Practice Essentials
Wilms tumor, treatment for which includes surgery (radical or partial nephrectomy), is the fifth most common pediatric malignancy and the most common type of renal tumor in children. Wilms tumor is rare in adults, accounting for only 0.5% of renal lesions. [1]
The outcome of current treatment for Wilms tumor is an example of success achieved through a multidisciplinary collaboration of the National Wilms' Tumor Study Group (NWTSG), which merged with other pediatric groups to form the Children's Oncology Group (COG), and the Societe Internationale d'Oncologie Pediatrique (SIOP). Note the image below.
Fifty years ago, when Wilms tumor was treated with surgery alone, the survival rate 2 years after nephrectomy was 20%. The introduction of adjuvant radiotherapy raised the survival rate to 50% overall. Owing to the cooperative efforts of oncologists, surgeons, and pathologists and with the introduction of chemotherapy with vincristine, dactinomycin (actinomycin D), and doxorubicin, the overall survival rate since the late 20th century has risen to 90%. [2]
Although the NWTSG/COG and SIOP guidelines concur that combined surgery, chemotherapy and radiotherapy is the initial treatment for Wilms tumor in children, they differ on the preferred strategy for delivery of therapy. For unilateral Wilms tumor, the NWTSG/COG guidelines, primarily followed in North America, recommend surgery prior to chemotherapy, whereas the SIOP guidelines, followed in Europe, recommend both preoperative and postoperative chemotherapy. [2]
See Wilms Tumor: A Pediatric Oncology Success Story, a Critical Images slideshow, to help identify the clinical features, staging evaluation, prognostic factors, and therapeutic options for this disease.
Anatomy
Wilms tumor arises from the primitive embryonal renal tissue. Grossly, Wilms tumor is typically an intrarenal solid or cystic mass, which may displace and, in rare cases, invade the renal collecting system. The tumor extends into the renal vein in 40% of cases. In very rare cases, it extends into the ureter and down to the bladder, where it may cause hematuria. Wilms tumor is bilateral in 6% of cases. Local invasion is rare and tumor spread is usually through lymphatic and vascular routes.
Surgical Indications and Contraindications
Indications
Indications for primary surgical excision of a Wilms tumor include tumors confined to the kidney, extending beyond the kidney but not crossing the midline, and with or without vascular extension. Postchemotherapy excision of the tumor is indicated in patients with bilateral tumors, tumors that extended beyond the midline and have shrunk, and tumors with vascular extension. Surgery alone is not recommended for Wilms tumor, based on the results of the NWTSG-5 study. [3, 4]
Contraindications
Contraindications to primary surgery for Wilms tumor include bilateral tumors and documented metastatic disease. Large tumors that extend beyond the midline, have vascular extension, or both are relative contraindications, since some surgeons elect to obtain tissue via surgical excision, but this may expose patients to increased surgical risks.
Surgical Approach
Radical nephrectomy
According to the NWTSG protocol, the first step in the treatment of Wilms tumor is surgical staging followed by radical nephrectomy, if possible. [5] During the surgical staging procedure, exploration of the contralateral kidney is considered unnecessary because of the diagnostic reliability of current imaging techniques (computed tomography [CT] scanning, magnetic resonance imaging [MRI]).
If imaging studies demonstrate bilateral disease, nephrectomy is not performed, but biopsy specimens are obtained. New protocols in the management of bilateral Wilms tumor are being explored.
Partial nephrectomy
In patients with bilateral Wilms tumors, solitary kidney, or kidney insufficiency, partial nephrectomy is a reasonable consideration. Although end-stage kidney disease after unilateral radical nephrectomy is uncommon (0.25% in the NWTSG trial), preserving healthy kidney tissue may prevent this complication, especially in patients with an underlying intrinsic kidney disease (eg, WAGR [Wilms tumor, aniridia, genitourinary anomalies, and mental retardation] syndrome, Denys-Drash syndrome). [6] Partial nephrectomy may be feasible in only 10%-15% of patients, however, as most tumors are too large at initial diagnosis.
