Overview
The normal function of the urinary bladder is to store and expel urine in a coordinated, controlled fashion. This coordinated activity is regulated by the central and peripheral nervous systems. [1] Neurogenic bladder is a term applied to urinary bladder malfunction due to neurologic dysfunction emanating from internal or external trauma, disease, or injury.
Symptoms of neurogenic bladder range from detrusor underactivity to overactivity, depending on the site of neurologic insult. The urinary sphincter also may be affected, resulting in sphincter underactivity or overactivity and loss of sphincter coordination with bladder function. The appropriate therapy for neurogenic bladder and a successful treatment outcome are predicated upon an accurate diagnosis through a careful medical and voiding history, together with a variety of clinical examinations, including urodynamics and selective radiographic imaging studies.
Neuroanatomy
Normal voiding is essentially a spinal reflex modulated by the central nervous system (brain and spinal cord), which coordinates function of the bladder and urethra. The bladder and urethra are innervated by 3 sets of peripheral nerves arising from the autonomic nervous system (ANS) and somatic nervous system. The central nervous system is composed of the brain, brain stem, and the spinal cord.
Brain
The brain is the master control of the entire urinary system.
Cognitive control of micturition is achieved by communication from a number of brain structures to the periaqueductal gray matter, which then exerts control over the pontine micturition center to suppress or trigger a voiding reflex. Overall, the brain receives input via afferent pathways that ascend from the bladder and provide feedback on how full the bladder is. Higher brain centers then determine whether it is socially acceptable to void and trigger downstream structures to permit or suppress the voiding reflex.
As a result of dependence upon higher brain centers, certain lesions or diseases of the brain (eg, stroke, cancer, dementia) can result in a loss of voluntary control of the normal micturition reflex as well as symptoms such as urinary urgency.
The signal transmitted by the brain is routed through 2 intermediate segments (the brainstem and the sacral spinal cord) prior to reaching the bladder.
Brainstem
The brainstem is located at the base of the skull. Within the brainstem is the pons, a specialized area that serves as a major relay center between the brain and the bladder (see the image below). The pons is responsible for coordinating the activities of the urinary sphincters and the bladder. The mechanical process of urination is coordinated in an area of the pons known as the pontine micturition center (PMC). The PMC coordinates the urethral sphincter relaxation and detrusor contraction to facilitate urination. The pons relays afferent information from the bladder to higher brain centers, which in turn communicate with the periaqueductal gray matter, a relay station that collects higher brain center intput and processes this in order to signal the PMC to trigger or suppress the voiding reflex.
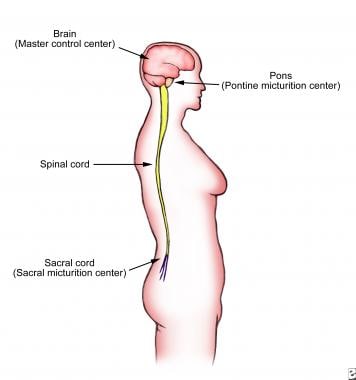 The pons is a major relay center between the brain and the bladder. The mechanical process of urination is coordinated by the pons in the area known as the pontine micturition center (PMC).
The pons is a major relay center between the brain and the bladder. The mechanical process of urination is coordinated by the pons in the area known as the pontine micturition center (PMC).
The conscious sensations associated with bladder activity are transmitted to the pons from the cerebral cortex. The interaction of a variety of excitatory and inhibitory neuronal systems influence the activity of the PMC, which by default attempts to trigger the voiding reflex. This voiding reflex causes the urethral sphincters to open while facilitating the detrusor to contract and expel the urine.
Emotions, experienced in higher brain centers, may exert downstream effects on the PMC, which is why some people can experience incontinence with excitment or fear. The ability of the brain to control the PMC is part of the social training that children experience during growth and development. Usually the brain takes over the control of the pons, via the periaqueducatal gray matter, when children undergo toilet training.
When the bladder becomes full, the stretch receptors of the detrusor muscle send a signal to the pons, which in turn notifies the brain. People perceive this signal (bladder fullness) as a sudden desire to urinate, or urinary urge. Under normal situations, the brain sends an inhibitory signal to the pons via the periaqueductal gray matter to inhibit the bladder from contracting until a bathroom is found.
When the PMC is deactivated, the urge to urinate disappears, allowing the patient to delay urination until finding a socially acceptable time and place. When urination is appropriate, the brain removes its suppression of the PMC via the periaqueductal gray matter, allowing the urinary sphincter to open and the detrusor to contract and empty the bladder.
Spinal cord
The spinal cord extends from the brainstem down to the lumbosacral spine. It is located in the spinal canal and is protected by the cerebrospinal fluid, meninges, and vertebral column. The spinal cord functions as a long communication pathway between the brainstem and the sacral spinal cord. When the sacral cord receives the sensory information from the bladder, this signal travels up the spinal cord to the pons and then ultimately to higher brain centers. The brain interprets this signal and sends a reply via the pons that travels down the spinal cord to the sacral cord and, subsequently, to the bladder. This 'reply' signal, part of the voiding reflex, can be suppressed by the periaqueductal gray matter inhibiting the pons.
In the normal cycle of bladder filling and emptying, the spinal cord acts as an important intermediary between the pons and the sacral cord. An intact spinal cord is critical for normal micturition.
Depending upon the level of lesion, a spinal cord injury can lead to urinary frequency, urgency, and urge incontinence, which may be complicated by difficulty emptying the bladder. This occurs because the urinary bladder and the sphincter are no longer coordinated and both exert overactivity, a condition termed detrusor sphincter dyssynergia (DSD).
The sacral spinal cord is the terminal portion of the spinal cord, situated at the lower back in the lumbar area. This is a specialized portion of the spinal cord known as the sacral reflex center. It is responsible for bladder contractions. The sacral reflex center is the primitive voiding center.
In infants, the higher center of voiding control (the brain) is not mature enough to command the bladder, so control of urination comes from signals sent from the sacral cord. When urine fills the infant bladder, an excitatory signal is sent to the sacral cord. When this signal is received by the sacral cord, the spinal reflex center automatically triggers the detrusor to contract. The result is involuntary detrusor contractions with coordinated voiding.
A continuous cycle of bladder filling and emptying occurs, which is why infants and young children are dependent on diapers until they are toilet trained. As the child's brain matures and develops, it gradually attains control of the bladder and the urinary sphincters to inhibit involuntary voiding. Control of the voiding process transitions from the sacral reflex center to the pontine micturition center, which is further modulated by higher brain centers that process emotion and social context.
Severe injury to the sacral cord results in loss of bladder function. Affected patients may develop urinary retention, termed detrusor areflexia. The detrusor will be unable to contract, so the patient will not be able to urinate and urinary retention will occur.
Peripheral nerves
Peripheral nerves form a network of pathways for sending and receiving information throughout the body. The nerves enter and exit the spinal cord, which then relays nerual information to and from the brain. The bladder and the urethral sphincters are under the influence of related but separate neural pathways.
The autonomic nervous system lies outside of the central nervous system. It regulates the actions of the internal organs under involuntary control. The autonomic nervous system is divided into the sympathetic and the parasympathetic systems.
When the sympathetic nervous system is active, it causes the bladder to increase its capacity without increasing detrusor resting pressure (accommodation) and stimulates the internal urinary sphincter to remain tightly closed. The sympathetic activity also inhibits parasympathetic stimulation, preventing bladder contractions. When the sympathetic nervous system is active, urinary accommodation occurs and the micturition reflex is suppressed.
The parasympathetic nervous system functions in a manner opposite to that of the sympathetic nervous system. In terms of urinary function, the parasympathetic nerves stimulate the detrusor to contract. Immediately preceding parasympathetic stimulation, the sympathetic influence on the internal urethral sphincter becomes suppressed so that the internal sphincter relaxes and opens. In addition, the activity of the pudendal nerve, a somatic nerve, is inhibited to cause the external sphincter to open. The result is facilitation of voluntary urination.
The somatic nervous system regulates the actions of the muscles under voluntary control. Examples of these muscles are the external urinary sphincter and the pelvic diaphragm. The pudendal nerve originates from the nucleus of Onuf and regulates the voluntary actions of the external urinary sphincter and the pelvic diaphragm. Activation of the pudendal nerve causes contraction of the external sphincter, which occurs with activities such as Kegel exercises (which also target the large pelvic floor muscles). Some reflex activity may also occur through the pudendal nerve, such as the sphincter contractions that occur during sneezing, coughing, and laughing—the so-called continence reflex.
Physiology and Pathophysiology
Physiology
During the course of a day, an average person will void approximately 4-8 times. The urinary bladder is in storage mode for most of the day, allowing the individual to engage in more important activities than urination.
Normal bladder function consists of 2 phases: filling and emptying. The normal micturition cycle requires that the urinary bladder and the urethral sphincter work together as a coordinated unit to store and empty urine. During urinary storage, the bladder acts as a low-pressure receptacle, while the urinary sphincter maintains high resistance to urinary flow, in order to keep the bladder outlet closed. During urine elimination, the bladder contracts to expel urine while the urinary sphincter opens (low resistance) to allow unobstructed urinary flow and bladder emptying.
Filling phase
During the filling phase, the bladder accumulates increasing volumes of urine while the pressure inside the bladder remains low. The pressure within the bladder must be lower than the urethral pressure during the filling phase. If the bladder pressure is greater than the urethral pressure (resistance), urine will leak out.
