Definition of Problem
In performing noncardiac surgery on patients on anticoagulation, the major concern is when it is safe to perform surgery without increasing the risk of hemorrhage or increasing the risk of thromboembolism (eg, venous, arterial) after discontinuing treatment. In treating patients on long-term warfarin perioperatively, consider the risks of hemorrhage or thromboembolism versus the benefit from the operation. When considering noncardiac surgery, these factors and the need to weigh the risk of hemorrhage against that of thromboembolism must be analyzed on an individual patient basis. Certain procedures (eg, oncologic procedures, threats to limb or life) are easy analyses. More complex discussions must be had for such cases as hernia repair of other elective nonurgent operations.
The perioperative management for these patients can be one of the following: continue warfarin therapy, withhold warfarin therapy for a period of time before and after the procedure, or temporarily withhold warfarin therapy and also provide a "heparin bridge" during the perioperative period. Which management option to follow is primarily determined by the characteristics of the patient and by the nature of the procedure.
Patients with prosthetic heart valves pose a particular problem. Arterial thromboembolism from the heart often results in death (40% of events) or major disability (20% of events). The greatest problem encountered is that no consensus exists regarding the optimal perioperative management of anticoagulation for patients who have been receiving long-term warfarin therapy. Some prospective studies have suggested that patients on long-term warfarin therapy who undergo minor invasive procedures and are taken off their oral anticoagulation for up to 5 days have a less than 1% risk of experiencing a thromboembolic event.
It has been suggested that patients on long-term warfarin therapy (including those with mechanical heart valves or atrial fibrillation) who are undergoing minor elective invasive outpatient procedures (eg, colonoscopy, dental procedures) may have a slightly higher risk of perioperative bleeding if placed on some form of heparin therapy (eg, heparin bridge) than those who have their oral anticoagulation withheld for 4-5 days (major hemorrhage 3.7% vs 0.2% and significant nonmajor hemorrhage 9% vs 0.6%, respectively). The perioperative risk of bleeding when using a heparin bridge appears to be higher and the risk of thromboembolic events appears to be lower when warfarin is stopped than what is reported elsewhere in the literature.
Studies
N-acetylcysteine is known to impair hemostasis when used for the prevention of perioperative inflammation and ischemia-reperfusion injury. Wijeysundera et al sought to determine whether N-acetylcysteine is associated with increased blood loss and blood product transfusion in 89 patients with preexisting moderate renal insufficiency undergoing cardiac surgery. [1] Another 88 patients received placebo.
The investigators found patients in the N-acetylcysteine group had a 261-mL greater mean 24-hour chest-tube blood loss and received 1.6 units more of red blood cell transfusions than the placebo group. [1] In addition, there was a significantly higher risk of receiving 5 or more units of red blood cells within 24 hours of surgery in the patients receiving N-acetylcysteine compared with the placebo group (P = 0.005). Wijeysundera et al therefore recommended clinicians and researchers consider the potential of impaired hemostasis in using N-acetylcysteine in the perioperative setting. [1]
A randomized study by Di Biase was the first study showing that performing catheter ablation of atrial fibrillation (AF) without warfarin discontinuation reduces the occurrence of periprocedural stroke and minor bleeding complications compared with bridging with low-molecular-weight heparin (LMWH). [2]
A National Heart, Lung, and Blood Institute (NHLBI)–sponsored study showed that a periprocedural bridging strategy with low-molecular-weight heparin (LMWH) offered no clinical advantages compared with interrupting warfarin treatment. [3, 4]
The Perioperative Anticoagulation Use for Surgery Evaluation (PAUSE) study included 3007 patients with nonvalvular atrial fibrillation who were receiving long-term therapy with dabigatran, rivaroxaban, or apixaban. The direct oral anticoagulant was stopped before and then resumed after elective surgery or procedures without heparin bridging. The investigators reported only about a 2% rate of perioperative major bleeding and less than 1% rate of thromboembolic events among the study’s participants. [5]
Clinical guidelines
The American College of Chest Physicians (ACCP) proposed guidelines for antithrombotic prophylaxis in patients with different risk factors, and it recommends that if the annual risk for thromboembolism is low, warfarin therapy can be withheld for 4-5 days before the procedure without bridging.
