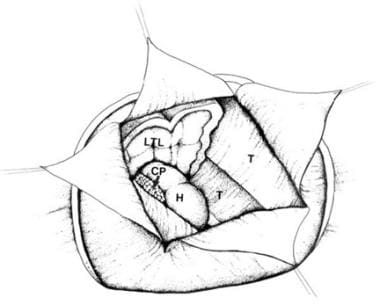 Lateral cortex is resected, leaving hippocampus (H). Tentorium (T) and choroid plexus (CH) should be identified.
Lateral cortex is resected, leaving hippocampus (H). Tentorium (T) and choroid plexus (CH) should be identified.
The hippocampus and the uncus are removed en bloc for pathologic examination. An incision into the parahippocampal gyrus is made through the choroidal fissure from the level of the posterior boundary of the cerebral peduncle anteriorly until the tip is reached. This serves as the superior-mesial margin of the resection.
The posterior incision is made at the level of the posterior margin of the cerebral peduncle laterally to the lateral hippocampal margin and then brought anteriorly and laterally until it meets the lateral resection margin. The hippocampus is separated carefully from the intact pial surface.
As the hippocampus is rolled posteriorly, care is taken to coagulate and cut the numerous small vessels that arise from the posterior communicating and cerebral arteries without damaging the vessels that supply the peduncle and thalamus and thereby causing traction hemiplegia and hemianopsias. With the hippocampus removed, the pial bank overlying the tentorial incisura and cerebral peduncle should be intact.
Below this pial barrier should be the cerebral peduncle, posterior cerebral artery, posterior communicating artery, and third nerve (see the image below). Pial bleeding is controlled carefully by surgical and gel foam at a low setting. Best results are obtained with removal of the hippocampus to the level of the superior colliculus. The resection cavity is irrigated completely free of blood, and the dura is closed in a watertight manner. No drain is needed, and blood loss should not exceed 300 mL.
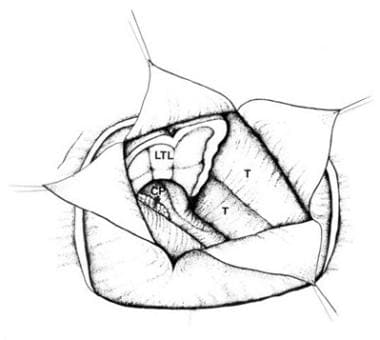 Hippocampus is removed as separate en-bloc specimen for pathologic evaluation. Resection is carried back to level of collicular plate. Peduncle and third nerve should be visible through intact pia.
Hippocampus is removed as separate en-bloc specimen for pathologic evaluation. Resection is carried back to level of collicular plate. Peduncle and third nerve should be visible through intact pia.
Corpus Callosotomy
Despite renewed interest in epilepsy surgery in general and corpus callosotomy in particular, reported seizure outcomes from this operation vary greatly. This is due to a lack of standardization of the anatomical structures disconnected, the forms of epilepsy treated, patient selection criteria, and the preoperative evaluations. Also, over the years, diagnostic equipment and surgical techniques have evolved and improved.
In his first group of operations, Van Wagenen approached the corpus callosum through a large right frontoparietal craniotomy. The structures cut varied among patients and included part or all of the corpus callosum with and without unilateral division of the fornix.
In contrast to Van Wagenen (who cut the entire callosum through one opening), Bogen and Vogel used 2 separate craniotomies to cut the anterior or posterior portions of the callosum and often included the anterior commissure. In a later series of patients, Bogen completely sectioned the corpus callosum, the anterior commissure, the hippocampal commissure, and the massa intermedia.
Since 1962, numerous other published series have described sectioning of various combinations of corpus callosum, massa intermedia, anterior and hippocampal commissures, and unilateral fornix. The wide variability of structures sectioned within individual surgical series makes comparison of outcomes and complications problematic.
In recent years, only the corpus callosum has been sectioned. The extent of sectioning necessary for maximum seizure control with minimum risk of disconnection syndrome (ie, mutism, left-sided apraxia that resembles hemiparesis, bilateral frontal lobe reflexes) is not known. Clearly, the best seizure results are achieved with a complete callosal section. However, the risk of disconnection is greatest with a complete section. Thus, an 80-90% section that spares the splenium seems optimal.