The filling of the urinary bladder depends on the intrinsic viscoelastic properties of the bladder and the inhibition of the parasympathetic nerves. Thus, bladder filling primarily is a passive event.
Sympathetic nerves also facilitate urine storage in the following ways:
-
Sympathetic nerves inhibit the parasympathetic nerves from triggering bladder contractions.
-
Sympathetic nerves directly cause relaxation and expansion of the detrusor muscle.
-
Sympathetic nerves close the bladder neck by constricting the internal urethral sphincter. This sympathetic input to the lower urinary tract is constantly active during bladder filling.
As the bladder fills, the pudendal nerve becomes excited. Stimulation of the pudendal nerve results in contraction of the external urethral sphincter. Contraction of the external sphincter, coupled with that of the internal sphincter, maintains urethral pressure (resistance) higher than normal bladder pressure. This increase in urethral pressure with filling is the continence reflex.
The pressure gradients within the bladder and urethra play an important functional role in normal micturition. As long as the urethral pressure is higher than that of the bladder, the person will remain continent. If the urethral pressure is abnormally low or if the intravesical pressure is abnormally high, urinary incontinence will result.
During some physical activities and with coughing, sneezing, or laughing, the pressure within the abdomen rises sharply. This rise is transmitted to the bladder, and in response the urethra both anatomically and functionally is designed to increase its pressure and maintain continence. When the pressure transmitted to the bladder is greater than that within the urethra, urine will leak out, resulting in stress incontinence.
Emptying phase
The storage phase of the urinary bladder can be switched to the voiding phase either involuntarily (reflexively) or voluntarily. Involuntary reflex voiding occurs in an infant when the volume of urine exceeds the voiding threshold. When the bladder is filled to capacity, the stretch receptors within the bladder wall signal the sacral cord. The sacral cord, in turn, sends a message back to the bladder to initiate urination.
At this point, the pudendal nerve causes relaxation of the urethral sphincter, which is also accompanied by broader pelvic floor relaxation. The sympathetic nerves send a message to the internal sphincter to relax and open, resulting in a lower urethral resistance. When the urethral sphincters relax and open, the parasympathetic nerves trigger contraction of the detrusor. When the bladder contracts, the pressure generated by the bladder overcomes the urethral pressure, resulting in urinary flow. These coordinated series of events allow unimpeded, automatic release of the stored urine. While conscious control of this reflex develops after infancy, the primitive voiding reflex may reappear with spinal cord injuries.
Delaying voiding or voluntary voiding
Bladder function is automatic but completely governed by the brain, which makes the final decision on whether or not to void. The normal function of urination means that an individual has the ability to stop and start urination on command. In addition, the individual has the ability to delay urination until a socially acceptable time and place. The healthy adult is aware of bladder filling and can willfully initiate or delay voiding.
In a healthy adult, the PMC functions as an on-off switch that is signalled by stretch receptors in the bladder wall and is, in turn, modulated by inhibitory and excitatory neurologic influences from the brain. When the bladder is full, the stretch receptors are activated. The individual perceives the activation of the stretch receptors as the bladder being full, which signals a need to void or the sensation of urinary urge.
When an individual cannot find a bathroom nearby, the brain bombards the PMC with a multitude of inhibitory signals, via the periaqueductal gray matter, to prevent detrusor contractions. At the same time, an individual may actively contract the levator muscles to keep the external sphincter closed or initiate distracting techniques to suppress urination.
Pathophysiology
If a problem occurs within the nervous system, the entire voiding cycle is affected. Any part of the nervous system may be affected, including the brain, pons, spinal cord, sacral cord, and peripheral nerves. A dysfunctional voiding condition results in different symptoms, ranging from acute urinary retention to an overactive bladder or to a combination of both.
Urinary incontinence results from a dysfunction of the bladder, the sphincter, or both. Overactive bladder is associated with the symptoms of urge incontinence, while sphincter underactivity (decreased resistance) results in symptomatic stress incontinence. A combination of detrusor overactivity and sphincter underactivity may result in mixed symptoms.
Brain lesions
Lesions of the brain above the pons interrupt the higher conscious control of voiding. The voiding reflexes of the lower urinary tract—the primitive voiding reflex—remain intact. Affected individuals show signs of urge incontinence and experience symptoms of overactive bladder. The bladder empties too quickly and too often, with relatively low volumes of urine, and deferring voiding or storing a large volume becomes difficult. Waking frequently at night to void is also common in such situations.
Typical examples of a brain lesion are stroke, brain tumor, and head trauma. Hydrocephalus, cerebral palsy, and Shy-Drager syndrome are also central nervous system pathologies that impact voiding function. Dementia can impact the socially appropriate control of voiding, as well.
Spinal cord lesions
Diseases or injuries of the spinal cord between the pons and the sacral spinal cord also result in overactive bladder, often accompanied by urge incontinence. The bladder empties too frequently and the overall picture can be similar to that of a brain lesion, except that the external sphincter may paradoxically contract. If both the bladder and external sphincter become spastic at the same time, the affected individual will sense an overwhelming desire to urinate but only a small amount of urine may dribble out. This is termed detrusor-sphincter dyssynergia, as the bladder and the external sphincter are not in synergy.
The causes of spinal cord injuries include physical trauma, tumors, ischemia, and multiple sclerosis (MS) as well as some other neurodegenerative conditions. Children born with myelomeningocele may have neurogenic bladder from birth or can develop it later upon growing due to tethering of the spinal cord.
Sacral cord injury
Selected injuries of the sacral cord and the corresponding nerve roots arising from the sacral cord may prevent the bladder from emptying and the patient from sensing a full bladder. Perosns who cannot sense a full bladder may be at risk for urinary retention and damage to the kidneys from the high pressure from storage of large urine volumes.
If the bladder cannot contract, a condition called detrusor areflexia is present, which also leads to the storage of large urine volumes and can be accompanied by overflow incontinence. Typical causes are a sacral cord tumor, herniated disc, and injuries that crush the pelvis. This condition also may occur after a lumbar laminectomy, radical hysterectomy, or abdominoperineal resection in some cases. Rapid growth in childhood can also lead to detrusor areflexia from a tethered spinal cord in patients with prior trauma or congenital malformations such as spina bifida.
Peripheral nerve injury
Diabetes mellitus, AIDS, and iatrogenic injury can result in peripheral neuropathy that causes urinary retention. These disorders interrupt the nerves to the bladder and may lead to silent, painless distention of the bladder. Patients with longstanding diabetes also often have an impaired sensation of bladder filling, complicating the situation further. As with sacral cord injury, affected individuals will have difficulty urinating and can develop a hypocontractile bladder. Other diseases resulting in this condition are poliomyelitis, Guillain-Barré syndrome, severe genitoanal herpes infection, pernicious anemia, and neurosyphilis (tabes dorsalis).
Types of Neurogenic Bladder
Types of neurogenic bladder can be classified in terms of the anatomic location of the causative lesion, as follows:
-
Supraspinal lesions
-
Spinal lesions
-
Peripheral nerve lesions
Supraspinal Lesions
Supraspinal lesions involve the central nervous system above the pons. They include stroke, brain tumor, Parkinson disease, and Shy-Drager syndrome.
Stroke
After a stroke, the brain may enter into a temporary acute cerebral shock phase. During this time, the urinary bladder will be in retention—detrusor areflexia. Almost 25% of affected individuals develop acute urinary retention after a stroke.
After the cerebral shock phase wears off, the bladder demonstrates detrusor hyperreflexia with coordinated urethral sphincter activity. This occurs because the PMC is released from the cerebral inhibitory center. Patients with detrusor hyperreflexia complain of urinary frequency, urinary urgency, and urge incontinence.
The treatment for the cerebral shock phase is indwelling Foley catheter placement or clean intermittent catheterization (CIC). Detrusor hyperreflexia is treated with anticholinergic medications to facilitate bladder filling and storage.
Brain tumor
Detrusor hyperreflexia with coordinated urethral sphincter is the most common observed urodynamic pattern associated with a brain tumor. These patients complain of urinary frequency and urgency and urge incontinence. First-line treatment for detrusor hyperreflexia includes anticholinergic medication.
Parkinson disease
This is a degenerative disorder of pigmented neurons of substantia nigra. It results in dopamine deficiency and increased cholinergic activity in the corpus striatum. Symptoms specific to the urinary bladder include urinary frequency, urinary urgency, nocturia, and urge incontinence.
Typical urodynamic findings for Parkinson disease are most consistent with detrusor hyperreflexia and urethral sphincter bradykinesia. The striated urethral sphincter often demonstrates poorly sustained contraction.
As with other supraspinal lesions, the treatment for Parkinson disease is to facilitate bladder filling and promote urinary storage with anticholinergic agents.
In men with Parkinson disease who exhibit symptoms of bladder outlet obstruction (BOO) due to benign prostatic hypertrophy (BPH), the diagnosis of BOO should be confirmed by multichannel urodynamic studies. The most common cause of postprostatectomy incontinence in the patient with Parkinson disease is detrusor hyperreflexia. If transurethral resection of the prostate (TURP) is performed without urodynamic confirmation of obstruction, the patient may become totally incontinent after the TURP procedure.
Shy-Drager syndrome
Shy-Drager syndrome is a rare, progressive, degenerative disease affecting the autonomic nervous system with multisystem organ atrophy. In addition to parkinsonlike symptoms, patients often exhibit cerebellar ataxia and autonomic dysfunction. Clinical manifestations include orthostatic hypotension, anhidrosis, and urinary incontinence.