The 8th and 9th edition of the American College of Chest Physicians Evidence-Based Clinical Practice Guidelines included the following key recommendations [6, 7] :
-
In patients with a mechanical heart valve or atrial fibrillation or venous thromboembolism (VTE):
At high risk for thromboembolism, bridging anticoagulation is recommended with therapeutic-dose subcutaneous (SC) low-molecular-weight heparin (LMWH) or intravenous unfractionated heparin (UFH) rather than no bridging during temporary interruption of vitamin K antagonist (VKA) therapy. [8]
At moderate risk for thromboembolism, it is proposed to base the plan for bridging versus no bridging on the individual patient rather than a generalized consensus. The bridging anticoagulation can be done with therapeutic-dose SC LMWH, therapeutic-dose IV UFH, or low-dose SC LMWH based on the patient.
At low risk for thromboembolism, it is recommended that no bridging with therapeutic-dose SC LMWH or IV UFH should occur.
-
In patients with a recently placed bare metal coronary stent who require surgery within 6 weeks of stent placement, the ACCP recommends to continue aspirin and clopidogrel in the perioperative period. It is recommended to defer elective surgeries until at least 6 weeks post stent placement.
-
In patients with a recently placed drug-eluting coronary stent who require surgery within 6 months of stent placement, the ACCP recommends to continue aspirin and clopidogrel in the perioperative period. It is recommended to defer elective surgeries until at least 6 months post stent placement.
-
In patients on Vitamin K antagonists (VKAs) who are undergoing minor dental procedures, it is recommended to continue the VKAs around the time of the procedure as well as coadminister an oral prohemostatic agent or to hold the VKA for 2-3 days prior to procedure based on individual patient risk assessment.
-
In patients on VKAs who are undergoing minor dermatologic procedures or cataract removal, continue the VKAs perioperatively.
The table below depicts which patients should receive heparin bridging before surgery.
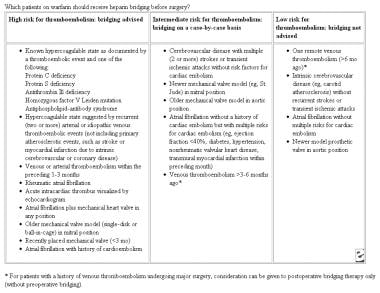 Which patients on warfarin should receive heparin bridging before surgery? Adapted from Cleveland Clinic Journal.
Which patients on warfarin should receive heparin bridging before surgery? Adapted from Cleveland Clinic Journal.
For excellent patient education resources, see eMedicineHealth's patient education article Deep Vein Thrombosis (Blood Clot in the Leg, DVT).
For a discussion of pathophysiology and laboratory findings, see Deep Venous Thrombosis.
Indications for Perioperative Management
Any patient who is on long-term anticoagulation and is to undergo a major surgery needs proactive management. [9] Some authors believe that patients can be maintained on oral anticoagulation for minor procedures, such as dental extractions, biopsies, ureterorenoscopy, Ho:YAG lithotripsy, and ophthalmic operations, as long as the therapeutic range of the prothrombin time (PT) value is not greater than 2.5. [10] A study revealed a higher rate of hemorrhagic complications after glaucoma surgery in patients on anticoagulation or antiplatelet therapy. Patients who continued anticoagulation during glaucoma surgery had a hemorrhagic complication rate of 31.8% compared to 3.7% of patients with no anticoagulation or antiplatelet therapy. [11] Local bleeding with dental surgery may be controlled with tranexamic acid or epsilon aminocaproic acid mouthwash.
The American Society of Gastrointestinal Endoscopy divided endoscopic procedures into low and high risk for bleeding in its 2002 guidelines on anticoagulation. Low bleeding-risk endoscopic procedures do not require a change in anticoagulation.