The procedure is performed from the vertex of the head, with the patient lying in the right lateral decubitus position. A lumbar drain can be implanted to allow cerebrospinal fluid (CSF) drainage for improved exposure until the callosum is sectioned (see the image below).
A rectilinear, U-shaped, vertex scalp incision is centered over the junction of the coronal and sagittal sutures (see the image below). A 4-hole bone flap which straddles the sagittal and coronal sutures is elevated. From this point, an operating microscope is used. To provide superior stability, the authors prefer to operate while seated, using a sterile, draped Mayo stand for elbow support.
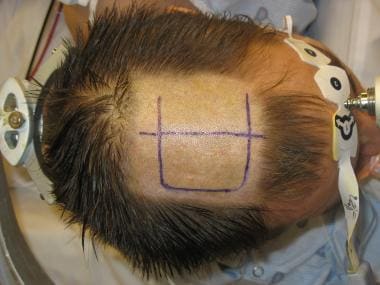 Surgical site preparation for corpus callosotomy. Right hemisphere is inferior, and left hemisphere is superior. In this position, left hemisphere will be held out of field by falx cerebri, and right hemisphere will fall away from field, minimizing need for traction.
Surgical site preparation for corpus callosotomy. Right hemisphere is inferior, and left hemisphere is superior. In this position, left hemisphere will be held out of field by falx cerebri, and right hemisphere will fall away from field, minimizing need for traction.
The dura over the dependent hemisphere is opened to the edge of the sagittal sinus. The dural flap is pulled tight with retention sutures to provide maximum exposure of the interhemispheric fissure. Lysis of midline adhesions between the arachnoid and dura is performed using bipolar cautery. Attempts are made to preserve bridging veins, but 1 or 2 veins (anterior to the coronal suture) can be sacrificed, if necessary.
Moist cottonoid strips are placed over the medial frontal cortex of the dependent frontal lobe, and any additional adhesions between the cortex and falx are cut with bipolar cautery. In this manner, dissection is carried down to the corpus callosum, which is identified only after clear visualization of both pericallosal arteries. Without this verification, an inexperienced surgeon may mistake the cingulate gyrus for the callosum.
After both pericallosal arteries are separated and protected and the callosum has been exposed, the callosum is opened along the midline of the body. This incision is carried deep until the cavum septum pellucidum is entered, with the ventricular ependyma left intact. In rare instances, one major pericallosal artery supplies both hemispheres and makes the dissection more difficult because the artery must be manipulated from side to side without damaging branches to either hemisphere.
A bipolar cautery is used to cut the callosum. Care is taken to stay within the cavum septum pellucidum. The entire rostrum, genu, and body are divided, and dissection is carried posteriorly until only the splenium remains intact.
The lumbar drain is removed, and the partial pressure of carbon dioxide (PCO2) is allowed to rise to 40 mm Hg. Nitrous oxide anesthesia should be discontinued at this point. The wound is irrigated generously to replace most of the drained CSF. If mannitol was used earlier, intravenous (IV) fluid should replace the volume loss from diuresis.
The dura is closed, dural tack-up sutures are secured, and the craniotomy is closed in layers. Blood loss should not exceed 150 mL. Experience has suggested that patients fare better postoperatively if the net fluid balance for the surgery is positive at the end of the case.
Multiple Subpial Transection
MST is still a relatively new surgical technique. Consequently, many questions remain unanswered. The following description is admittedly based on personal experience, and thus subject to bias, but to date the authors have found it to be effective in the author’s experience. Over time, these recommendations will no doubt be improved on, as many of the questions posed are answered.
As with all surgery for partial epilepsy, the margins of the epileptic focus must be clearly defined. Although interictal recordings define the foci of Landau-Kleffner syndrome (LKS) cases (because most children with LKS have no seizures), all other cases should have long-term ictal recordings using a subdural grid electrode.
Because most patients considered for MST have primary motor cortex involvement, the focus is near the central sulcus or, more commonly, the junction of the central sulcus with the sylvian fissure. Thus, a craniotomy that provides adequate exposure of this area is required whenever possible. A 64-electrode contact grid, centered over the suspected region of ictal onset, is recommended.