Degeneration of the nucleus of Onuf results in denervation of the external striated sphincter. Sympathetic nerve atrophy causes nonfunctional bladder and an open bladder neck.
Urodynamic evaluation often reveals detrusor hyperreflexia, although a few patients may have detrusor areflexia or poorly sustained bladder contractions. Often, the bladder neck (internal sphincter) will be open at rest, with striated sphincter denervation.
The treatment for Shy-Drager syndrome is to facilitate urinary storage with anticholinergic agents coupled with CIC or indwelling catheter. Patients with Shy-Drager syndrome should avoid undergoing TURP because the risk of total incontinence is high.
Spinal Cord Lesions
Neurogenic bladder from spinal cord lesions may take various forms, depending on the mechanism and site of injury.
Spinal cord trauma
When an individual sustains a spinal cord injury (eg, from a diving accident or motor vehicle injury), the initial neurologic response is spinal shock. During this spinal shock phase, the affected individual experiences flaccid paralysis below the level of injury, and the somatic reflex activity is either depressed or absent.
The anal and bulbocavernosus reflex typically is absent. The autonomic activity is depressed, and the individual experiences urinary retention and constipation. Urodynamic findings are consistent with areflexic detrusor and rectum. The internal and external urethral sphincter activities, however, are normal.
The spinal shock phase typically lasts 6-12 weeks but may persist longer in some cases. During this time, the urinary bladder must be drained with CIC or indwelling urethral catheter.
When the spinal shock phase wears off, bladder function returns but the detrusor activity increases in reflex excitability to an overactive state (ie, detrusor hyperreflexia). Depending on the level of the lesion, detrusor sphincter dyssynergia–detrusor hyperreflexia (DSD-DH) may develop. Thus, these patients must be monitored for leaking between CIC, and periodic urodynamic testing must be performed for this alteration in detrusor behavior. During urodynamic studies, intravesical instillation of cold saline may indicate return of reflex activity or help better characterize the lesion.
Realizing that suprasacral lesions exhibit detrusor areflexia at initial insult but progress to hyperreflexic state over time is important. Conversely, sacral cord lesions are associated with areflexic bladders that may become hypertonic over time.
Spinal cord lesions above the sixth thoracic vertebra
Individuals who sustain a complete cord transection above the sixth thoracic vertebra (T6) most often will have urodynamic findings of detrusor hyperreflexia, striated sphincter dyssynergia, and smooth sphincter dyssynergia. A unique complication of T6 injury is autonomic dysreflexia, which is an exaggerated sympathetic response to any stimuli below the level of the lesion. This occurs most commonly with lesions of the cervical cord. Often, the inciting event is instrumentation of the urinary bladder or the rectum, causing visceral distention.
Signs and symptoms of autonomic dysreflexia include sweating, headache, hypertension, and reflex bradycardia. Acute management of autonomic dysreflexia is to decompress the rectum or bladder. Decompression usually will reverse the effects of unopposed sympathetic outflow. If additional measures are required, parenteral ganglionic or adrenergic blocking agents, such as chlorpromazine, may be used.
Oral blocking agents, including terazosin, may be used for prophylaxjis in patients with autonomic dysreflexia. Alternatively, spinal anesthesia may be used as a prophylactic measure whenever bladder instrumentation is to be performed.
Spinal cord lesions below T6
Individuals who sustain spinal cord lesions below T6 level will have urodynamic findings of detrusor hyperreflexia, striated sphincter dyssynergia, and smooth sphincter dyssynergia but no autonomic dysreflexia. Neurologic evaluation will reveal skeletal muscle spasticity with hyperreflexic deep tendon reflexes. Affected patients will demonstrate extensor plantar response and a positive Babinski sign.
These individuals will experience incomplete bladder emptying secondary to detrusor sphincter dyssynergia, or loss of facilitatory input from higher centers. The cornerstones of treatment are CIC and anticholinergic medications.
Multiple sclerosis
MS is caused by focal demyelinating lesions of the central nervous system. It most commonly involves the posterior and lateral columns of the cervical spinal cord. Usually, poor correlation exists between the clinical symptoms and urodynamic findings. Thus, using urodynamic studies to evaluate patients with MS is critical. [2]
The most common urodynamic finding is detrusor hyperreflexia, occurring in as many as 50-90% of patients with MS. As many as 50% of patients will demonstrate DSD-DH. Detrusor areflexia occurs in 20-30% of cases. The optimum therapy for a patient with MS and incontinence must be individualized and based on the urodynamic findings.
Peripheral nerve lesions
Peripheral nerve lesions resulting in detrusor areflexia may be due to any of the following:
-
Diabetes mellitus
-
Tabes dorsalis (neurosyphilis)
-
Herpes zoster
-
Herniated lumbar disc disease
-
Radical pelvic surgery
Diabetic cystopathy
Usually, neurogenic bladder dysfunction occurs 10 or more years after the onset of diabetes mellitus. Neurogenic bladder occurs because of autonomic and peripheral neuropathy. A metabolic derangement of the Schwann cell results in segmental demyelination and impaired nerve conduction.
The first symptoms of diabetic cystopathy are loss of sensation of bladder filling followed by loss of motor function. Classic urodynamic findings associated with this condition are elevated residual urine level, decreased bladder sensation, impaired detrusor contractility, and, eventually, detrusor areflexia. Paradoxically, detrusor hyperactivity with impaired contractile function (DHIC) also has been observed. Treatment of diabetic cystopathy is with CIC, long-term indwelling catheterization, or urinary diversion.
Tabes dorsalis
In tabes dorsalis, central and peripheral nerve conduction is impaired. Affected patients experience decreased bladder sensation and increased voiding intervals. The most common urodynamic finding associated with neurosyphilis is detrusor areflexia with normal sphincteric function.
Herpes zoster
Herpes zoster is a neuropathy associated with painful vesicular eruptions in the distribution of the affected nerve. The herpes virus lies dormant in the dorsal root ganglia or the sacral nerves.
Sacral nerve involvement leads to impairment of detrusor function. The early stages of herpes infection are associated with lower urinary tract symptoms of urinary frequency, urgency, and urge incontinence. Later stages include decreased bladder sensation, increased residual urine, and urinary retention. Urinary retention is self-limited and will resolve spontaneously with clearing of the herpes infection.
Lumbar disc herniation
Slow and progressive herniation of the lumbar disc may cause irritation of the sacral nerves and detrusor hyperreflexia. Conversely, acute compression of the sacral roots associated with deceleration trauma will prevent nerve conduction and result in detrusor areflexia.
A typical urodynamic finding in sacral nerve injury is detrusor areflexia with intact bladder sensation. Associated internal sphincter denervation may occur. If the peripheral sympathetic nerves are damaged, the internal sphincter will be open and nonfunctional. Peripheral sympathetic nerve damage often occurs in association with detrusor denervation. The striated sphincter, however, is preserved.
Pelvic surgery
Patients undergoing major pelvic surgery, such as radical hysterectomy, abdominoperineal resection, proctocolectomy, or total exenteration will experience bladder dysfunction postoperatively. Most commonly, postsurgical patients will manifest symptoms of detrusor areflexia. However, as many as 80% of affected patients will experience spontaneous recovery of function within 6 months after surgery.
Workup
Laboratory Studies
These include the following:
-
Urinalysis and urine culture – Urinary tract infection can cause irritative voiding symptoms and urge incontinence.
-
Urine cytology – Irritative voiding symptoms out of proportion to the overall clinical picture and/or hematuria warrant urine cytology and cystoscopy, as these may indicate carcinoma in situ of the urinary bladder
-
Kidney function studies – Blood urea nitrogen (BUN) and creatinine (Cr) are checked if compromise of kidney function is suspected.
Other Tests
A voiding diary is a daily record of the patient's bladder activity. It is an objective documentation of the patient's voiding pattern, incontinent episodes, and inciting events associated with urinary incontinence.
The pad test is an objective test that documents and can quantify urine loss. It may be helfpul to assess the severity of incontinence.
Diagnostic Procedures
Procedures used to investigate possible neurogenic bladder include the following:
-
Postvoid residual (PVR) bladder volume
-
Uroflow rate
-
Filling cystometrogram
-
Voiding cystometrogram (pressure-flow study)
-
Cystogram
-
Electromyography (EMG)
-
Cystoscopy
-
Videourodynamics
Postvoid residual bladder volume
PVR measurement is a part of the basic evaluation for urinary incontinence. If the PVR is high, the bladder may be poorly contractile or the bladder outlet may be obstructed. Both of these conditions can cause urinary retention with overflow incontinence.
Uroflow rate
Uroflow rate is a useful screening test used mainly to evaluate bladder outlet obstruction, but will also identify detrusor weakness. Uroflow rate is volume of urine voided per unit of time.
Low uroflow rate may reflect urethral obstruction, a weak detrusor, or a combination of both. This test alone cannot distinguish an obstruction from a contractile detrusor.
Filling cystometrogram
A filling cystometrogram (CMG) assesses the bladder capacity, compliance, and the presence of phasic contractions (detrusor instability). Most commonly, liquid filling medium is used.
An average adult bladder holds approximately 50-500 mL of urine. During the test, provocative maneuvers help to unveil bladder instability.
