Practice Essentials
Japanese encephalitis is a neurologic infection with a broad range of manifestations. It can range from subtle changes in behavior to serious problems, including blindness, ataxia, weakness, and movement disorders. Japanese encephalitis is caused by the Japanese encephalitis virus (JEV), a flavivirus, and is closely related to St. Louis encephalitis and West Nile encephalitis. It occurs primarily in rural areas of Asia and as of March 2022 has been declared a "Communicable Disease Incident of National Significance" in Australia. [1] Japanese encephalitis is spread through these regions by bites of culicine mosquitoes, most often Culex tritaeniorhynchus.
Epidemiology
In the United States, Japanese encephalitis mostly develops among travelers returning from endemic countries. Countries with endemic Japanese encephalitis virus include Malaysia, Philippines, China, Taiwan, Bangladesh, Thailand, India, Japan, Pakistan, and several other countries in the neighboring regions. See the Epidemiology section for more detail.
Signs and Symptoms
Individuals infected with Japanese encephalitis virus have a history of mosquito exposure in an endemic area. Such individuals may present with fever, headache, nausea, diarrhea, vomiting, and/or myalgia, followed by altered mental status, seizures, flaccid paralysis, hyperpneic breathing, extrapyramidal signs, and cranial nerve findings.
See Clinical Presentation for more detail.
Diagnosis
Japanese encephalitis virus–specific immunoglobulin M (IgM) capture enzyme-linked immunoassay (ELISA) on serum or cerebrospinal fluid (CSF) is the standard diagnostic test for Japanese encephalitis. Virus isolation from clinical specimens is difficult because viremia in humans is transient and low level. Lumbar puncture is performed to obtain CSF and to rule out other potential etiologies of encephalitis. The opening pressure may be high, CSF protein level may be high, and CSF glucose level is often normal. Potential bloodwork findings may include mild leukocytosis and hyponatremia. MRI and CT scanning of the brain may show bilateral thalamic lesions with hemorrhage. Electroencephalography (EEG) may show diffuse slowing.
See Workup for more detail.
Differential Diagnoses
Differential diagnoses include West Nile virus encephalitis, St. Louis encephalitis, Murray Valley encephalitis, herpes simplex virus encephalitis, dengue fever, Nipah virus infection, California encephalitis, pyogenic focal brain abscess, tuberculous meningitis, Rocky Mountain spotted fever, fungal infections, central nervous system (CNS) lupus, CNS tumors, and cerebrovascular accident (CVA).
Management
No antiviral agent is effective against Japanese encephalitis virus. Care is supportive, including management of intracranial pressure, if needed, airway protection, and seizure control.
See Treatment and Medication for more detail.
Prevention
Japanese encephalitis vaccine is available. Measures to prevent mosquito bites and to decrease the mosquito population and viral spread should be implemented.
See Treatment and Medication for more detail.
Prognosis
Mortality rates associated with Japanese encephalitis may exceed 35% in less-developed countries. The prognosis varies depending on several factors. Most cases improve between 6 months and 12 months. Approximately 33-50% of survivors have major neurologic sequelae. Good prognostic factors include high CSF concentration of neutralizing antibodies. Poor prognostic factors include low Glasgow coma scale (GCS), hyponatremia, and age younger than 10 years.
See Prognosis for more detail.
Etiology
Japanese encephalitis virus, a flavivirus (single-stranded ribonucleic acid [RNA]), represents the most significant etiology of arboviral encephalitis worldwide. Japanese encephalitis virus belongs to the Japanese encephalitis serocomplex, which is composed of 9 genetically and antigenically related viruses of the Flaviviridae family. Japanese encephalitis serocomplex flaviviruses include Alfuy virus, Cacipacore virus, Japanese encephalitis virus, Koutango virus, Murray Valley encephalitis virus, St. encephalitis virus, Usutu virus, West Nile virus including Kunjin virus, and Yaounde virus. [2]
In 1934, a Japanese scientist, Hayashi, inoculated monkey brains with the virus, reproducing the disease. This virus was named Japanese B encephalitis virus, after its association with the summer type (or type B) encephalitis.
Japanese encephalitis virus is transmitted to humans via the bite of infected Culex mosquitoes, especially C tritaeniorhynchus. Other Culex vectors include Culexvishnui (India), Culexgelidus, and Culexfuscocephala (Thailand, India, Malaysia). They prefer to bite outdoors and are extremely active in the evening and night, when the risk for infection is greatest.