Low bleeding-risk endoscopic procedures are as follows:
-
Upper endoscopy with or without biopsy
-
Flexible sigmoidoscopy with or without biopsy
-
Colonoscopy with or without biopsy
-
Endoscopic retrograde cannulation of the pancreatic duct without sphincterotomy
-
Biliary stent insertion without sphincterotomy
-
Endosonography without fine-needle aspiration
-
Push enteroscopy of the small bowel
High bleeding-risk endoscopic procedures are as follows:
-
Polypectomy
-
Laser ablation and coagulation
-
Endoscopic sphincterotomy
-
Pneumatic or bougie dilation
-
Percutaneous endoscopic gastrostomy tube placement
-
Treatment of varices
In general, antithrombotic therapy is indicated for venous thromboembolic disease (ie, deep venous thrombosis [DVT]; pulmonary embolism [PE]; primary prophylaxis of DVT or PE; antithrombin III [ATIII], protein C, and protein S deficiency); arterial thromboembolic disease (ie, prosthetic heart valves, atrial fibrillation, congestive cardiomyopathies, mural cardiac thrombus, acute myocardial infarction, mitral valve disease); disseminated intravascular coagulation; and maintaining patency of vascular grafts, shunts, and bypasses. [12, 13]
Currently, it is generally recommended that patients with the highest risk of arterial or venous thromboembolism, who require interruption of oral anticoagulant therapy for surgery, should receive therapeutic-dose heparin therapy (eg, unfractionated heparin [UFH], low molecular weight heparin [LMWH]) during much of the interval when the international normalized ratio (INR) is subtherapeutic.
Usually, unless accompanied by significant cardiomyopathy or recent arterial embolus, patients with atrial fibrillation can have their warfarin stopped 4 days prior to surgery, then resumed at the usual dose the night of surgery.
Patients with prosthetic heart valves usually are treated with perioperative LMWH, although randomized controlled trials validating this method are lacking. Warfarin can be stopped 4-5 days preoperatively, with LMWH started the next day at a therapeutic dose. The last dose should be 12 hours preoperatively. LMWH and warfarin can be retitrated the evening of the operative day. LMWH is stopped when the warfarin reaches the target range. For patients at higher risk of valve thrombosis (ie, patients with 2 prosthetic valves or with caged-ball type of valves), whether LMWH provides adequate anticoagulant protection is unclear. For these patients, consider use of perioperative UFH instead of LMWH. Preoperatively, the heparin should be stopped 6 hours before the procedure. Postoperatively, the heparin can be restarted when the surgeon agrees that it is safe, usually 6-12 hours postoperatively.
Prophylactic and therapeutic doses of LMWH in perioperative anticoagulation management are tabulated below.
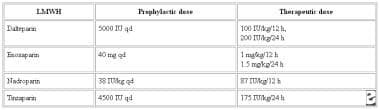 Perioperative anticoagulation management. Low molecular weight heparin, prophylactic doses, and therapeutic doses.
Perioperative anticoagulation management. Low molecular weight heparin, prophylactic doses, and therapeutic doses.
Contraindications to antithrombotic therapy are relative, and the risks and benefits need to be weighed. Relative contraindications are bleeding abnormality (eg, thrombocytopenia, platelet defect, peptic ulcer disease), CNS lesion (eg, stroke, surgery, trauma), spinal anesthesia or lumbar puncture, malignant hypertension, and advanced retinopathy. Contraindications specific to warfarin are early or late pregnancy, poor patient cooperation, and occupational risk. LMWH should be avoided in patients with renal insufficiency, because it is cleared primarily by the kidney. If used, the anticoagulant effect of LMWH should be measured with an antifactor Xa level done 4 hours after the LMWH dose. The targeted therapeutic antifactor Xa level is 0.5-1.5 U/mL.
Preoperative Treatment
Several protocols have been developed to care for patients taking oral anticoagulants. Regardless of the protocol used, the period of subtherapeutic oral anticoagulation should be kept to a minimum in patients with previous embolism and in others who are at highest risk for embolism. Kearon formulated a preoperative and postoperative strategy divided into sites of embolic disease. [14] His recommendations are summarized below.