Voiding cystometrogram
Pressure-flow study simultaneously records the voiding detrusor pressure and the rate of urinary flow. This is the only test able to assess bladder contractility and the extent of a bladder outlet obstruction.
Pressure-flow studies can be combined with voiding cystogram and videourodynamic study for complicated cases of incontinence.
Cystogram
A static cystogram (anteroposterior and lateral) helps to confirm the presence of stress incontinence, the degree of urethral motion, and the presence of a cystocele. Intrinsic sphincter deficiency will be evident by an open bladder neck. The presence of a vesicovaginal fistula or bladder diverticulum also may be noted.
A voiding cystogram can assess bladder neck and urethral function (internal and external sphincter) during filling and voiding phases. A voiding cystogram can identify a urethral diverticulum, urethral obstruction, and vesicoureteral reflux.
Electromyography
EMG helps to ascertain whether voiding is coordinated or uncoordinated. Failure of urethral relaxation during bladder contraction results in uncoordinated voiding (detrusor sphincter dyssynergia). EMG allows accurate diagnosis of the detrusor sphincter dyssynergia that is common in spinal cord injuries.
Cystoscopy
The role of cystoscopy in the evaluation of neurogenic bladder is to allow discovery of bladder lesions (eg, bladder cancer, bladder stone) that would remain undiagnosed by urodynamics alone.
General agreement is that cystoscopy is indicated for patients complaining of persistent irritative voiding symptoms or hematuria. The physician can easily diagnose obvious causes of bladder overactivity, such as cystitis, stone, and tumor. This information is important in determining the etiology of the incontinence and may influence treatment decisions.
Videourodynamics
Videourodynamics is the criterion standard for evaluation of a patient with incontinence. Videourodynamics combines the radiographic findings of voiding cystourethrogram (VCUG) and multichannel urodynamics.
Videourodynamics enables documentation of lower urinary tract anatomy, such as vesicoureteral reflux and bladder diverticulum, as well as the functional pressure-flow relationship between the bladder and the urethra.
Treatment & Management
Medical Care
Treatment of urinary incontinence varies by type, as follows:
-
Stress incontinence may be treated with surgical and some non-surgical approaches
-
Urge incontinence may be treated with behavioral modification, pharmacotherapy, or third-line procedures
-
Mixed incontinence may require medications as well as surgery
-
Overflow incontinence is generally treated by emptying the bladder with a catheter
-
Other forms of incontinence may be resolved by treating the underlying cause (eg, urinary tract infection, constipation)
Do not consider anti-incontinence products to be a cure-all for urinary incontinence; however, judicious use of pads and devices to contain urine loss and maintain skin integrity are extremely useful in selected cases. Absorbent pads and internal and external collecting devices have an important role in the management of chronic incontinence. The criteria for use of these products are fairly straightforward, and they are beneficial in certain situations:
-
Failure of all other treatments and persistent incontinence
-
Illness or disability that prevents participation in care
-
Inability to benefit from medications
-
Incontinence disorders that cannot be corrected by surgery
-
Awaiting surgery
Absorbent products
Absorbent products are pads or garments designed to absorb urine to protect the skin and clothing. Available in both disposable and reusable forms, they are a temporary means of keeping the patient dry until a more permanent solution becomes available. By reducing wetness and odor, they help maintain the patient's comfort and allow them to function in normal activities. They may be used temporarily until a definitive treatment takes effect or if the treatment yields less-than-perfect results. Absorbent products are helpful during the initial assessment and workup of urinary incontinence. As an adjunct to behavioral and pharmacologic therapies, they play an important role in the care of persons with intractable incontinence.
Do not use absorbent products instead of definitive interventions to decrease or eliminate urinary incontinence. Early dependency on absorbent pads may be a deterrent to achieving continence, providing the wearer a false sense of security. The improper use of absorbent products may contribute to skin breakdown and urinary tract infections. Thus, appropriate use, meticulous care, and frequent pad or garment changes are needed when absorbent products are used.
Catheters
Urinary diversion, using various catheters, has been one of the mainstays of anti-incontinence therapy. The use of catheters for bladder drainage has withstood the test of time. Bladder catheterization may be a temporary measure or a permanent solution for urinary incontinence. Different types of bladder catheterization include indwelling urethral catheters, suprapubic tubes, and self-intermittent catheterization. [3]
Indwelling urethral catheters
Commonly known as Foley catheters, indwelling urethral catheters historically have been the mainstay of treatment for bladder dysfunction. If urethral catheters are used for a long-term condition, they must be changed at least monthly. These catheters may be changed at an office, a clinic, or at home by a visiting nurse. The standard catheter size for treating urinary retention is 16F or 18F, with a balloon filled to 10 mL of sterile water. Larger catheters (eg, 22F, 24F) with bigger balloons are used for treating grossly bloody urine found in other urologic conditions or diseases. Proper management of indwelling urethral catheters varies per individual.
The usual practice is to replace indwelling catheters and collection bags at least once monthly. However, catheters that develop encrustations and problems with urine drainage must be changed more frequently. All indwelling catheters that remain in the urinary bladder for more than 2 weeks become colonized with bacteria. Bacterial colonization does not mean the patient has clinical bladder infection. Symptoms of bladder infection include foul odor, purulent urine, and hematuria. Fever with flank pain often is present if upper tracts are involved. If bladder infection occurs, change the entire catheter and the drainage system. The urinary drainage bag does not need to be disinfected to prevent infection.
Routine irrigation of catheters is not required. However, some authors favor the use of 0.25% acetic acid irrigation because it is bacteriostatic, minimizes catheter encrustation, and diminishes the odor. When this method is used, 30 mL is instilled into the bladder and allowed to freely drain on a twice-daily basis.
Continuous antibiotic prophylaxis is not only unnecessary for patients with indwelling catheters, it is contraindicated, because it promotes the generation of bacteria that are resistant to common antibiotics. Use of an indwelling Foley catheter in individuals who are homebound requires close supervision by a visiting nurse and additional personal hygiene care.
In spite of its apparent advantages, the use of a Foley catheter for a prolonged period of time (eg, months to years) is strongly discouraged. Long-term use of urethral catheters poses significant health hazards. Indwelling urethral catheters are a significant cause of urinary tract infections that involve the urethra, bladder, and kidneys. Within 2-4 weeks after catheter insertion, bacteria will be present in the bladder of most women. Asymptomatic bacterial colonization is common and does not pose a health hazard. However, untreated symptomatic urinary tract infections may lead to urosepsis and death. The death rate of nursing home residents with urethral catheters has been found to be three times higher than that of residents without catheters; this may be more a reflection of the severity of comorbid conditions that lead to the clinical decision to use chronic bladder drainage than causation from the use of chronic bladder drainage. [4]
The use of a urethral catheter is contraindicated in the treatment of urge incontinence. Other problems associated with indwelling urethral catheters include encrustation of the catheter, bladder spasms resulting in urinary leakage, hematuria, and urethritis. More severe complications include formation of bladder stones, development of periurethral abscess, renal damage, and urethral erosion.
Another problem of long-term catheterization is bladder contracture, which occurs with urethral catheters as well as suprapubic tubes. Anticholinergic therapy (for patients with significant detrusor hyperreflexia) and intermittent clamping of the catheter in combination have been reported to be beneficial for preserving the bladder integrity with long-term catheter use. [5] Individuals who did not use the medication and daily clamping regimen experienced a decrease in bladder capacity. For this reason, some physicians recommend using anticholinergic medications with intermittent clamping of the catheter if lower urinary tract reconstruction is anticipated in the future.
Restrict the use of indwelling catheters to the following situations:
-
As comfort measures for the terminally ill
-
To avoid contamination or to promote healing of severe pressure sores
-
In cases of inoperable urethral obstruction that prevents bladder emptying
-
In individuals who are severely impaired and for whom alternative interventions are not an option
-
When an individual lives alone and a caregiver is unavailable to provide other supportive measures
-
For acutely ill patients who require accurate monitoring of fluid balance
-
For severely impaired persons for whom bed and clothing changes are painful or disruptive
Suprapubic catheters
A suprapubic tube is an attractive alternative to long-term urethral catheter use. The most common use of a suprapubic catheter is in individuals with spinal cord injuries and a malfunctioning bladder. Both paraplegic and quadriplegic individuals have benefited from this form of urinary diversion. When suprapubic tubes are needed, usually smaller (eg, 14F, 16F) catheters are placed. [6] Like the urethral catheter, suprapubic tubes should be changed once a month on a regular basis.
Suprapubic catheters have many advantages. With a suprapubic catheter, the risk of urethral damage is eliminated. Multiple voiding trials may be performed without having to remove the catheter. Because the catheter comes out of the lower abdomen rather than the genital area, a suprapubic tube is more patient-friendly. Bladder spasms occur less often because the suprapubic catheter does not irritate the trigone as does the urethral catheter. In addition, suprapubic tubes are more sanitary for the individual, and bladder infections are minimized because the tube is away from the perineum.
Suprapubic catheters are changed easily by either a nurse or a doctor. Unlike the urethral catheter, a suprapubic tube is less likely to become dislodged because the exit site is so small. When the tube is removed, the hole in the abdomen quickly seals itself with scar formation.
Indications for suprapubic catheters include short-term use following gynecologic, urologic, and other types of surgery. Suprapubic catheters may be used whenever the clinical situation requires the use of a bladder drainage device; however, suprapubic catheters are contraindicated in persons with chronic unstable bladders or intrinsic sphincter deficiency because involuntary urine loss is not prevented. A suprapubic tube does not prevent bladder spasms from occurring in unstable bladders nor does it improve the urethral closure mechanism in an incompetent urethra.