Within the first few hours of monitoring, somatosensory evoked potentials (SSEPs) are performed to orient the grid sensory-motor cortex with respect to the grid. Direct cortical mapping by electrical stimulation is performed as needed for each individual. Once sufficient ictal recordings identify the focus, the patient is returned to surgery for grid removal and MST.
Often, the ictal region is no larger than a few square centimeters; it is usually bordered by sulci (ie, contained within 1-2 gyri). Seizure propagation is commonly observed along the long axis of the involved gyri. The entire region of ictal onset should undergo MST, as well as the 1-2 cm bordering the ictal zone. If a discrete ictal zone is not evident from long-term recordings, MST results are not as good.
The authors’ preference is to use a specially designed MST knife (see the image below; Ad-Tech Medical, Racine, Wis) with the point angled downward (rather than upward, as originally described). In addition, the cutting portion of this knife is sharpened to a blade to minimize tissue damage; such damage can be excessive when blunt instruments such as right-angle dissectors are used. The actual cuts should be performed under direct vision through the operating microscope.
After the surrounding cortex is protected with cotton patties, the insertion point is chosen; it can be either at the side or at the crest of the gyrus. After a small pial spot is cauterized, the knife blade is inserted and pushed subpially towards the gyrus edge, making a right-angle cut to the long axis of the gyrus. The horizontal arm to the blade should be barely visible through the pia at all times.
If the insertion point is centered in the gyrus, then, after the first half-cut, the instrument is removed and replaced and the remainder of the slice is completed. Parallel cuts then are made 5 mm apart until the entire proposed ictal zone and surrounding area have been sliced.
Take care when encountering a gyrus that curves; the outer length of the curve will be much longer than the inner length. When a curving gyrus is encountered, the authors suggest staggering the cut lengths so that slices converging at the center of the curve do not all join at a common point or come so close together as to severely damage the cortex. Pial bleeding at the blade insertion point is usually controlled with a bipolar cautery or a small square of thrombin-soaked Gelfoam. Significant subpial hemorrhage should not occur.
Functional Hemispherectomy
Functional hemispherectomy dates back to 1950, when Krynauw first described an operation for children with infantile hemiplegia in which the entire hemisphere (excluding basal ganglia) was anatomically removed from the cranium (anatomic hemispherectomy). [102]
In 1969, Falconer and Wilson reported on long-term follow-up of early cases and disclosed symptomatic hydrocephalus and progressive hemosiderosis as 2 potentially lethal complications. [103] As these lethal complications became known, enthusiasm for hemispherectomy declined; it remained low until 1983, when Rasmussen reported that preserving disconnected frontal or occipital lobe(s) lessened the incidence of these adverse consequences. [104]
From this observation, functional hemispherectomy was developed. In a functional hemispherectomy, the cortex is disconnected from all subcortical structures and the interhemispheric commissures are divided, but the brain remains in place.
This procedure still involved considerable time in the operating room (OR) and often required blood transfusions, and these factors led to further refinement of the functional hemispherectomy, frequently referred to as the hemispherotomy, by a number of authors. [105, 106, 14, 107, 11]
These variations on the original functional hemispherectomy all include the following in their techniques: disconnection of the corona radiata and the internal capsule, resection or disconnection of the mesial temporal lobe structures, intraventricular callosotomy and deafferentation of the medial-basal occipital and frontal lobes. Such refinements have reduced the time needed for the operation and the blood loss associated with the operation, making the operation less morbid and easier to perform.
Post-Procedure
Postoperative Care
A patient who has undergone anteromedial temporal resection (AMTR) is moved to the intensive care unit (ICU) for overnight observation unless he or she can be returned to a seizure monitoring room in the epilepsy unit for careful monitoring. On the night of surgery, the patient sits at the bedside and performs deep breathing for pulmonary toilet. Should a fever occur, an incentive spirometer is used. The Foley catheter and arterial line are removed as soon as possible, usually before the patient leaves the recovery room.