Mosquitoes breed in collections of water (typically rice paddies), increasing the risk for infection in rural areas.
Aedes mosquitoes also have been implicated in Japanese encephalitis virus infection.
Humans are incidental and dead-end hosts, producing a low-grade, short-term viremia. Therefore, mosquitoes are unable to transmit the virus from one person to another.
Pigs and aquatic birds (eg, egrets, herons) serve as amplifying hosts. They develop persistent, high-grade viremia and represent the main vertebrate hosts and the principal reservoir for the virus. Cattle develop only relatively low-grade viremia or none at all; these animals are not part of the natural transmission cycle of the virus.
Horses and piglets (not adult pigs) may develop clinical illness with a symptom spectrum similar to that in humans (eg, fever, locomotion difficulty, confusion).
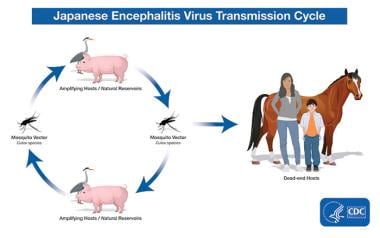 Transmission of Japanese encephalitis virus. Courtesy of the Centers for Disease Control and Prevention (CDC) [https://www.cdc.gov/japaneseencephalitis/transmission/index.html].
Transmission of Japanese encephalitis virus. Courtesy of the Centers for Disease Control and Prevention (CDC) [https://www.cdc.gov/japaneseencephalitis/transmission/index.html].
The main genotypic variants of Japanese encephalitis virus include the following [3] :
-
Japanese encephalitis virus genotype I isolates have been identified in northern Thailand, Cambodia, and Korea.
-
Japanese encephalitis virus genotype II isolates have been identified in southern Thailand, Malaysia, Indonesia and Northern Australia. This Genotype was the cause of the cases seen in Australia in the 1990s. [4]
-
Japanese encephalitis virus genotype III isolates have been identified in Japan, China, Taiwan, Philippines, and the Asian subcontinent, including India and Nepal.
-
Japanese encephalitis virus genotype IV isolates are the least common worldwide. Previously its distribution was limited to Indonesia and Papua New Guinea; however, it is the causative agent of the most recent 2022 outbreak in Australia. [4]
-
Japanese encephalitis virus genotype V isolate was identified in 1952 from a patient who originated in Muar, Malaysia (Muar strain). There is a report of its possible reemergence in the Republic of Korea. [5]
Pathophysiology
Innoculation occurs at the site of a mosquito blood meal. In cases where symptoms arise, the incubation period is believed to range from 5-15 days.
After attachment of the Japanese encephalitis virus to a host cell membrane, local membrane disruption may lead to entry of the virus into the cell itself. The virus initially propagates within antigen presenting cells present at the site of the mosquito bite and in regional lymph nodes. Two cellular characteristics are critical to the pathogenesis: (1) the M protein, which contains hydrophobic domains that help to anchor the virus onto the host cell, and (2) the E protein, which is the principal immunogenic feature and which is expressed on the membrane of infected cells. The E protein mediates membrane fusion of the viral envelope and the cellular membrane, promoting viral entry into the host cell. The Japanese encephalitis virus replication cycle includes initial host cell receptor interaction of the virus followed by receptor-mediated endocytosis, fusion of the viral and host cell membranes, subsequent cytoplasmic release of viral genome, and several other transcription and pretranslation steps. Maturation of virus particles occurs in the Golgi complex, followed by ultimate release of the virus. [6, 7]
Subsequently, viremia develops, leading to inflammatory changes in the heart, lungs, liver, and reticuloendothelial system. Most infections are cleared before the virus can invade the CNS, leading to subclinical disease.
Subclinical or mild forms of Japanese encephalitis resolve in a few days if the CNS is not involved. In such cases, the infection may not produce symptoms and therefore remains undetected. However, given the neurotropic character of Japanese encephalitis virus, neurologic invasion can develop, possibly by growth of the virus across vascular endothelial cells, leading to involvement of large areas of the brain, including the thalamus, basal ganglia, brain stem, cerebellum (especially the destruction of the cerebellar Purkinje cells), hippocampus, and cerebral cortex. Persistent infection and congenital transmission may occur. The levels of varying immune response (intrinsic, cellular, humoral) have been characterized. Higher levels of certain cytokines (interferon-alpha, interleukins 6 and 8) have been associated with an increased mortality risk. The types of response implicate impaired T-helper-cell immunity in patients with severe advanced disease.