Potential complications of long-term suprapubic catheterization are similar to those associated with indwelling urethral catheters, including leakage around the catheter, bladder stone formation, urinary tract infection, and catheter obstruction.
During the initial placement of a suprapubic tube, a potential for bowel injury exists. Although uncommon, bowel perforation is known to occur with first-time placement of suprapubic tubes. Other potential complications include cellulitis around the tube site and hematoma.
If the suprapubic tube falls out inadvertently, the exit hole of the tube will seal up and close quickly within 24 hours if the tube is not replaced with a new one. If tube dislodgment is recognized promptly, a new tube can be reinserted quickly and painlessly as long as the tube site remains patent.
A suprapubic catheter is an alternative solution to an indwelling urethral catheter in patients who require long-term bladder drainage. Potential problems unique to suprapubic catheters include skin infection, hematoma, bowel injury, and problems with catheter reinsertion. Long-term management of a suprapubic tube also may be problematic if the healthcare provider lacks the knowledge and expertise of suprapubic catheter management or if the homebound individual lacks quick access to a medical center in case of an emergency. Nevertheless, in the appropriate situation, the suprapubic catheter affords many advantages over long-term urethral catheters.
Intermittent catheterization
Intermittent catheterization or self-catheterization is a mode of draining the bladder at timed intervals, as opposed to continuous bladder drainage. A prerequisite for self-catheterization is patients' ability to use their hands and arms; however, in a situation in which a patient is physically or mentally impaired, a caregiver or health professional can perform intermittent catheterization for the patient. Of all 3 possible options (ie, urethral catheter, suprapubic tube, intermittent catheterization), intermittent catheterization is the best solution for bladder decompression of motivated individuals who can physically and cognitively participate in their care.
Many studies of young patients with spinal cord injuries have shown that intermittent catheterization is preferable to indwelling catheters (ie, urethral catheter, suprapubic tube) for both men and women. Intermittent catheterization has become a healthy alternative to indwelling catheters for individuals with chronic urinary retention due to an obstructed, weak, or nonfunctioning bladder. Young children with myelomeningocele have also benefited from the use of intermittent catheterization.
For those children, antibiotic prophylaxis (low-dose chemoprophylaxis) has commonly been prescribed for urinary tract infections. A study by Zegers et al found that this practice can be safely discontinued, especially in boys, patients with low urinary tract infection rates, and patients without vesicoureteral reflux. [7]
Intermittent catheterization may be performed using a soft, red, rubber catheter or a short, rigid, plastic catheter. Plastic catheters are preferable to red rubber catheters because they are easier to clean and last longer.
The bladder must be drained on a regular basis, either based on a timed interval (eg, on awakening, every 3-6 hours during the day, and before bed) or based on bladder volume. Remember that the average adult bladder holds approximately 400-500 mL of urine. Ideally, the amount drained each time should not exceed 400-500 mL. This drainage limit may require decreasing the patient's fluid intake or increasing the frequency of catheterizations. For example, if catheterization is performed every 6 hours and the amount drained is 700 mL, increase the frequency of catheterization to, perhaps, every 4 hours to maintain the volume drained at 400-500 mL.
Intermittent catheterization is designed to simulate normal voiding. Usually, the average adult empties the bladder four to five times a day. Thus, catheterization should be done four to five times a day; however, individual catheterization schedules may vary, depending on the amount of fluid taken in during the day.
Candidates for intermittent catheterization must have motivation and intact physical and cognitive abilities. Anyone with good manual dexterity and an accessible urethra, including young children and older adults, can perform self-catheterization every day without problems. For individuals who are unable to self-catheterize, a home caregiver or a visiting nurse can be instructed to perform intermittent catheterization. Self-catheterization may be performed almost anywhere, including at home and at work.
Intermittent catheterization may be performed using either a sterile catheter or a nonsterile clean catheter. Intermittent catheterization, using a clean technique, is recommended for young individuals with a bladder that cannot empty and without any other available options. Patients should wash their hands with soap and water. Sterile gloves are not necessary. Clean intermittent catheterization results in lower rates of infection than the rates noted with indwelling catheters.
Studies show that in patients with spinal cord injuries, the incidence of bacteria in the bladder is 1-3% per catheterization, and one to four episodes of bacteriuria occur per 100 days of intermittent catheterization performed four times a day. Furthermore, the infections that do occur usually are managed without complications. In general, routine use of long-term suppressive therapy with antibiotics in patients regularly using clean intermittent catheterization is not recommended, because it may result in the emergence of resistant bacterial strains.
A study of a patients with acute spinal cord injury at 15 North American centers revealed that using a hydrophilic-coated catheter for intermittent catheterization delayed the onset of first antibiotic-treated symptomatic urinary tract infections. In addition, a reduction in incidence of symptomatic urinary tract infection was noted during inpatient rehabilitation for these patients. [8]
For older individuals and those with a weak immune system, the use of sterile technique for intermittent catheterization has been recommended. Older persons are at higher risk than younger persons for developing bacteriuria and other complications of intermittent catheterization because they do not have a strong defense system against infection. Although the incidence of infection and other complications for older patients using sterile versus clean intermittent catheterization is not well established, sterile intermittent catheterization appears to be the safest method for this high-risk population.
Potential advantages of performing intermittent catheterization include patient autonomy, freedom from indwelling catheter and bags, and unimpeded sexual relations. Potential complications of intermittent catheterization include bladder infection, urethral trauma, urethral inflammation, and stricture. Concurrent use of anticholinergic therapy will maintain acceptable intravesical pressures and prevent bladder contracture. Studies have demonstrated that long-term use of intermittent catheterization appears to be preferable to indwelling catheterization (ie, urethral catheter, suprapubic tube) with respect to urinary tract infections and the development of stones within the bladder or kidneys.
Overall, the management of infections in the setting of catheters and drainage tubes is challenging. Experimental use of bacterial interference (bladder colonization with low-virulence bacteria, such as Escherichia coli HU2117) represents a novel and perhaps effective method at the prevention of infections; however, at the present time, it is difficult to do clinically outside of the research setting. Further studies may prove this modality more clinically useful in practice environments. [9, 10]
Surgical Care
Surgical care for stress incontinence involves procedures that increase urethral outlet resistance, which include the following:
Surgical care for urge incontinence involves procedures that improve bladder compliance or bladder capacity, which include the following:
-
Sacral neuromodulation
The US Food and Drug Administration (FDA) has approved botulinum toxin A for neurogenic detrusor overactivity in adults who have an inadequate response to or are intolerant of an anticholinergic drug. However, a Cochrane review that included four randomized controlled trials of botulinum A toxin injection as a treatment for detrusor-sphincter dyssynergia (DSD) found that intraurethral injections might improve some urodynamic measures after 30 days, but the studies had a high risk of bias, the quality of the evidence was limited, and the need for reinjection is a significant drawback. The authors advised that more study of effectiveness needed; optimal dose and mode of injection remain to be determined, and sphincterotomy might be a more effective option for long-term treatment. [13]
Fluid Intake
The quantity and quality of fluids consumed will influence urinary voiding symptoms. Fluids refer to all the beverages a person consumes in a day, including water, soda, and milk. The human body receives water from beverages consumed and from food eaten. The recommended amount of fluids consumed (all types) in 24 hours totals 6-8 glasses. The benefits of adequate fluid intake include prevention of dehydration, constipation, urinary tract infection, and kidney stone formation.
Some patients tend to drink water excessively. Others take medication that makes their mouths dry, so they drink more water. Some patients who are trying to lose weight are on a diet that requires consuming abundant amounts of water. Excessive water intake worsens irritative bladder symptoms. The exact amount of fluid needed per day is calculated on the basis of the patient's lean body mass. Thus, the amount of fluid requirement will vary per individual.
Some older patients do not drink enough fluids to keep themselves well hydrated. They minimize their fluid intake to unacceptable levels, thinking that if they drink less, they will experience less incontinence. Trying to prevent incontinence by restricting fluids excessively may lead to bladder irritation and actually worsen urge incontinence. In addition, dehydration contributes to constipation. If a patient has a problem with constipation, recommend eating a high-fiber diet, receiving adequate hydration, and administering laxatives.
Many drinks contain caffeine. Caffeine is a natural diuretic, and it has a direct excitatory effect on bladder smooth muscle. Thus, caffeine-containing products produce excessive urine and exacerbate symptoms of urinary frequency and urgency. Caffeine-containing products include coffee, tea, hot chocolate, and sodas. Even chocolate milk and many over-the-counter medications contain caffeine.
Of caffeine-containing products, coffee contains the most caffeine. Drip coffee contains the most caffeine, followed by percolated coffee and then instant coffee. Even decaffeinated coffee contains a small amount of caffeine. Decaffeinated coffee contains an amount of caffeine similar to the amount in chocolate milk. Persons who consume a large amount of caffeine should slowly decrease the amount of caffeine consumed to avoid significant withdrawal responses such as headache and depression.
Studies have shown that drinking carbonated beverages, citrus fruits drinks, and acidic juices may worsen irritative voiding or urge symptoms. Consumption of artificial sweeteners also has been theorized to contribute to urge incontinence.