Several groups have proposed criteria for selecting patients for partial nephrectomy. [7] In children with bilateral Wilms tumor, the COG and the SIOP Renal Tumour Study Group (RTSG) advocate nephron-sparing surgery, if feasible, with preoperative chemotherapy for up to 12 weeks to shrink the tumor and improve outcome. [8, 9]
For unilateral Wilms tumor, the SIOP-RTSG considers nephron-sparing surgery acceptable for nonsyndromic cases with small tumor volume (< 300 mL) and the expectation of a substantial remnant kidney function in patients with tumors < 300 mL who never had lymph node involvement. [8] A meta-analysis of nephron-sparing surgery for Wilms tumor concluded that it provides higher survivability and postoperative kidney function and a lower incidence of relapse, compared with radical nephrectomy. [10]
The main concern regarding a nephron-sparing procedure is that of local recurrence. The NWTS-4 study showed an 8% local recurrence rate following partial nephrectomy for patients with bilateral disease. [11]
Laparoscopic Nephrectomy
Laparoscopy has been used since the 1990s in pediatric surgical oncology. [12, 13, 14, 15] More recently, several authors have recommended the use of a laparoscopic approach in children with Wilms tumor that has favorable histology and has not spread locally or metastasized. [16, 17, 18] In addition to decreased lengths of hospital stay and analgesic requirements, the faster recovery with a laparoscopic procedure offers the further benefit of reducing the time required to begin or reinstitute chemo- or radiotherapy postoperatively. [19]
Laparoscopic nephrectomy can be performed when the tumor is small, allowing a first approach to the renal vessels without mobilization of the kidney. In some reports, all the procedures could be completed when the tumor did not cross the lateral edge of the vertebra on the CT scan. [20, 21]
Although laparoscopic nephrectomy for Wilms tumor is becoming more common, it remains controversial and accounts for only a minority of procedures. [7, 22] A review of the National Cancer Data Base from 2010 to 2012 found that minimally invasive surgery was used in only 5% of children with Wilms tumor. Compared with open surgery, minimally invasive surgery, less often evaluated lymph nodes and had smaller lymph node harvest; after propensity matching, however, there was no difference in 30-day mortality, readmissions, surgical margin status, and 1- and 3-year survival. [23] In contrast, a systematic review by the American Pediatric Surgery Association found a lack of evidence to support minimally invasive surgery for pediatric renal tumors, including Wilms tumor. [22]
Robotic nephrectomy
In recent years, robotic-assisted laparoscopic surgery (RAS) has gained popularity in both adult and pediatric urology. RAS has several advantages over conventional laparoscopic surgery, especially in the enhanced exposure with magnified three-dimensional view and simplification of suturing with the increased degree of freedom and movement of the robotic arm. [24]
Robotic-assisted nephrectomy is possible in children with Wilms tumor and offers some advantages but should be, at this time, considered experimental. In addition, expense currently limits its use. [25]
Preoperative Details
If the tumor is unresectable, biopsies are performed and the nephrectomy is deferred until after chemotherapy, which, in most cases, will shrink the tumor. Contiguous involvement of adjacent organs is frequently overdiagnosed. The overall surgical complication rate for Wilms tumor is approximately 20%. If inferior vena cava (IVC) thrombus is present, preoperative chemotherapy will reduce the cavotomy rate by 50%.
With bilateral Wilms tumor (6% of cases), surgical exploration, biopsy of both sides, and accurate surgical staging (including lymph node biopsy of both sides) are performed. This is followed by 6 weeks of chemotherapy that is appropriate to the stage and histology of the tumor. Reassessment is then performed using imaging studies, followed by definitive surgery with one of the following:
-
Unilateral radical nephrectomy and partial nephrectomy on the contralateral side
-
Bilateral partial nephrectomy
-
Unilateral nephrectomy only, if the response was complete on the opposite side
This approach dramatically reduces the kidney failure rate following bilateral Wilms tumor therapy.The overall 2-year survival rate is higher than 80% with this approach, and the nephrectomy rate drops by 50% in patients with bilateral Wilms tumor. Bilateral partial nephrectomy is possible after chemotherapy or, if the tumor on one side responds completely to chemotherapy, with no subsequent need for nephrectomy.
Chemotherapy
Multimodal therapy (ie, surgery, radiation, chemotherapy) is the key to success when treating Wilms tumor. [12, 26] The NWTSG recommends preoperative chemotherapy (after initial exploratory laparotomy and biopsy) in the following situations [13, 14, 16] :
-
Intracaval tumor extension - This occurs in 5% of cases of Wilms tumor and is associated with a 40% rate of surgical complications, even in experienced hands; up-front chemotherapy after staging and biopsy reduces tumor and thrombus size, which account for 25% of surgical complications
-
Inoperable tumors - Large tumors that involve vital structures make resection difficult; the complication rate is high, and the incidence of tumor spill soilage is also high, although up-front chemotherapy reduces soilage by 50%
-
Bilateral Wilms tumor
SIOP advocates up-front chemotherapy without previous laparotomy and biopsy. The NWTSG suggests that this approach results in a 1-5% risk of treating a benign disease. [17]
Chemotherapy without proper surgical staging (eg, staging by means of imaging studies only) may alter the actual initial stage of the disease by the time of surgery and may subsequently alter decisions regarding the adjuvant chemotherapy and radiation therapy, which is based on the surgical staging.
