Overview
Impairment of the excretory function of the kidney results in an elevation in levels of blood urea nitrogen (BUN), creatinine, and various protein metabolic products. Impairment in the synthetic function results in a decrease in the production of erythropoietin (causing anemia) and active vitamin D-3 (causing hypocalcemia, secondary hyperparathyroidism, hyperphosphatemia, and renal osteodystrophy). Impairment in synthetic function also results in a reduction in acid, potassium, salt, and water excretion (causing acidosis, hyperkalemia, hypertension, and edema) and in platelet dysfunction (promoting bleeding).
The aforementioned complications must be identified and corrected perioperatively. [1] In addition, drugs normally excreted by the kidney can accumulate to toxic levels in patients with chronic kidney disease (CKD). Therefore, adjusting dosages or avoiding such drugs, including iodinated contrast in high-risk patients, is a key management principle in patients with CKD. Of note is the avoidance of meperidine in patients with CKD or end-stage renal disease (ESRD) because the active metabolite (normeperidine) can accumulate and cause seizures.
CKD can be associated with excess surgical morbidity, the most important of which include acute renal failure, hyperkalemia, volume overload, and infections. [2, 3] The above underscore the need for early involvement of a nephrologist.
Preparation
Anesthesia
The administration of general anesthesia may induce a reduction in renal blood flow in up to 50% of patients, resulting in the impaired excretion of nephrotoxic drugs. In addition, the function of cholinesterase, an enzyme responsible for breaking down certain anesthetic agents, may be impaired, resulting in prolonged respiratory muscle paralysis if neuromuscular blocking agents are used.
N -acetyl-procainamide, a metabolite of procainamide, accumulates in persons with chronic kidney disease (CKD) and, when used in combination with histamine-2 (H2) blockers, causes prolongation of the QTc. [4] The dose of procainamide should be adjusted, or a substitute should be used.
Fluorinated compounds, such as methoxyflurane and enflurane, are nephrotoxic and should be avoided in patients with CKD. Succinylcholine, a depolarizing blocker, causes hyperkalemia.
Complication prevention
Hyperkalemia may be precipitated by tissue breakdown, transfusions, acidosis, angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, heparin, rhabdomyolysis, and the use of Ringer lactate solution as a replacement fluid. Ringer lactate solution contains potassium, which is often disregarded but can cause hyperkalemia. Third-space fluid loss, diarrhea, vomiting, and nasoenteric suction result in both volume contraction and hypokalemia. Hypokalemia is sometimes followed concomitantly with hypomagnesemia.
Most patients with CKD have chronic acidosis; surgical disease can further complicate the acidemia. Such patients are at a higher risk for hyperkalemia, myocardial depression, and cardiac arrhythmia.
Hypocalcemia and hyperphosphatemia may be caused by rhabdomyolysis. Hyponatremia may occur from hypotonic fluids or inappropriate secretion of antidiuretic hormone.
Cardiovascular risk assessment
Up to 50% of patients with stage 5 CKD have cardiovascular disease. [5, 6] In light of the high prevalence of cardiovascular disease and increased perioperative morbidity in patients with CKD, a thorough cardiovascular risk assessment is indicated and should be performed in accordance with the American College of Cardiology/American Heart Association (ACC/AHA) guidelines, as outlined below. [7, 8]
Clinical predictors of preoperative cardiovascular risk (eg, myocardial infarction[MI], chronic heart failure [CHF]) can be described as major, intermediate, or minor risk factors.
Major predictors include the following:
-
Unstable coronary syndromes - Recent MI with evidence of important ischemic risk based on clinical symptoms or the results of noninvasive testing or unstable or severe angina (Canadian Heart Association class III or IV)
-
Decompensated CHF
-
Significant arrhythmias - High-grade atrioventricular block, symptomatic ventricular arrhythmias in the presence of underlying heart disease, supraventricular arrhythmias with uncontrolled ventricular rate
-
Severe valvular disease [9]
-
Intermediate predictors include the following:
-
Mild angina pectoris (Canadian Heart Association class I or II)
-
Prior MI based on history findings or the presence of pathological Q waves
-
Compensated or prior CHF
-
Diabetes mellitus
-
Minor predictors include the following:
-
Advanced age
-
Abnormal ECG findings (eg, left ventricular hypertrophy, left bundle-branch block, ST-T abnormalities)
-
Rhythm other than sinus (eg, atrial fibrillation)
-
Low functional capacity (eg, inability to climb one flight of stairs with bag of groceries)
-
History of stroke
-
Uncontrolled systemic hypertension
Surgical risk for noncardiac procedures can be divided into high-risk, intermediate-risk, or low-risk surgery. The type of surgery, the duration of the surgical procedure, and, occasionally, the choice of anesthesia can make a difference in patient outcome. Allowing the anesthesiologist to choose the mode of anesthesia is always advisable.