If kept overnight in the ICU, the patient is moved to a surgical floor the next morning. The intravenous (IV) drip is converted to a saline lock as soon as the patient takes oral fluids. Ambulation is encouraged, as is sitting in a chair. Unless a problem arises, the patient is discharged on the third postoperative day.
If the patient is taking multiple antiepileptic drugs (AEDs), an attempt is made to reduce the doses to nontoxic (but effective) levels or to diminish the most poorly tolerated medication some time after surgery. If the patient is seizure free after about 2 years, the provider may discuss with the patient the pros and cons of discontinuing multiple AEDs. Patients are strongly urged to continue medications for many years postoperatively; there is a 1 in 3 chance of having a seizure in the 5 years following medication withdrawal after epilepsy surgery.
A patient who has undergone corpus callosotomy is observed in the ICU for the first evening following surgery. During this time, neurologic parameters may fluctuate and be complicated by the disconnection syndrome. The patient may not verbalize readily or respond quickly and may have unexplained pupillary inequality. These findings may prompt computed tomography (CT) scanning, which can help rule out a clot or tension pneumocephalus.
By the second postoperative day, the patient’s normal baseline neurologic status should begin to return. Magnetic resonance imaging (MRI) in the midsagittal plane is an excellent method for evaluating the extent of sectioning.
Expected Outcomes
A 2011 study identified long-term outcomes of epilepsy surgery in adults. A cohort of 615 adults who underwent surgery was followed up for a median of 8 years. Five years after surgery, 52% of patients remained seizure-free, apart from simple partial seizures (SPS). Patients who had anterior temporal resections were less likely to experience seizure recurrence than those who underwent extratemporal resections. The longer a patient was seizure free, the less likely the chance of relapse. [108]
The results of one randomized controlled study conclusively show that patients with medial temporal lobe epilepsy who have medication-resistant seizures (who have failed at least two AEDs approved for treatment of focal onset seizures at appropriate doses), even early on in the course of their epilepsy, benefit from antromedial temporal resection with regard to seizure outcome. [109] Presently, patients wait an average of over 20 years before undergoing AMTR. What was not demonstrated, because of closure of the study due to slow recruitment, was a benefit on quality of life. However, other studies have demonstrated a significant improvement in quality of life in patients rendered seizure-free with surgery. One note of caution is that a proportion of those patients who underwent dominant temporal lobectomy had a statistically-significant decline in verbal funtion on neuropsychological testing. Several prior studies strongly suggest that the latter occurs over years with persistent complexpartial seizures, with or without progression to tonic-clonic seizures. Therefore, delaying surgery may not prevent verbal deficits and likely exposes the patient to all the negative consequences of continued seizures. [110]
Corpus callosotomy
The patient is maintained on the same anticonvulsant regimen as before surgery. Seizures may increase transiently during the postoperative first week. Although seizure outcome varies from patient to patient, the authors’ results seem typical of other published reports.
In general, this operation should decrease but not stop seizures for most patients. A 60-70% decrease can be expected for more than 80% of patients. Approximately 10-15% of patients receive no worthwhile benefit.
The major problem with callosotomy is its unpredictable and/or incomplete seizure control. Nevertheless, for patients with frequent seizures (especially frequent status epilepticus) or seizure-related injuries, it may provide significant benefit. These patients are often observed to continue to improve in seizure control over the years after surgery, though this observation has never been objectively documented. Whether this is a long-term effect of surgery or the natural history of epilepsy is not clear.
Neurologic sequelae are few. Transient left-sided hemiparesis has been reported. Lateral decubitus positioning may eliminate much of this problem. Temporary bladder incontinence has been associated with damage to the cingulate gyrus.
Numerous psychological studies have been performed following various degrees of midline commissurotomy. Although results can demonstrate problems in transfer of interhemispheric information, the changes do not seem to hinder patients in activities of daily living. Many of the earlier studies involved patients with more extensive commissurotomies, rather than partial or complete callosotomy. Memory difficulties have been reported after complete callosotomy.
A deterioration of impulse control and an increase in aggressive outbursts has been observed after callosotomy in some children with mental handicaps. Although no formal measurements of this behavior have been reported, the authors found that some patients are more alert and more aware of their surroundings after surgery and, hence, may be frustrated more easily.