Overall, Japanese encephalitis virus is believed to result in increased CNS pathology because of its direct neurotoxic effects in brain cells and its ability to prevent the development of new cells from neural stem/progenitor cells (NPCs). Japanese encephalitis virus likely represents the first mosquito-transmitted viral pathogen to affect neural stem cells. These cells can serve important roles in injury recovery; consequently, Japanese encephalitis–induced disruption of neural stem cell growth may be particularly important to further morbidity and mortality.
Studies have found, in addition to neurons, other CNS cells such as astrocytes and microglial cells may also serve as reservoirs for viral replication, resulting in potential damage to the blood-brain barrier. [8, 7]
Recent research indicates that matrix metalloproteinases and inhibitors of metalloproteinases likely play a role in pathogenesis during viral encephalitis by modulating the blood-brain barrier and affecting the entry of immune cells into the CNS. A 2016 study found that matrix metalloproteinases were up-regulated in mice infected with Japanese encephalitis virus, and inhibitors of metalloproteinases were down-regulated in the infected mice. [9]
Epidemiology
Serologic evidence of Japanese encephalitis virus infection in endemic rural areas is found in nearly all inhabitants by early adulthood. Most symptomatic infections in endemic areas occur in young children (aged 2-10 years) and elderly people. In nonendemic areas, Japanese encephalitis virus infection has no age predilection.
Occurrence in the United States
In the United States, Japanese encephalitis develops mostly among military personnel, expatriates, and, rarely, returning travelers. Before 1973, more than 300 cases of Japanese encephalitis were reported among US military personnel or their family members. [10] From 1973-2017, 84 cases were reported to the Center for Disease Control and Prevention (CDC) among travelers and expatriates from nonendemic countries. Between 1993 - 2017, only 12 cases among US travellers were reported. The approximate risk estimate is less than 0.2 cases per 1 million US travelers. Overall risk is increased by prolonged travel to endemic areas and prolonged time spent outdoors and nighttime exposure in rural areas. Outbreaks are rare in the US territories of Guam and Saipan. [11, 12]
International occurrence
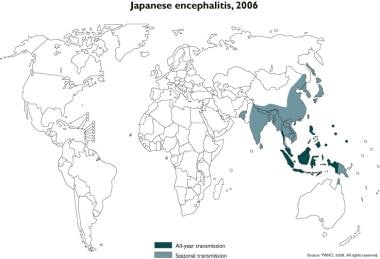
Approximately 3 billion people currently live in areas endemic for Japanese encephalitis; these areas extend from Pakistan to maritime Siberia and Japan. Japanese encephalitis is a seasonal disease, with most cases occurring in temperate areas from June to September. Further south, in subtropical areas, Japanese encephalitis virus transmission begins as early as March and extends until October. Transmission may occur all year in some tropical areas (eg, Indonesia). The annual incidence of Japanese encephalitis differs among affected countries. In endemic countries, the annual incidence is estimated at 5.4/100,000 in children aged 0-14 years and 0.6/100,000 in individuals older than 15 years. Approximately 68,000 global cases are reported annually. Reported annual global deaths from Japanese Encephalitis range from 13,600-20,400. [13]
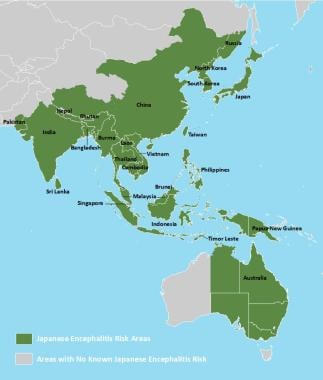 Geographic distribution of Japanese encephalitis virus. Courtesy of the Centers for Disease Control and Prevention (CDC) [https://www.cdc.gov/japaneseencephalitis/maps/index.html].
Geographic distribution of Japanese encephalitis virus. Courtesy of the Centers for Disease Control and Prevention (CDC) [https://www.cdc.gov/japaneseencephalitis/maps/index.html].
Areas of seasonal and year-round transmission of Japanese encephalitis virus are shown in the map below.