High-risk (reported cardiac risk often >5%) noncardiac procedures include the following:
-
Emergency operations, particularly in elderly persons
-
Aortic and other major vascular procedures
-
Peripheral vascular procedures
-
Anticipated prolonged surgical procedures associated with large fluid shifts, blood loss, or both
Intermediate-risk (reported cardiac risk generally < 5%) noncardiac procedures include the following:
-
Carotid endarterectomy
-
Head and neck procedures
-
Intraperitoneal and intrathoracic procedures
-
Orthopedic procedures
-
Prostate surgery
Low-risk (reported cardiac risk generally < 1%) noncardiac procedures include the following:
-
Endoscopic procedures
-
Superficial procedures
-
Cataract surgery
-
Breast surgery
Patients with major clinical predictors of cardiac morbidity can be assessed using the flow chart from the ACC/AHA guidelines on preoperative management (see the image below).
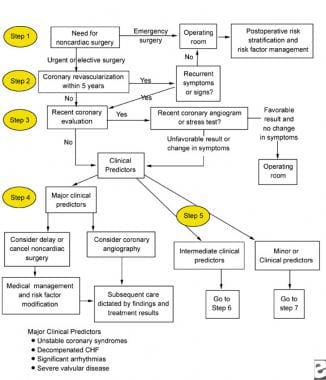 Major clinical predictors to be used for the perioperative management of a patient with chronic renal failure.
Major clinical predictors to be used for the perioperative management of a patient with chronic renal failure.
Preoperatively, patients who are undergoing elective surgery can be treated by identifying their risk profile for surgery and their risk with the intended procedure, as indicated above. Patients with decompensated heart failure or unstable coronary syndromes should have their procedures postponed until their medical management is optimized.
Patients with intermediate or minor clinical predictors (see images below) who are undergoing elective noncardiac surgery should be evaluated based on their functional capacity and type of surgery.
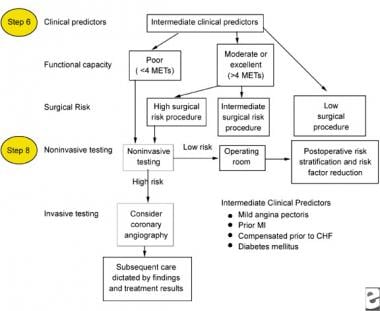 Intermediate clinical predictors to be used for the perioperative management of a patient with chronic renal failure.
Intermediate clinical predictors to be used for the perioperative management of a patient with chronic renal failure.
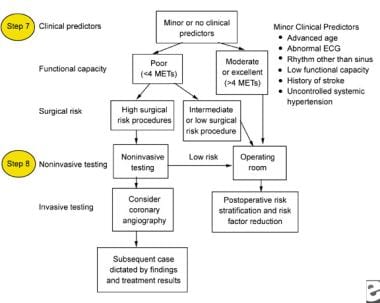 Minor clinical predictors to be used for the perioperative management of a patient with chronic renal failure.
Minor clinical predictors to be used for the perioperative management of a patient with chronic renal failure.
Functional capacity is defined in metabolic equivalents of task (METs), which are usually self-reported; however, this capacity can also be assessed based on the results from exercise treadmill testing. Patients who achieve at least 6 METs have a significantly better prognosis. Patients with poor functional capacity should be evaluated with noninvasive testing methods, while patients with good functional capacity can proceed to surgery.
Based on the updated ACC/AHA guidelines on perioperative cardiovascular evaluation of noncardiac surgery, all patients with renal insufficiency who have a creatinine level of 2 mg/dL or higher are considered to have an intermediate clinical predictor of increased perioperative cardiovascular risk.
Renal risk assessment and interventions
Patients with CKD treated conservatively
Rapidly establish the duration of CKD; level of renal function impairment; and whether the elevation in BUN and creatinine is prerenal, intrarenal, postrenal, or a combination of these on a background of CKD. Patients who are euvolemic, responsive to diuretic therapy, and/or have no significant electrolyte abnormalities or bleeding tendencies have uncomplicated cases and do not require dialysis before surgery.
Patients with edema, CHF, or pulmonary congestion or those who are responsive to diuretic therapy require further cardiovascular evaluation. If the results of the cardiovascular evaluation are optimal, then fluid overload can be attributed to CKD. Combination diuretic therapy can help treat these patients to achieve euvolemia prior to surgery.
Patients with diabetes have a greater tendency of having volume overload or cardiovascular disease. CKD may be so advanced that the patient develops diuretic resistance, with progressive edema. Preoperative dialysis may be considered in these patients.
If postoperative dialysis is imminent, the surgeons should be advised to place a temporary catheter intraoperatively. This avoids the use of femoral cannulation, which carries a higher risk of infection. Permanent vascular access placement can then be arranged when the patient is more stable.
Further deterioration in renal function can be avoided by identifying and eliminating potential nephrotoxic agents. These include substitution or dosage adjustment for antibiotics (eg, aminoglycosides, acyclovir, amphotericin), sedatives, and muscle relaxants. Nonsteroidal anti-inflammatory drugs (NSAIDs) should be avoided, as should radiocontrast material.