Nighttime voiding and incontinence are major problems in the older population. Women who have nocturia more than twice a night or experience nighttime bed-wetting may benefit from fluid restriction and the elimination of caffeine-containing beverages from their diet in the evening. Patients should restrict fluids after dinnertime so they can sleep uninterrupted through the night.
Pelvic floor exercise
Anti-incontinence exercises emphasize rehabilitating and strengthening the pelvic floor muscles that are critical in maintaining urinary continence. Pelvic floor muscles also are known as levator ani muscles because they function to levitate or elevate the pelvic organs into their proper place. When levator muscles weaken and fail, pelvic prolapse and stress incontinence result. An anatomic defect of the levator ani musculature requires physical rehabilitation. If aggressive physical therapy does not work, surgery is warranted.
Pelvic floor exercises, sometimes called Kegel exercises, are a rehabilitation technique used to tighten and tone the pelvic floor muscles. Kegel exercises may be performed to eliminate urge incontinence. Contraction of the external urinary sphincter induces reflex bladder relaxation. Pelvic floor muscle rehabilitation may be used to reprogram the urinary bladder to decrease the frequency of incontinence episodes. [14, 15]
Individuals who benefit the most from pelvic floor exercises tend to be young, healthy, and able to identify the levator muscles accurately. These rehabilitation exercises may be used for urge incontinence as well as mixed incontinence. For urge incontinence, pelvic floor muscle exercises are used to retrain the bladder. When the patient contracts the external urethral sphincter, the bladder automatically relaxes, so the urge to urinate eventually subsides. Strong contractions of the pelvic floor muscles will suppress bladder contractions. Whenever patients feel urinary urgency, they may try to stop the feeling by contracting the pelvic floor muscles. These steps will provide the patient more time to walk slowly to the bathroom with urinary control.
By regularly training the external sphincter, patients can gradually increase the time between urination from 1-3 hours. Patients should begin to see improvement in 3-4 weeks. Thus, this technique may be used for urge symptoms, urge incontinence, and mixed incontinence (stress and urge incontinence). Patients should practice contracting the levator ani muscles immediately before and during situations when leakage may occur. This will condition the external sphincter instinctively to contract with increases in abdominal pressure or when the need to urinate is imminent. This is known as the guarding reflex. When the patient tightens the external urinary sphincter just as a sneeze is about to occur, the involuntary urine loss is thwarted. By squeezing the levator ani muscles when the sense of urgency arises, the sensation of impending bladder contraction will dissipate. By making this maneuver a habit, patients will develop a protective mechanism against stress and urge incontinence.
The beneficial effects of pelvic floor muscle exercises alone have been well documented in medical literature. [16] Successful reduction in urinary incontinence has been reported to range from 56-95%. Pelvic floor exercises are effective, even after multiple anti-incontinence surgeries.
Electrical stimulation
Electrical stimulation is an area of active research in treating neurogenic bladder. It has been successfully applied to the genital nerve with overactive bladder and shown to decrease detrusor contractions and improve bladder capacity. [17] Furthermore, this treatment was deemed effective and tolerable by the patients who participated in the study. [18] It remains unclear whether sacral neuromodulation has a role in treating neurogenic detrusor overactivity, but this is an area of ongoing study. [19] Overall, electrical stimulation has notable potential as a treatment for neurogenic bladder.
Stem Cell Therapy
A number of clinical trials (mainly phase I and II) on the effects and safety of stem cell therapy for neurogenic bladder after SCI have shown promising results. However, most were not randomized trials, did not have a control group, and included a small number of patients. High-quality randomized trials are still needed before stem cell therapy can be considered for use in clinical settings. [20]
Medications Used to Treat Neurogenic Bladder
The role of pharmacologic therapy in neurogenic bladder, and the selection of drugs, varies with the type of neurogenic bladder. Pharmacologic therapy for overactive bladder may be most effective when combined with a pelvic exercise regimen. The 3 main categories of drugs used to treat urge incontinence include anticholinergic drugs, antispasmodics, and tricyclic antidepressants.
All drugs with anticholinergic adverse effects are contraindicated if patients have documented narrow-angle glaucoma. Wide-angle glaucoma is not a contraindication to their use. Urinary retention, bowel obstruction, ulcerative colitis, myasthenia gravis, and severe heart disease are contraindications for anticholinergic use. These agents may impair the patient's ability to perform hazardous activities, such as driving or operating heavy machinery, because of the potential for drowsiness. Anticholinergic drugs should not be taken in combination with alcohol, sedatives, or hypnotic drugs. However, the use of anticholinergics in urinary retention with a catheterization regimen may help improve bladder capacity.
When a single drug treatment does not work, combination therapy may be used. Generally, agents with different mechanisms of action must be combined in order to improve urge incontinence; for instance, the combination of a beta-3 agonist with an anticholinerigic can be used to treat detrusor overactivity. Together, those drugs produce a synergistic effect that relaxes the unstable bladder to hold in urine and prevents urge incontinence. However, anticholinergic adverse effects may be additive because both drugs have similar adverse reactions.
Anticholinergic and antispasmodic drugs
Anticholinergic drugs are the first-line pharmacologic therapy in urge incontinence. They are effective in treating urge incontinence because they inhibit involuntary bladder contractions. They are also useful in treating urinary incontinence associated with frequency, urgency, and nocturnal enuresis. All anticholinergic drugs have similar performance profiles and toxicity. Potential adverse effects of all anticholinergic agents include blurred vision, dry mouth, heart palpitations, drowsiness, and facial flushing. When anticholinergic drugs are used in excess, acute urinary retention may occur.
Antispasmodic drugs
Antispasmodic drugs relax the smooth muscles of the urinary bladder. By exerting a direct spasmolytic action on the smooth muscle of the bladder, antispasmodic drugs have been reported to increase bladder capacity and effectively decrease or eliminate urge incontinence. The adverse-effect profile of antispasmodic drugs is similar to that of anticholinergic agents. These drugs may impair the patient's ability to perform activities requiring mental alertness and physical coordination. Drinking alcohol and using sedatives in combination with these antispasmodic drugs is contraindicated.
Solifenacin succinate (VESIcare)
Solifenacin succinate elicits competitive muscarinic receptor antagonist activity, which results in anticholinergic effect and inhibition of bladder smooth muscle contraction. It is indicated for overactive bladder with symptoms of urgency, frequency, and urge incontinence.
Darifenacin (Enablex)
Darifenacin is an extended-release product that elicits competitive muscarinic receptor antagonistic activity. It reduces bladder smooth muscle contractions. It has a high affinity for M3 receptors involved in bladder and gastrointestinal (GI) smooth muscle contraction, saliva production, and iris sphincter function. Darifenacin is indicated for overactive bladder with symptoms of urge incontinence, urgency, and frequency. The product should be swallowed whole; do not chew, divide, or crush.
Oxybutynin chloride (Ditropan IR, Ditropan XL)
Oxybutynin chloride has both anticholinergic and direct smooth-muscle relaxant effects on urinary bladder. It provides a local anesthetic effect on irritable bladder. Urodynamic studies have shown that oxybutynin increases bladder size, decreases frequency of symptoms, and delays initial desire to void.
Ditropan XL has an innovative drug delivery system, the oral osmotic delivery system (OROS). The Ditropan XL tablet has a bilayer core that contains a drug layer and a push layer that contains osmotic components. The outer tablet is composed of a semipermeable membrane with a precision laser-drilled hole that allows the drug to be released at a constant rate.
When the drug is ingested, the aqueous environment in the GI tract causes water to enter the tablet via the semipermeable membrane at constant rate. Introduction of water inside the tablet liquifies the drug and causes the push layer to swell osmotically. As the push layer swells, it forces the drug suspension out of the hole at a constant rate over a 24-h period.
Ditropan XL achieves steady-state levels over a 24-h period. It avoids first-pass metabolism of the liver and upper GI tract to avoid cytochrome P450 enzymes. It has excellent efficacy with minimal adverse effects.
Studies have shown that oxybutynin chloride reduces incontinence episodes by 83-90%. The total continence rate has been reported to be 41-50%. The mean reduction in urinary frequency was 23%. In clinical trials, only 1% stopped taking Ditropan XL because of dry mouth, and less than 1% stopped taking Ditropan XL due to CNS adverse effects.
Tolterodine L-tartrate (Detrol and Detrol LA)
Tolterodine L-tartrate is a competitive muscarinic receptor antagonist for overactive bladder. It differs from other anticholinergic types in that it has selectivity for urinary bladder over salivary glands. It exhibits high specificity for muscarinic receptors and has minimal activity or affinity for other neurotransmitter receptors and other potential targets such as calcium channels. In clinical studies, the mean decrease in urge incontinence episodes was 50% and the mean decrease in urinary frequency was 17%.
Trospium (Sanctura)
Trospium is a quaternary ammonium compound that elicits antispasmodic and antimuscarinic effects. It antagonizes the acetylcholine effect on muscarinic receptors. Parasympathetic effect reduces smooth muscle tone in the bladder. Trospium is indicated to treat symptoms of overactive bladder (eg, urinary incontinence, urgency, frequency).
Fesoterodine (Toviaz)
Fesoterodine is a competitive muscarinic receptor antagonist. The antagonistic effect results in decreased bladder smooth muscle contractions. It is indicated for symptoms of overactive bladder (eg, urinary urge incontinence, urgency, and frequency). Fesoterodine is available as a 4- or 8-mg extended-release tab.