Intraoperative Details
Through a transperitoneal approach, mobilize the ipsilateral colon and enter the Gerota (perinephric) fascia to examine the kidney. In cases of a unilateral tumor, perform a radical nephrectomy if the opposite side is normal. Evaluate the liver, lymph nodes, and peritoneum for metastases. To ensure accurate staging, the SIOP recommends a goal of sampling seven locoregional lymph nodes. [8] The renal vein and inferior vena cava should be palpated to assess intravascular extension (present in 6% of the cases).
In cases of bilateral disease, excisional biopsy of visible tumor is indicated, followed by re-resection with nephron preservation after chemotherapy. Identify the involved nodes with clips to facilitate postoperative radiation therapy.
Integrity of the surgical specimen is of paramount importance, as tumor spillage has a deleterious impact on prognosis (six-fold increase in local abdominal recurrence).
Postoperative Details
The NWTSG has published guidelines for postoperative chemotherapy and radiotherapy protocols, based on the surgical staging, as follows:
-
Stage I, with favorable or unfavorable histology: Vincristine and actinomycin D for 18 wk
-
Stage II with favorable histology: Vincristine and actinomycin D for 18 wk
-
Stage II with focal anaplasia: Abdominal radiation (1000 cGy); vincristine, actinomycin D, and doxorubicin for 24 wk
-
Stage III with favorable histology and focal anaplasia: Abdominal radiation (1000 cGy); vincristine, actinomycin D, and doxorubicin for 24 wk
-
Stage IV with favorable histology or focal anaplasia: Abdominal irradiation according to local stage; bilateral pulmonary irradiation (1200 cGy); trimethoprim/sulfamethoxazole prophylaxis for Pneumocystis jiroveci; c hemotherapy with vincristine, actinomycin D, and doxorubicin
-
Stage IV with diffuse anaplasia: Abdominal irradiation; whole-lung irradiation; chemotherapy for 24 months with vincristine, actinomycin D, doxorubicin, etoposide, and cyclophosphamide
A study by Pritchard-Jones et al on 583 children from 251 hospitals in 26 countries aimed to assess whether doxorubicin can be omitted safely from chemotherapy for stage II-III, histological intermediate-risk Wilms' tumor in order to avoid doxorubicin-related cardiotoxicity effects. The study concluded that doxorubicin does not need to be included in treatment of stage II-III intermediate risk Wilms' tumour when the histological response to preoperative chemotherapy is incorporated into the risk stratification. [27]
Radiation Therapy
The National Comprehensive Cancer Network (NCCN) guidelines recommend radiation therapy begin 10 days (preferred) but no later than 14 days post surgery. The guidelines note that there are studies that show an increased risk for recurrence in patients with later start of RT. [28]
Stage V
Wilms tumor is designated as stage V if cancer cells are found in both kidneys when the disease is first diagnosed. After bilateral kidney biopsies and staging of each kidney, treatment of stage V Wilms tumor varies by stage, as follows:
-
Stage I or II disease in both kidneys: Vincristine and dactinomycin, preoperatively and for 18 weeks postoperatively
-
Stage III or IV disease in both kidneys: Vincristine, dactinomycin, and doxorubicin, preoperatively and for 24 weeks postoperatively
-
Stage III or IV disease in both kidneys with favorable histology: Vincristine, doxorubicin, cyclophosphamide, and etoposide preoperatively and for 24 weeks postoperatively
Bilateral, high-stage tumors with unfavorable histology are associated with a poor prognosis in spite of multimodal therapy.
Follow-up
Follow-up care after treatment must be long (if possible, lifelong), because Wilms tumor may recur after several years. Follow-up consists of chest radiography and abdominal ultrasonography, CT scanning, or MRI every 3 months for the first 2 years, every 6 months for another 2 years, and once every 2 years thereafter.