Arterial thromboembolism
In patients with previous arterial embolism, only 4 daily doses of warfarin should be withheld preoperatively and the INR should be measured the day before surgery to determine if a small dose of vitamin K is needed to accelerate the reversal of anticoagulation. If the INR is more than 1.7 on the day before surgery, administer 1 mg of vitamin K subcutaneously and repeat the INR the morning of the surgery. If on the day of surgery the INR is 1.3-1.7, administer 1 unit of frozen plasma; administer 2 units of frozen plasma if the INR is 1.7-2. The active reversal of oral anticoagulants should be discouraged in patients with mechanical valves, especially with the use of fresh frozen plasma.
For a patient who has had an arterial thromboembolism within a month of surgery, start intravenous UFH when the INR drops to less than 2 to minimize the risk of recurrent embolism. Discontinue the intravenous heparin 6 hours before surgery.
Venous thromboembolism
After an acute episode of venous thromboembolism (VTE), defer surgery, if feasible, until patients have received at least 1 month, and preferably 3 months, of anticoagulation. If surgery must be performed within 1 month of an acute VTE, intravenous UFH should be administered while the INR is less than 2. If surgery must be performed within 2 weeks after an acute episode, intravenous heparin may be withheld 6 hours preoperatively and 12 hours postoperatively, if the surgery is short. If the acute event was within 2 weeks of major surgery and/or patients have a higher risk of postoperative bleeding, a vena caval filter should be inserted preoperatively or intraoperatively.
Warfarin should be withheld for only 4 doses if the most recent episode of VTE occurred 1-3 months before surgery. If the patient has been anticoagulated for 3 or more months, 5 doses of warfarin can be withheld before surgery. Preoperatively, subcutaneous UFH or LMWH is needed only for immobilized inpatients with an INR of less than 1.8.
Jaffer formulated the Cleveland Clinic Anticoagulation Clinic Protocol and defined the following 3 risk categories for thromboembolism [15] :
-
High - 1-year risk of arterial embolism greater than 10%, or 1-month risk of venous thromboembolism greater than 10%
-
Intermediate - 1-year risk of arterial embolism greater than 5-10%, or 1-month risk of venous thromboembolism at 2-10%
-
Low - 1-year risk of arterial embolism less than 5%, or 1-month risk of venous thromboembolism less than 2%
The table below gives a protocol for LMWH as a bridge to surgery in patients on warfarin.
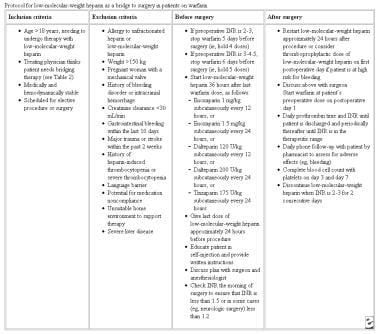 Protocol for low molecular weight heparin as a bridge to surgery in patients on warfarin. Adapted from Cleveland Clinic Journal.
Protocol for low molecular weight heparin as a bridge to surgery in patients on warfarin. Adapted from Cleveland Clinic Journal.
Lai addresses perioperative management of patients on new oral anticoagulants. Novel oral anticoagulants (NOACs) offer an alternative to warfarin for preventing stroke in patients with atrial fibrillation. Management of NOACs in elective and emergency conditions requires knowledge of time of last intake of drug, current renal function, and the planned procedure in order to assess the overall risk of bleeding. [16]
The first of several NOAC reversal agents, idarucizumab (Praxbind), was approved by the FDA in October 2015. Idarucizumab is a monoclonal antibody that binds specifically to dabigatran (it does not affect other NOACs). It is approved for patients treated with dabigatran when reversal of the anticoagulant effects are needed for emergency surgery or urgent procedures, or in the event of life-threatening or uncontrolled bleeding.