Tricyclic antidepressants
Treatment of bladder dysfunction is an off-label use of tricyclic antidepressants. These drugs function to increase norepinephrine and serotonin levels. In addition, they exhibit anticholinergic and direct muscle relaxant effects on the urinary bladder.
Imipramine (Tofranil)
Imipramine is a typical tricyclic antidepressant. It facilitates urine storage by decreasing bladder contractility and increasing outlet resistance. It has alpha-adrenergic effect on the bladder neck and antispasmodic effect on detrusor muscle. Imipramine hydrochloride has a local anesthetic effect on bladder mucosa.
Amitriptyline (Elavil)
Amitriptyline is a tricyclic antidepressant with sedative properties. It increases circulating levels of norepinephrine and serotonin by blocking their reuptake at nerve endings. It is ineffective for use in urge incontinence but is extremely effective in decreasing symptoms of urinary frequency in women with pelvic floor muscle dysfunction. Amitriptyline restores serotonin levels and helps break the cycle of pelvic floor muscle spasms. It is well tolerated and effective in most women with urinary frequency.
Beta-3 adrenergic receptor
Mirabegron was approved in 2012 by the US Food and Drug Administration (FDA) for the treatment of overactive bladder. In an initial study of its effectiveness for the treatment of neurogenic detrusor overactivity in 15 patients with spinal cord injury, a significant reduction of the frequency of bladder evacuation per 24 h (8.1 vs 6.4, P=0.003), and of incontinence episodes per 24 h (2.9 vs 1.3, P=0.027) was observed. However due to the limited size of the study, more research is needed. [21]
A systematic review of seven studies of mirabegron used as a treatment for neurogenic bladder concluded that it appeared to be an effective second-line treatment, particularly for patients with storage symptoms who were unresponsive to antimuscarinics . However, there was no evidence yet available to support its use as a first-line treatment. [22]
Follow-up
Complications
Prolonged contact of urine with unprotected skin causes contact dermatitis and skin breakdown. If left untreated, these skin disorders may lead to pressure sores and ulcers, possibly resulting in secondary infections.
For individuals with a decompensated bladder that does not empty well, the postvoid residual urine can lead to overgrowth of bacteria and subsequent urinary tract infection (UTI). In patients with neurogenic bladder, UTIs often do not produce classic symptoms; instead, these patients may present with abdominal or back pain, increased spasticity, and urinary incontinence. Untreated UTIs can quickly lead to life-threatening autonomic dysreflexia or sepsis, whereas overtreatment promotes antibiotic resistance. However, there are few evidence-based practices for preventing UTI in this population. [23]
Complications of specific interventions include the following:
-
Long-term indwelling catheters - Recurrent bladder infection, bladder stones, ascending pyelonephritis, and urethral erosion
-
Intermittent catheterization - Bladder infections or urethral injury
-
Long-term suprapubic tubes - Bladder spasms, bladder stone formation, and bladder infection
-
Potential problems unique to suprapubic catheters include skin infection, hematoma, bowel injury, and problems with catheter reinsertion
Prognosis
The prognosis of patients with incontinence from neurogenic bladder is excellent with modern health care. With improvement in information technology, well-trained medical staff, and advances in medical knowledge, patients who are incontinent should not experience the morbidity and mortality of the past. Although the patient's ultimate well-being depends on the underlying condition that has precipitated urinary incontinence, urinary incontinence itself is easily treated and prevented by properly trained health care personnel.
Patient education
For patient education information, see Bladder Control Problems, Bladder Control Medications, Inability to Urinate, and Foley Catheter.
Patients may well conduct Internet searches for information on their condition. Using the search terms "neurogenic bladder intermittent catheter" and "spinal cord injury intermittent catheter", Ho et al found 71 videos online that covered these topics. However, most of the videos provided poor-quality information, and some offered information contradictory to European Urological Association guidelines for intermittent catheterization. Videos that the authors deemed of good quality were not prominently ranked by the YouTube search algorithm, suggesting that users would be less likely to access those. [24]
Guidelines Summary
In 2021, the American Urological Association (AUA) and the Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction (SUFU) released joint guidelines for the diagnosis and treatment of neurogenic lower urinary tract dysfunction (NLUTD) in adults. [25, 26] The guidelines provide an algorithm for determining whether patients are at low or unknown risk for damage to the upper urinary tract, and stratifying those at unknown risk into moderate- or high-risk categories. The guidelines include surveillance schedules based on level of risk. New or worsening signs and symptoms should trigger reevaluation and repeated risk stratification. [25]
Diagnosis and Evaluation
The recommended initial evaluation of patients with NLUTD comprises the following [25] :
-
History and physical exam
-
Urinalysis
-
Post-void residual (PVR) measurement
-
Optional diary, pad test, noninvasive uroflow, as indicated
-
Cystoscopy is not routinely indicated
Upper tract imaging, kidney function assessment, and multichannel urodynamic studies (UDS) are not routinely indicated for low-risk NLUTD but should be performed in patients with unknown risk. Patients at risk for autonomic dysreflexia must be hemodynamically monitored during UDS and/or cystoscopy. Testing and/or cystoscopy should be terminated if autonomic dysreflexia develops and the bladder immediately drained. Hemodynamic monitoring should be continued. Care should be escalated and pharmacotherapy initiated if autonomic dysreflexia continues following bladder drainage.
Criteria for low risk are as follows:
-
Suprapontine lesion (eg, stroke, Parkinson disease, brain tumor, traumatic brain injury, cerebral palsy) with no potentially related NLUTD complications identified
-
Lesions distal to the spinal cord (eg, disk disease, pelvic surgery, diabetes) with no potentially related NLUTD complications identified
-
Able to void spontaneously (no indwelling catheter or clean intermittent catheterization)
-
Low PVR
-
No other potentially related complications identified (eg, bladder stones, recurrent UTIs)
-
Kidney function normal/stable
-
UDS (if done): synergistic voiding
-
Upper tract imaging (if done): normal/stable
-
Stable lower urinary tract symptoms (LUTS)
Patients are initially classified as being at unknown risk if they have a suprasacral spinal cord lesion, other neurologic lesions with identified genitourinary complications potentially related to NLUTD, or a change in LUTS. They are then stratified into moderate or high risk.
Criteria for moderate risk are as follows:
-
UDS demonstrating urinary retention, bladder outlet obstruction, or detrusor overactivity with incomplete emptying
-
PVR elevated
-
Upper tract imaging normal
-
Kidney function normal/stable
Criteria for high risk are as follows:
-
UDS: Poor bladder compliance, elevated detrusor storage pressures with detrusor overactivity, detrusor sphincter dyssynergia, vesicoureteral reflux (if done with fluoroscopy)
-
Upper tract imaging: Hydronephrosis, new renal scarring, parenchyma loss, staghorn calculi, large or increased stone burden
-
Kidney function abnormal/unstable
Surveillance
Surveillance is not indicated for low-risk patients.
Moderate-risk patients should receive the following monitoring:
-
Annual history, exam, and symptom assessment
-
Annual kidney function assessment
-
Upper tract imaging every 1-2 years
High-risk patients should receive the following monitoring:
-
Annual history, exam, and symptom assessment
-
Annual kidney function assessment
-
Annual upper tract imaging
-
Multichannel urodynamic studies, with or without fluoroscopy, which may be repeated when clinically indicated
Cystoscopy should be performed on any patient with concomitant hematuria, recurrent UTI, or suspected anatomic anomaly (eg, strictures, false passage). Cystoscopy should NOT be performed for general screening/surveillance or in patients with a long-term indwelling catheter.
Evaluate the upper and lower urinary tracts with imaging and cystoscopy in patients with recurrent UTI. If the findings are unremarkable, clinicians may perform UDS.
In patients with febrile UTI, upper tract imaging is indicated in the following settings:
-
The condition is unresponsive to appropriate antibiotic therapy.
-
The patient is at moderate or high risk and is not up to date with routine upper tract imaging, regardless of response to therapy.
Surveillance for patients with a long-term indwelling catheter includes the following:
-
Interval physical examination of the catheter and the catheter site (suprapubic or urethral)
-
If patient is at risk for upper and lower urinary tract calculi (eg, patients with spinal cord injury, recurrent UTI, immobilization, hypercalciuria), urinary tract imaging every 1-2 years
-
If a UTI is suspected, obtain a urine culture specimen after changing the catheter and after allowing for urine accumulation while plugging the catheter. Do not obtain urine from the extension tubing or collection bag.
-
Use of daily antibiotic prophylaxis to prevent UTI is NOT recommended
Nonsurgical Treatment
The following are the key nonsurgical treatment recommendations [26] :
-
Appropriately selected patients, particularly those with multiple sclerosis or stroke, should consider pelvic floor muscle training to improve urinary symptoms and quality of life.
-
Antimuscarinics, beta-3 adrenergic receptor agonists, or a combination of both may be used to improve bladder storage parameters.
-
Alpha-blockers may be used to improve voiding parameters in patients who can void spontaneously.
-
Intermittent catheterization is preferred over indwelling catheters to facilitate bladder emptying; patients with recurrent UTIs may be offered oral antimicrobial prophylaxis or bladder instillations to reduce the rate of UTIs.
-
For appropriately selected patients who require a long-term indwelling catheter, suprapubic catheterization is preferred over a urethral catheter.
-
Patients whose neurogenic bladder is refractory to oral medications may be offered onabotulinumtoxinA to improve bladder storage parameters, decrease episodes of incontinence, and improve quality of life.