Callosotomy disrupts electroencephalographic (EEG) bilateral synchrony but does not eliminate epileptiform discharges. Corpus callosotomy may be used diagnostically to help define the side of seizure onset in some patients with frontal lobe epilepsy and secondary bilateral synchrony. However, the authors had little success with this approach when it was tried on 6 patients.
In 1993, Spencer et al reported more intense focal seizures or emergence of focal seizures after corpus callosotomy, especially in patients with asymmetric bilateral epileptogenic foci. [111] The authors have observed 1 patient in whom this occurred, but seizures were reduced with alterations in medications, so that the overall benefits of surgery were favorable.
Multiple subpial transection
In 1989, Morrell et al reported on multiple subpial transaction (MST) in 32 patients but were able to document 5-year seizure outcomes in only 20 patients. [8] Complete seizure control was obtained in 11 (55%) patients. Taken in the context of extratemporal epilepsy, these results are comparable with those of cortical resection. MST involved the precentral gyrus in 16 patients, the postcentral cortex in 6, Broca’s area in 5, and Wernicke’s area in 5; none of the 32 patients experienced clinically significant behavioral deficits.
Evaluation of the antiseizure efficacy of MST was confounded by the inclusion of patients with progressive neurologic diseases and structural lesions (eg, tumors). For example, whether continued seizures in a patient with chronic encephalitis represented failure of MST or progression of the disease is unknown. Whether seizure control after MST and lesionectomy was attributable to lesionectomy or MST is unknown. On the other hand, this pioneering report documented the minimal cognitive or behavioral sequelae of the procedure.
Subsequent reports support the findings of Morrell et al. In 1991, Shimizu et al reported on 12 cases of MST, with fair results. [112] In 1995, Sawhney et al reviewed 21 cases of MST, with 18 patients having epilepsy and 3 having Landau-Kleffner syndrome (LKS). [113] Of the 18 patients with epilepsy, 8 demonstrated focal abnormalities on MRI, including 6 with chronic encephalitis and 1 with a tumor. Cortical resection plus MST was performed in 12 patients, with 11 showing worthwhile seizure decreases. None of the 21 patients developed chronic neurologic deficits.
In 1996, Pacia et al reported on a series of 21 patients. [114] Only 3 of the 21 underwent MST alone; the other 18 had additional cortical resections.
In 1997, Hufnagel et al described less satisfactory results in a series of 22 patients with a mixture of resections and MST. [115] Also in 1997, Devinsky et al reported on 13 patients with MST in language cortex whose cases were followed for longer than 1 year (11 patients underwent additional resective surgery). [116] Although 11 of the 13 had greater than 90% seizure reduction, MST patients had significantly poorer postoperative naming, verbal fluency, and oral reading than patients with dominant temporal lobe resections that spared language function.
These additional reports make assessing the true antiseizure effect of MST alone difficult: assessment is hindered by the inclusion of patients with progressive neurodegenerative diseases or a combination of lesionectomy and cortical resection plus MST.
In 1995, Wyler et al reported on 6 patients who underwent only precentral and/or postcentral MST without resection. [117] In a longer follow-up of that series, 2 patients (30%) were seizure free; 3 (50%) showed greater than 90% seizure reduction; and 1 did not benefit significantly. Four patients had MST of primary language cortex. Patients with MST of precentral language areas showed subjective improved fluency, and those in postcentral speech areas showed no deficits.
Wyler changed the technique from Morrell’s original description by pointing the instrument tip downward (away from the pia) rather than toward the pia. He believes this is technically easier and results in less subpial hemorrhage. A special knife has been developed (Ad-Tech Medical, Racine, Wis) for this purpose.
In 1997, Patil et al reported good results in patients undergoing MST only from large multilobar areas or bilateral areas of cortex; they also reported a series of patients with bilateral foci treated with MST plus other minimally resective procedures. [9]
Perhaps the most sensitive validation of the antiepileptic effects of MST with preservation of underlying cortical function is its application to patients with LKS. LKS, also known as acquired epileptiform aphasia, is characterized by acquired verbal auditory agnosia in childhood with epileptiform discharges in central regions in most cases and superior temporal regions in some.