Countries with epidemic or endemic Japanese encephalitis include the following:
-
Malaysia
-
Burma
-
Singapore (rare cases)
-
Philippines
-
Indonesia
-
China
-
Taiwan
-
Russia (maritime Siberia)
-
Bangladesh
-
Laos
-
Cambodia
-
Thailand
-
Vietnam
-
India
-
Nepal (especially the Terai region)
-
Sri Lanka
-
Korea
-
Japan
-
Australia (possibly in islands of Torres Strait [14] )
-
Brunei
-
Pakistan
-
Papua New Guinea
-
Pacific Islands (rare outbreaks in Guam and Saipan)
-
Himalayas (under investigation as new emergence or improved detection) [15]
In 2005, a Japanese encephalitis epidemic occurred in the Indian states of Uttar Pradesh and Bihar and throughout Nepal, resulting in more than 5000 cases and approximately 1000 deaths. [16]
Two outbreaks of Japanese encephalitis have occurred in Australia, the first in 1995 on islands in the Torres Strait [14] and the second in 1998 on the Cape York Peninsula. In addition, in 2004, one Japanese encephalitis virus isolate was detected from a pool of Culex mosquitoes trapped on the Cape York Peninsula.
The incidence of Japanese encephalitis in China has decreased since the introduction of vaccination in 1980. Whereas the incidence is decreasing in children, there is a higher incidence in adults, which is emerging as a public health problem. Epidemiologic research into the spatial and temporal distribution of Japanese encephalitis virus revealed that children aged 0-15 years tend to become infected more commonly south of the Yangtze River and adults older than 40 years tend to become infected more commonly north of the Yangtze River. [17]
Overall, as with other emerging pathogens, many of which are zoonotic viruses, a very complicated interplay of ecologic, climatic, environmental, and human behavioral factors have resulted in widespread distribution of Japanese encephalitis virus. Even mosquitoes pushed along by wind currents have been considered contributory to viral spread, eg, from Papua New Guinea to the Torres Strait islands and the Australian mainland. However, no evidence shows that Japanese encephalitis epidemics are likely part of postflooding infectious disease outbreaks.
As of March 2022, Japanese Encephalitis has been declared a "Communicable Disease Incident of National Significance" in Australia. Between January 2021 and February 2023, there have been 45 cases of Japanese Encephalitis in Australia, with 7 deaths attributed to the infection. [18]
Prognosis
The prognosis of symptomatic Japanese encephalitis virus infection varies. Two factors that portend a good prognosis include high concentrations of neutralizing antibodies in the cerebrospinal fluid (CSF) and high levels of Japanese encephalitis virus immunoglobulin G (IgG) in the CSF.
Overall, poor prognostic factors include the following:
-
Age younger than 10 years
-
Low Glasgow coma scale
-
Hyponatremia
-
Shock
-
Presence of immune complexes in CSF
-
Presence of increased amounts of antineurofilament antibodies
-
Increased levels of tumor necrosis factor
-
Coexisting neurocysticercosis
Proven risk factors for mortality include demonstration of virus in the CSF, low levels of IgG/IgM in the CSF or serum, and a decreased sensorium.
Mortality rates in locales with intensive care capabilities are 5-10%. In less-developed areas, mortality rates may exceed 35%. Worldwide, more than 10,000 deaths attributable to Japanese encephalitis are reported per year. The main causes of Japanese encephalitis–related mortality include aspiration, seizures, increased intracranial pressure, and hypoglycemia. [19]
Most cases improve between 6 months (55%) and 12 months (78%). [20]
Approximately 33-50% of survivors of symptomatic disease have major neurologic sequelae at 1 year, including seizure disorders, motor or cranial nerve paresis, or movement disorders. At 5 years, nearly 75% of such patients score lower on standardized tests than control subjects.
Japanese encephalitis virus infection in the first or second trimester of pregnancy may lead to fetal death. Infection in the third trimester, although not systematically evaluated, appears to be associated with a normal fetal outcome.
Previous dengue infection may be associated with decreased morbidity and mortality rates, possibly due to partial protection of cross-reacting antiflaviviral antibodies.
Patient Education
For patient education information, see the Brain and Nervous System Center, as well as Encephalitis.
-
Japanese encephalitis, 2006. Courtesy of WHO.
-
Geographic distribution of Japanese encephalitis virus. Courtesy of the Centers for Disease Control and Prevention (CDC) [https://www.cdc.gov/japaneseencephalitis/maps/index.html].
-
Transmission of Japanese encephalitis virus. Courtesy of the Centers for Disease Control and Prevention (CDC) [https://www.cdc.gov/japaneseencephalitis/transmission/index.html].