Surgical Treatment
The following are the key surgical treatment recommendations [26] :
-
For appropriately selected male patients, sphincterotomy may be considered to facilitate emptying, but patients must be made aware of the high risk of failure or potential need for additional treatment or surgery.
-
Urethral bulking agents may be offered to treat stress urinary incontinence, but patients must be made aware that efficacy is modest and cure is rare.
-
Offer slings to select patients with stress urinary incontinence and acceptable bladder storage parameters.
-
Offer artificial urinary sphincter to select patients with stress urinary incontinence and acceptable bladder storage parameters.
-
Offer posterior tibial nerve stimulation to select spontaneously voiding patients with urgency, frequency, and/or urgency incontinence.
-
Offer sacral neuromodulation to select patients with urgency, frequency, and/or urgency incontinence.
-
Do not offer sacral neuromodulation to patients with spinal cord injury or spina bifida.
-
Offer augmentation cystoplasty to select patients who are refractory to, or intolerant of, less invasive therapies for detrusor overactivity and/or poor bladder compliance.
-
Offer continent catheterizable channels, with or without augmentation, to select patients to facilitate catheterization.
-
Offer urinary diversion to patients in whom other options have failed, or are inappropriate, in order to improve long-term quality of life.
Questions & Answers
Overview
What are the signs and symptoms of neurogenic bladder?
What is the neuroanatomy of normal bladder voiding?
How is the urinary system controlled?
How is cognitive control of micturition achieved?
Which CNS disorders are associated with urinary complications such as neurogenic bladder?
How are brain signals transmitted to the urinary system?
What is the role of the brainstem in the neuroanatomy of neurogenic bladder?
What is the role of the pontine micturition center (PMC) in the neuroanatomy of neurogenic bladder?
How does the brain signal bladder fullness in the neuroanatomy of neurogenic bladder?
What is the role of the spinal cord play in the neuroanatomy of neurogenic bladder?
What is the effect of a spinal cord injury on the neuroanatomy of neurogenic bladder?
What is the role of the sacral spinal cord in neurogenic bladder?
What is the neuroanatomy of involuntary detrusor contractions in infants?
How does the brain attain control of the urinary system?
What causes urinary retention?
What is the role of the peripheral nerve networks in the neuroanatomy of neurogenic bladder?
What is the autonomic nervous system?
What is the role of the sympathetic nervous system in the neuroanatomy of neurogenic bladder?
What is the role of the parasympathetic nervous system in the neuroanatomy of neurogenic bladder?
What is the role of the somatic nervous system in the neuroanatomy of neurogenic bladder?
How often is the urinary bladder voided?
What are the phases of normal bladder function?
What happens during the filling phase of urinary bladder function?
What does bladder filling depend upon?
How do sympathetic nerves facilitate urine storage?
What is the continence reflex?
What is the role of pressure gradients within the bladder?
How does the urinary bladder switch from the storage phase to the voiding phase?
What are characteristics of the voiding phase of the urinary bladder?
How is the automatic function of the bladder controlled by the brain?
How does the pontine micturition center (PMC) function in normal adults?
What is the pathophysiology of neurogenic bladder?
Which brain lesions are associated with neurogenic bladder?
Which spinal cord lesions are associated with neurogenic bladder?
What is the role of sacral cord injuries in the pathogenesis of neurogenic bladder?
What is the role of peripheral nerve injury in the pathogenesis of neurogenic bladder?
How are the types of neurogenic bladder classified?
Which supraspinal lesions cause neurogenic bladder?
What is the role of stroke in the etiology of neurogenic bladder?
What is the role of brain tumor in the etiology of neurogenic bladder?
What is the role of Parkinson disease in the etiology of neurogenic bladder?
What is the role of Shy-Drager syndrome in the etiology of neurogenic bladder?
What is the relationship between spinal cord lesions and neurogenic bladder?
What is the pathogenesis of neurogenic bladder caused by a spinal cord injury (SCI)?
Which treatments are used prophylactically for autonomic dysreflexia in neurogenic bladder?
Which findings are characteristic of neurogenic bladder in patients with multiple sclerosis (MS)?
What can cause peripheral nerve lesions resulting in detrusor areflexia in neurogenic bladder?
What causes neurogenic bladder dysfunction in patients with diabetes?
What are the symptoms of diabetic cystopathy?
Which findings are characteristic of neurogenic bladder in patients with tabes dorsalis?
Which findings are characteristic of neurogenic bladder in patients with herpes zoster?
Which findings are characteristic of neurogenic bladder in patients with lumbar disc herniation?
Which findings are characteristic of neurogenic bladder following pelvic surgery?
Which procedures are used to investigate possible neurogenic bladder?
What is the role of lab studies in the diagnosis of neurogenic bladder?
What is the role of a voiding diary in the diagnosis of neurogenic bladder?
What is the role of the pad test in the diagnosis of neurogenic bladder?
What is the uroflow rate and how is it used in the diagnosis of neurogenic bladder?
What is a filling cystometrogram (CMG)?
How is a voiding cystometrogram (CMG) used in the diagnosis of neurogenic bladder?
What is the role of static cystogram in the diagnosis of neurogenic bladder?
What is the role of a voiding cystogram in the diagnosis of neurogenic bladder?
What is the role of electromyography in the diagnosis of neurogenic bladder?
What is the role of cystoscopy in the diagnosis of neurogenic bladder?
What is the role of videourodynamics in the diagnosis of neurogenic bladder?
What are the different types and treatment options for urinary incontinence in neurogenic bladder?
What are the criteria for use of anti-incontinence products in the treatment of neurogenic bladder?
How are absorbent products used in the treatment of neurogenic bladder?
What are the limitations of absorbent products for the treatment of neurogenic bladder?
What is the role of catheters in the treatment of neurogenic bladder?
How are Foley catheters used in the treatment of neurogenic bladder?
How are indwelling catheters maintained in the treatment of neurogenic bladder?
What is the role of antibiotic prophylaxis in the treatment of neurogenic bladder?
What are the adverse effects of Foley catheters for the treatment of neurogenic bladder?
What are the indications for use of indwelling catheters for the treatment of neurogenic bladder?
What is a suprapubic catheter for the treatment of neurogenic bladder?
What are the advantages of using suprapubic catheters for the treatment of neurogenic bladder?
How are suprapubic catheters changed during the treatment of neurogenic bladder?
What are the indications for suprapubic catheters for the treatment of neurogenic bladder?
What are limitation of suprapubic catheters for the treatment of neurogenic bladder?
What is intermittent catheterization for the treatment of neurogenic bladder?
How is intermittent catheterization performed for the treatment of neurogenic bladder?
What is the role of intermittent catheterization for neurogenic bladder?
Who are candidates for intermittent catheterization for neurogenic bladder?
How is intermittent catheterization performed for neurogenic bladder?
What is the frequency of infections with intermittent catheterization for neurogenic bladder?
What are the advantages of intermittent catheterization for neurogenic bladder?
What is the role of bacterial interference in the treatment of neurogenic bladder?
What are the surgical options for treatment of stress incontinence in neurogenic bladder?
What are the surgical options for urge incontinence in neurogenic bladder?
What is the role of botulinum A toxin injection in the treatment of neurogenic bladder?
What is the role of fluid intake in the treatment of neurogenic bladder?
What is the effect of excessive water intake in neurogenic bladder?
How is constipation treated in patients with neurogenic bladder?
What effect does caffeine have on neurogenic bladder?
What effect do carbonated drinks have on neurogenic bladder?
What are the treatment options for nighttime voiding and incontinence in neurogenic bladder?
What is the role of anti-incontinence exercises for the treatment of neurogenic bladder?
What is the role of pelvic floor exercises in the treatment of neurogenic bladder?
How are pelvic floor exercises performed in the treatment of neurogenic bladder?
What are the beneficial effects of pelvic floor exercises for the treatment of neurogenic bladder?
What is the role of electrical stimulation in the treatment of neurogenic bladder?
What is the role of stem cell therapy in the treatment of neurogenic bladder?
Which medications are used to treat urge incontinence in neurogenic bladder?
Which medications are contradicted in patients with neurogenic bladder?
What is the role of combination therapy in the treatment of neurogenic bladder?
What is the first-line pharmacologic therapy for urge incontinence in neurogenic bladder?
What is the role of antispasmodic drugs in the treatment of neurogenic bladder?
What is the role of solifenacin succinate (VESIcare) in the treatment of neurogenic bladder?
What is the role of darifenacin (Enablex) in the treatment of neurogenic bladder?
What is the role of oxybutynin (Ditropan IR, Ditropan XL) in the treatment of neurogenic bladder?
What is the role of trospium (Sanctura) in the treatment of neurogenic bladder?
What is the role of fesoterodine (Toviaz) in the treatment of neurogenic bladder?
What is the role of tricyclic antidepressants in the treatment of neurogenic bladder?
What is the role of imipramine (Tofranil) in the treatment of neurogenic bladder?
What is the role of amitriptyline (Elavil) in the treatment of neurogenic bladder?
What is the role of mirabegron in the treatment of neurogenic bladder?
What are the complications of decompensated bladder in neurogenic bladder?
What are the complications of specific treatments for neurogenic bladder?
What is the prognosis of neurogenic bladder?
What information about neurogenic bladder should patients receive?
-
The pons is a major relay center between the brain and the bladder. The mechanical process of urination is coordinated by the pons in the area known as the pontine micturition center (PMC).