Children often become mute and unresponsive to verbal commands after initially acquiring rudimentary language. Some children show no overt seizures in spite of obvious epileptiform discharges that become more plentiful during sleep; at times these discharges are so diffuse and continuous that they resemble the epilepsy syndrome of continuous spike waves in slow-wave sleep. AED therapy to help the patient regain speech is usually ineffective. Steroid therapy may provide temporary improvement but seldom yields a long-lasting benefit.
In 1995, Sawhney et al reported on 3 preoperatively mute patients with LKS who showed “substantial recovery of speech” months after MST. [113] In 1995, Morrell et al reported on 14 children with LKS who were treated with MST. [118] Of these, 7 recovered age-appropriate speech and were taking regular classes in school, and 4 have shown marked improvement. Thus, 11 of the 14, none of whom possessed communicative language for at least 2 years preoperatively, are now speaking.
These results have several important implications. First, they add additional evidence that this novel technique is effective in eliminating epileptiform electrical activity from cortex while preserving function. Second, they support the concept of early surgical intervention in well-defined epileptic childhood syndromes for fear that delays result in irreversible cognitive and/or behavioral sequelae.
The authors have applied MST to several children with LKS, with mixed benefit. Interestingly, in most cases, the disruptive behavior displayed by these children improves after surgery.
Functional hemispherectomy
Functional hemispherectomy has a seizure free outcome of between 54-90%, depending on the series reported and the type of syndrome for which the procedure is performed. Some patients have minor seizures after surgery that are not considered disabling. When overall improvement includes nondisabling seizures, the percentage of patients who are significantly improved by functional hemispherectomy rises to 80-90% in most series. [12, 105, 13, 119]
Some authors have found the underlying pathologic substrate to have a bearing on postoperative outcome, with patients with migrational disorders and hemimegalencephaly doing less well than other disease processes. [15, 120] In addition, higher preoperative cognitive ability, shorter duration of seizures and better postoperative seizure control are more likely to correlate with higher postoperative developmental gains. [12]
Seizure freedom is important in improving postoperative cognition, but quality of life scores have improved even when some minor seizures persist. [15, 120, 105]
Physicians and parents are often concerned about the motor consequences of functional hemispherectomy. Usually, the decision to proceed to hemispherectomy is taken after the patient has a complete hemiparesis. However, in the case of a progressive disease process, such as Rasmussen encephalitis, some centers advocate early surgery, before complete hemiparesis develops, so as to avoid developmental delay that might be caused by epileptic encephalopathy. [15]
Several series have reported on motor outcomes after functional hemispherectomy. [15, 120, 119] Most children remain unchanged by surgery, with a return to baseline motor function in the contralateral leg within 1 year of surgery. The exception to this generalization is that increased tone in the contralateral hand compromises function of that hand and does not return to baseline.
A small percentage of children will have a worsening of hemiparesis, and a few will have an improvement in motor function. Most patients (89%) are ambulatory after surgery: those patients who walked before surgery are likely to be ambulatory after surgery as well. [105]
Overall, gains in activities of daily living, quality of life, and social interactions outweigh the increased motor deficit in the hand that occurs after functional hemispherectomy. [119]
Complications
In a large surgical series compiled prospectively by Kraemer, serious complications occurred after AMTR in less than 4% of patients. Two cases of hemiparesis occurred in 160 temporal lobectomies (1.25%), due to damage of the perforators to the anterior choroidal artery that supplies the internal capsule. Paralysis was present immediately and resolved completely in one patient, but it prevented another patient from returning to work.
Use of the operating microscope, careful attention to en-passant blood vessels around the brainstem and thalamus, minimal retraction of the mesial structures and restraint of the bipolar cautery on the choroid plexus can minimize the risk of this complication occurring after AMTR.
The anticipated visual-field deficit from AMTR is a contralateral superior quadrantanopsia resulting from damage to Meyer’s loop as it courses anteriorly around the temporal horn of the lateral ventricle. When this deficit is present, it rarely causes significant disability and often goes unnoticed by the patient unless a formal visual-field evaluation is performed. It typically is not a barrier to driving or work.