Outcome and Prognosis
Tumor biomarkers, histology, and stage are the most important prognostic factors in cases of unilateral disease.
With the advent of multimodal therapy, patients with Wilms tumor have a good prognosis; the disease is considered an example of success in cancer therapy. The overall survival rate with Wilms tumor is 90%. Patients with cases that involve diffuse anaplasia and stage III or IV disease that recur despite complex therapy have a much poorer prognosis. However, the addition of newer chemotherapeutic agents, such as cyclophosphamide, ifosfamide, cisplatin, carboplatin, and etoposide, especially the ICE combination (ifosfamide, carboplatin, etoposide), have contributed to significantly increased postrelapse survival rates to 50%-60%. [29, 32, 33]
Complications may include the following:
-
Small-bowel obstruction (7%)
-
Hemorrhage (6%)
-
Wound infection, hernia (4%)
-
Vascular complications (2%)
-
Splenic and intestinal injury (1.5%)
-
Impaired kidney function
Bilateral Wilms tumor
Approximately 5% of children with Wilms tumor will have bilateral involvement. [34] In these patients, the goals of treatment are oncologic control plus preservation of renal parenchyma to ensure sufficient kidney function. [35] Kidney insufficiency may adversely affect both overall health outcomes as well as quality of life. [36] This impact may be especially pronounced in children with bilateral Wilms tumor, who are often younger than their counterparts with unilateral disease and may also be afflicted with syndromes associated with poor kidney function at baseline. [37, 38] Bilateral disease is an independent risk factor for the development of renal insufficiency, due in large part to the loss of renal parenchyma.
Overall, about 15% of children with bilateral involvement who were enrolled in National Wilms Tumor Studies 1–4 developed kidney insufficiency in the two decades following diagnosis; children with metachronous disease and those with underlying syndromes such as WAGR or Denys–Drash had 50% increased risk of kidney failure. In particular, risk was increased in children who had not undergone upfront chemotherapy or initial nephron-sparing surgery. [35]
Chemotherapy and nephron-sparing surgery (NSS) provide a safe and effective means of providing oncologic control. [39, 40] Current collaborative studies for patients with Wilms tumor aim at optimizing therapy while decreasing morbidity. Although NSS preserves renal parenchyma and thus may prevent kidney failure, questions remain over whether recurrence rates are higher with this approach. [41]
Future considerations
Using risk stratification based on the molecular profile of the tumor may allow treatment to be tailored for each patient individually. Clinical outcomes may be further improved with newer cytotoxic agents, such as the camptothecin analogue topotecan. A promising class of chemotherapeutic drugs is the antiangiogenesis agents, which target the vascular endothelial growth factor (VEGF) pathway.
Questions & Answers
Overview
What is the anatomy of Wilms tumor?
When is surgery indicated in the treatment of Wilms tumor?
What are the contraindications to surgery for the treatment of Wilms tumor?
What is the role of radical nephrectomy in the treatment of Wilms tumor?
What is the role of partial nephrectomy in the treatment of Wilms tumor?
What is the role of laparoscopic nephrectomy in the treatment of Wilms tumor?
What is the role of robotic-assisted laparoscopic surgery (RAS) in the treatment of Wilms tumor?
How are unresectable Wilms tumors treated?
How are bilateral Wilms tumors treated?
What is the role of chemotherapy in the treatment of Wilms tumor?
How is radical nephrectomy performed for the treatment of Wilms tumor?
What are the NWTSG guidelines on postoperative chemotherapy and radiotherapy for Wilms tumor?
What is a stage V Wilms tumor?
What is included in the long-term monitoring of Wilms tumor?
What is the prognosis of Wilms tumor?
What is the prognosis of bilateral Wilms tumor?
Which treatments are under investigation for Wilms tumor?
-
CT scan in a patient with a right-sided Wilms tumor with favorable histology.
-
CT scan of child with a stage IV Wilms tumor with favorable histology. Note the bilateral pulmonary metastases.
-
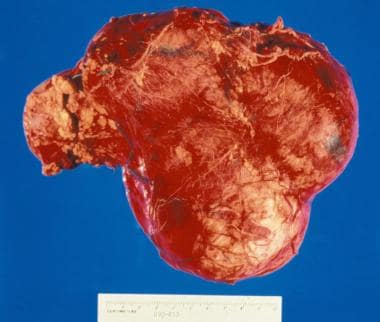
Gross nephrectomy specimen shows a Wilms tumor pushing the normal renal parenchyma to the side.