Accelerated approval for idarucizumab was based on interim analysis of the Re-VERSE AD trial. Investigators found that, among 39 patients who had been receiving dabigatran and required an urgent procedure were then given idarucizumab, 36 underwent their urgent procedure—with 33 (92%) having normal hemostasis during the event. Two of the remaining patients had mildly abnormal bleeding (with slight oozing), while just one had moderately abnormal yet controlled bleeding. Among 35 of 51 patients who had serious bleeding were able to be assessed, hemostasis, as determined by local investigators, was restored at a median of 11.4 hours. [17]
In May 2018, coagulation factor Xa recombinant (AndexXa) was approved for patients treated with rivaroxaban or apixaban, when reversal of anticoagulation is needed because of life-threatening or uncontrolled bleeding. Approval was supported by data from two Phase 3 ANNEXA studies (ANNEXA-R and ANNEXA-A), which evaluated the safety and efficacy of Andexxa in reversing the anticoagulant activity of the Factor Xa inhibitors rivaroxaban and apixaban in healthy older volunteers. Results demonstrated a rapid and significant reversal of anti-Factor Xa (FXa) activity. Anti-FXa activity was reduced among apixaban-treated participants by 94% compared with 21% for placebo (p< 0.001). A 92% reduction of anti-FXa activity was observed in the rivaroxaban-treated participants compared with 18% for placebo (p< 0.001). [18]
In the ANNEXA-4 trial, 67 patients who had acute major bleeding within 18 hr after administration of an FXa inhibitor received coagulation factor Xa recombinant. After the IV bolus plus 2 hour IV infusion, the median anti-FXa activity decreased by 89% from baseline among patients receiving rivaroxaban and by 93% among patients receiving apixaban. Assessment at 12 hours after the infusion adjudicated clinical hemostasis as excellent or good in 37 of 47 patients in the efficacy analysis (79%; 95% CI, 64 to 89). Thrombotic events occurred in 12 of 67 patients (18%) during the 30-day follow-up. [19]
Postoperative Management
Arterial thromboembolism
If surgery is performed within 1 month after an episode of arterial thromboembolism, intravenous heparin is warranted until the INR reaches 2 if the risk of bleeding is not very high. Administer intravenous UFH without a loading dose 12 hours after surgery at a rate of no more than 18 U/kg/h. Defer the first activated partial thromboplastin time (aPTT) for 12 hours to attain a stable anticoagulant response. Postoperative intravenous heparin is not recommended for patients who undergo major surgery and who are at high risk for anticoagulant-induced bleeding, even if an episode of arterial embolism has occurred within 1 month before surgery. Instead, administer subcutaneous UFH or LMWH (3000 U bid) until the INR reaches 1.8.
Venous thromboembolism
If the patient had an episode of VTE within 3 months before surgery, intravenous UFH is recommended until the INR is greater than or equal to 2. Patients who have a vena caval filter are protected from pulmonary embolism, and intravenous heparin can be avoided in their early postoperative period. If no previous episodes of VTE occurred within 3 months, postoperative intravenous heparin is not indicated. Subcutaneous heparin is recommended.
Madura et al recommend discontinuing warfarin 5 days before surgery and beginning intravenous heparin at 1000 U/h, while adjusting to maintain the aPTT at therapeutic levels. [20] Heparin is discontinued 6-12 hours before surgery and restarted at 200-400 U/h at 4-6 hours after surgery. Warfarin is restarted as soon as tolerated by the patient.
Stop oral anticoagulants at least 5 days preoperatively, and do not perform the procedure until the PT is in the reference range. Substitute intravenous heparin infusion for oral anticoagulant therapy preoperatively to prevent thromboembolic complications in the perioperative period. Stop the intravenous heparin infusion 6-12 hours preoperatively to allow the aPTT to return to normal for adequate intraoperative hemostasis. Restart the intravenous heparin infusion within 6 hours of completion of the surgical procedure to prevent postoperative thromboembolism. Resume oral warfarin therapy as soon as the patient is able to tolerate oral liquids. Do not release the patient until the PT is once again in the therapeutic range.
Consensus Conference on Antithrombotic Therapy
Perioperative management of anticoagulation entails an understanding of all thromboembolic events, indications for treatment, and duration of treatment. [21, 22, 23] The American College of Chest Physicians for Prevention of Thromboembolism published the following guidelines. [24] An updated guideline was published in 2008 [6] and 2012 [7] . A full listing is available at American College of Chest Physicians.
-
Low-risk general surgery patients - Early ambulation
-
Moderate-risk general surgery patients - Low-dose unfractionated heparin (LDUH), LMWH, intermittent pneumatic compression (IPC), or elastic stockings (ES)
-
Higher-risk general surgery patients - LDUH or higher-dose LMWH
-
Higher-risk general surgery patients prone to wound complications (eg, hematomas, infection) - IPC is an alternative.