Iodinated radiocontrast material can induce acute renal failure by causing vasoconstriction and direct renal tubular epithelial cell damage and should be avoided as much as possible. The use of gadolinium in patients with CKD or ESRD has been shown to be associated with diffuse fibrosis, mostly of the skin, known as nephrogenic systemic fibrosis. [10] Therefore, unless there is no alternative method of diagnosis, gadolinium should be avoided as much as possible. If iodinated radiocontrast material must be used, prophylactic oral administration of the antioxidant acetylcysteine, or administration of sodium bicarbonate along with hydration (0.45% saline), may reduce the risk of acute renal failure. [11]
Controversy exists on the effectiveness of acetylcysteine and sodium bicarbonate and the superiority of one over the other; nonetheless, until rigorous clinical trials are conducted, the use of these agents in clinical practice is likely to continue, as these agents are benign and have been shown in some (if not most) studies to reduce acute renal failure.
Use of meperidine for postoperative pain control should be avoided because accumulation of its metabolite normeperidine can cause seizures in patients with CKD, especially those on dialysis.
All drug interactions and potential nephrotoxicity must be identified and either the drug must be stopped or its dose adjusted for the level of renal function. Electrolyte abnormalities must be identified and corrected perioperatively.
Dialysis and renal transplant patients
For patients already on dialysis, the following need to be determined [12] :
-
Dialysis adequacy
-
Preoperative dialysis needs
-
Postoperative dialysis timing
-
Dosage requirements for all medications
Patients on hemodialysis usually require preoperative dialysis within 24 hours before surgery to reduce the risk of volume overload, hyperkalemia, and excessive bleeding. Patients with peritoneal dialysis who are undergoing abdominal surgery should be switched to hemodialysis until wound healing is complete. Peritoneal dialysis should be continued for those undergoing nonabdominal surgery.
Because of complicated interactions and immunosuppressive dosing, monitoring, and adjustment, a nephrologist with specialized knowledge of renal transplantation should be involved in the preoperative evaluation of patients with CKD who have received kidney transplantation. Cyclosporine or tacrolimus taken by renal transplant recipients for immunosuppression are metabolized by the cytochrome P-450 system in the liver and, thus, interact with a wide variety of agents.
Diltiazem, hepatic 3-methylglutaryl coenzyme A reductase inhibitors (statins), macrolides, and antifungal drugs inhibit the system, elevate levels, and can precipitate nephrotoxicity. Others, such as carbamazepine, barbiturates, and theophylline, induce the system, reduce levels, and can precipitate rejection. Drug levels must be monitored in this setting. Intravenous cyclosporine or tacrolimus should be given at one third the oral dose until the patient is able to tolerate oral medications.
Post-Procedure
Postoperative decision making and management
No preoperative cardiac assessment is indicated for emergency surgery; however, postoperative cardiac assessment must be performed and continued for 3-5 days with daily ECGs and screening of cardiac enzyme levels to detect and treat possible perioperative MI. Perioperative MI occurs mostly within the first 72 hours; however, most occurrences are silent. The incidence rate of perioperative MI is approximately 1% but the mortality rate is almost 50%.
Be aware of discordant testing results of total creatine kinase (CK), myocardial band enzymes of CK (CK-MB), and troponin. [13] Total CK levels are elevated in patients with CKD, but CK-MB levels are not; thus, elevation in CK-MB levels is due to myocardial injury. Elevation of troponin levels without a corresponding elevation in total CK levels has been shown to reflect enzyme elimination kinetics due to renal failure or cross-reactivity of the troponin I assay with noncardiac antigens.
Therefore, any cardiac enzyme elevations are not diagnostic in and of themselves. The diagnosis of postoperative MI should be made based on a combination of clinical, laboratory, and ECG evidence.
The preoperative use of beta-blocker therapy (eg, metoprolol, atenolol) may be beneficial. At least 2 randomized placebo-controlled trials have demonstrated reduced perioperative cardiac events and up to 6 months' improved survival. The risks of developing or worsening hyperkalemia have been mentioned previously.
The ACC/AHA guidelines for perioperative cardiovascular evaluation for noncardiac surgery indicate definite benefit (ie, class 1 classification) of beta-blocker therapy in patients at high cardiac risk for events because of the presence of ischemia in those undergoing vascular surgery or who have required them in the past for angina or hypertension disease; however, no data are available on patients with CKD. [7, 8]
The authors suggest beta-blocker therapy with a target heart rate of 50-60 beats per minute for patients at high or intermediate risk for cardiac events, provided that no absolute or significant relative contraindications exist. However, larger placebo-controlled trials are needed to better define benefits and risks in patients with CKD or ESRD.
-
Major clinical predictors to be used for the perioperative management of a patient with chronic renal failure.
-
Intermediate clinical predictors to be used for the perioperative management of a patient with chronic renal failure.
-
Minor clinical predictors to be used for the perioperative management of a patient with chronic renal failure.