In the series by Kraemer (see above), 3 unanticipated visual-field deficits occurred: 1 patient had an infarct to the optic radiation, and 2 others developed homonomous hemianopsia when the temporal lobe resection extended beyond 7 cm from the temporal pole (1.8%). Homonomous hemianopsia can occur from vascular damage to the geniculate body or optic tract by a mechanism similar to what causes hemiparesis.
Again, careful microsurgical technique can minimize this risk. Minimal lateral cortical and maximal medial resection can minimize the risk of injury to Meyer’s loop.
In the above series, infection developed in 2% of patients. All infections occurred locally within the suture line and necessitated removal of the bone flap and prolonged IV antibiotics. No infection resulted in a permanent neurologic injury, and bacterial meningitis was not encountered.
Cranial nerve III or IV palsies are less common if surgeons use the operating microscope. Ocular motor paresis after AMTR has become much less common; however, it may occur in up to 20% of cases. Because cranial nerve III lies directly beneath the pial surface of the uncus, it can develop temporary dysfunction from mild manipulation during dissection.
Cranial nerve IV can be damaged if the current of the bipolar cautery is set too high when coagulating near the edge of the tentorium, from traction on the tentorium during the surgery, or possibly even by drainage of cerebrospinal fluid (CSF) during the procedure.
Temperature may be elevated for the first few days after most craniotomies. However, if fever continues longer than approximately 72 hours in the setting of good pulmonary toilet, aseptic or bacterial meningitis should be suspected. A sudden headache associated with a sharp rise in temperature can, in some cases of aseptic meningitis, mar an uneventful convalescence.
The diagnosis is made if findings from lumbar puncture demonstrate sterile xanthochromic CSF under pressure (with several hundred to several thousand leukocytes per cubic millimeter) and CSF cultures are negative. Treatment includes an antipyretic and dexamethasone.
Verbal memory problems have been noted, particularly in patients with speech-dominant temporal lobe resection. These problems are more severe than the deficits in visual-spatial memory that are thought to occur after nondominant AMTR. Patients without hippocampal sclerosis are at greatest risk for this complication.
The most consistent and reliable clinical indicator for or against this complication is age at first seizure: if the patient’s first seizure (including a febrile seizure) occurred before age 6 years, the risk of increased memory problems postoperatively is slight. A history of severe alcohol abuse also places a person at higher risk for global memory problems after AMTR.
-
Examples of various grid electrodes available for specific needs. These range in size and number of contacts.
-
Examples of 3 depth electrodes with varying numbers of contacts. Note that stylus is in place and is removed once electrode has been inserted.
-
Method for positioning patient for temporal lobectomy. Head is angled so that malar prominence is highest portion of patient's head.
-
Pterional incision is made, exposing temporalis muscle and skull.
-
After muscle is retracted and small craniotomy performed, dura is opened to expose temporal tip and small portion of suprasylvian cortex.
-
Lateral cortex is resected, leaving hippocampus (H). Tentorium (T) and choroid plexus (CH) should be identified.
-
Hippocampus is removed as separate en-bloc specimen for pathologic evaluation. Resection is carried back to level of collicular plate. Peduncle and third nerve should be visible through intact pia.
-
Craniotomy showing intracranial grid in place. Grid lies over left primary motor and sensory cortex, near vertex of skull. Patient is positioned laterally, and face is at right. Intracranial study was performed to document seizure onsets in patient who had previous oligodendroglioma removed. Seizures began just posterior to leg motor cortex, in primary sensory cortex.
-
Primary motor cortex is located with use of extraoperative somatosensory evoked potentials and intraoperative cortical stimulation, as well as grid previously described (see above). Penfield instrument in field is positioned over primary motor cortex.
-
Lateral position for insertion of lumbar drain.
-
Patient positioned for lumbar drain insertion before corpus callosotomy.
-
Surgical site preparation for corpus callosotomy. Right hemisphere is inferior, and left hemisphere is superior. In this position, left hemisphere will be held out of field by falx cerebri, and right hemisphere will fall away from field, minimizing need for traction.
-
Incision of dura mater, adjacent to sagittal sinus.
-
Lateral and medial surfaces of cerebrum, showing major sulci and gyri.