-
Very high-risk general surgery patients with multiple risk factors - LDUH or LMWH combined with IPC
-
Selected very high-risk general surgery patients - Perioperative warfarin (goal INR 2.5, range 2-3)
-
Patients undergoing total hip replacement surgery - LMWH started 12-24 hours after surgery or warfarin started before or immediately after surgery (goal INR 2.5, range 2-3) if adjusted-dose heparin is started preoperatively; possible adjuvant use of ES or IPC
-
Patients undergoing total knee replacement surgery - LMWH, warfarin, or IPC
-
Patients undergoing hip fracture surgery - LMWH or warfarin (goal INR 2.5, range 2-3) started preoperatively or immediately after surgery
-
High-risk patients undergoing orthopedic surgery - Inferior vena cava (IVC) filter placement only if other forms of anticoagulant-based prophylaxis are not feasible because of active bleeding (should rarely be necessary)
-
Patients undergoing intracranial neurosurgery - IPC with or without ES; LMWH and LDUH may be acceptable alternatives; consider IPC or ES, with LMWH or LDUH, for high-risk patients
-
Patients with acute spinal cord injury - LMWH; although ES and IPC appear ineffective when used alone, ES and IPC may have benefit when used with LMWH or if anticoagulants are contraindicated; during rehabilitation, consider continuation of LMWH or conversion to full-dose oral anticoagulation
-
Trauma patients with an identifiable risk factor for thromboembolism - LMWH, as soon as considered safe; consider initial prophylaxis with IPC if administration of LMWH is delayed or is contraindicated; in high-risk patients with suboptimal prophylaxis, consider screening with duplex ultrasonography or filter placement in the IVC
-
Patients with myocardial infarction - LDUH or full-dose anticoagulation; IPC and possibly ES may be useful when heparin is contraindicated
-
Patients with ischemic stroke and lower extremity paralysis - LDUH or LMWH; IPC with ES also probably is effective
-
General medical patients with clinical risk factors for VTE, particularly those with congestive heart failure (CHF) or chest infections - LDUH or LMWH
-
Patients with long-term indwelling central vein catheters - Warfarin (1 mg/d) or daily LMWH to prevent axillary-subclavian venous thrombosis
-
Patients having spinal puncture or epidural catheters placed for regional anesthesia or analgesia - LMWH should be used with caution (additional data are now reported on timing of catheter removal), ES, LDUH
A retrospective study by Wamala et al that included 150 patients receiving new oral anticoagulants undergoing elective surgery reported that 41.5% of the decisions to interrupt anticoagulation were considered consistent with guidelines and based on low bleeding risk in all cases and high thrombotic risk in one-third. [25]
Questions & Answers
Overview
Which risks must be considered in surgical patients taking anticoagulants?
What are the options for perioperative anticoagulation management?
What are the risks of N-acetylcysteine in perioperative anticoagulation management?
What are the indications for perioperative anticoagulation management?
When is antithrombotic therapy indicated in perioperative anticoagulation management?
What is the perioperative anticoagulation management for patients with prosthetic heart valves?
What is the perioperative anticoagulation management of patients with previous arterial embolism?
What is the perioperative anticoagulation management of patients with venous thromboembolism (VTE)?
What are the risk categories for thromboembolism?
What is the protocol for use of LMWH as a bridge to surgery?
What is the role of novel oral anticoagulants (NOACs) in perioperative anticoagulation management?
What is the role of idarucizumab in perioperative anticoagulation management?
What is the role of IV heparin in postoperative anticoagulation management?
What is the basis for decisions to interrupt anticoagulation during perioperative management?
Which lab studies are needed for perioperative anticoagulation management?
-
Perioperative anticoagulation management. Low molecular weight heparin, prophylactic doses, and therapeutic doses.
-
Which patients on warfarin should receive heparin bridging before surgery? Adapted from Cleveland Clinic Journal.
-
Protocol for low molecular weight heparin as a bridge to surgery in patients on warfarin. Adapted from Cleveland Clinic Journal.