Background
Vasovasostomy (VV) and vasoepididymostomy (VE) are surgical procedures designed to bypass an obstruction in the male genital tract. These procedures are usually performed to restore fertility, although they are occasionally undertaken to relieve pain, such as in postvasectomy pain syndromes.
Vasovasostomy involves the anastomosis of segments of the vas deferens above and below an obstruction. The vast majority of vasovasostomies are performed to reverse a prior vasectomy, but the procedure is occasionally indicated for repair of an iatrogenic vasal injury secondary to prior surgery (eg, inguinal herniorrhaphy).
Vasoepididymostomy is a technically more demanding procedure than vasovasostomy. It involves anastomosis of the vas deferens to the epididymis in order to bypass an epididymal obstruction. This obstruction may be secondary to long-standing vasal obstruction resulting in damage to an epididymal tubule (epididymal blowout) or may result from epididymal infections or trauma.
History of the Procedure
Surgical procedures to remove genital tract obstructions and to restore fertility have been attempted for almost 100 years. In 1903, Martin et al first reported a technique for vasoepididymostomy to treat an obstructed epididymis due to gonococcal infection. He described anastomosing the vas to a cut end of the epididymis using fine silver wires. This fistula technique of anastomosis remained the standard for nearly 75 years, until advances in technique and instrumentation made direct, single tubule anastomosis feasible.
In 1978, Silber first reported a technique for directly anastomosing the mucosa of the vas deferens to a single epididymal tubule. [1] While this required more technical skill and magnification than the fistula technique, it allowed precise alignment of the vasal and epididymal lumens, resulting in markedly improved fertility rates.
Quinby reported the first successful vasovasostomy for vasectomy reversal in 1919. The anastomosis was performed over a strand of silkworm gut that was later removed. By 1948, O'Conor reported a practice survey revealing that 18% of urologists had performed a vasal anastomosis procedure at least once and that the operation was successful in up to 40% of patients. [2] Various techniques have been tried since O'Conor's survey, including the use of stents and adhesive materials to improve patency rates for the macroscopic anastomosis.
The next major advance occurred when Owen [3] and Silber [4] separately reported their techniques for microsurgical anastomosis in 1977. Silber reported patency rates of up to 94% using the 2-layer microsurgical technique for vasal anastomosis. This is the current gold standard for vasectomy reversal.
More recently, newer literature has emerged investigating the use of robotic-assisted vasovasostomy with promising results. The handful of studies available, notably from Parekattil et al and Kavoussi, have found similar rates of patency compared with microsurgical technique. [5, 6] A systematic literature review reported vasal patency rates of 88-100% for robot-assisted vasovasostomy and 55-61% for robot-assisted vasoepididymostomy. [7]
The potential benefits of the robotic technique vs the microsurgical approach appear to be decreased operative time, mildly increased patency rates, and decreased learning curve for the surgeon. Increased cost, as well as decreased magnification compared to the microsurgical technique are the main disadvantages. [8]
Problem
Azoospermia (the absence of sperm in the ejaculate) can result from an obstructed genital tract or a failure of spermatogenesis in the testicle. Vasovasostomies are indicated for an obstruction at the level of the vas deferens, while vasoepididymostomies are used to treat epididymal obstructions. The site of obstruction can often be discerned by examination of the fluid from the vasal end or from the epididymal tubule, as described below. The goal of both procedures is to restore genital tract patency and ultimately to allow conception. These procedures are not indicated for nonobstructive causes of azoospermia.
Epidemiology
Frequency
Vasectomy remains one of the most commonly performed operations in the United States and throughout the world. Despite careful preoperative counseling, between 3-6% of the men who undergo vasectomy ultimately desire a vasectomy reversal (VR). Restoring fertility and relief from postvasectomy pain syndrome (PVSP) have been cited as the top reasons for a reversal. [8]
Primary genital tract obstruction occurs in 7.4% of infertile males who have not undergone a prior vasectomy. While the cause may be multifactorial (eg, including epididymal trauma, infection, congenital hypoplasia of the ductal system), a significant number of these patients are candidates for vasoepididymostomy for restoration of the patency of the seminal tract.
Etiology
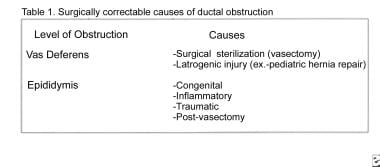
Causes of vasal and epididymal obstruction are outlined in Table 1 (see image below). Vasal obstruction is usually the result of an intentional division for sterilization, although it occasionally may be caused by iatrogenic injury during surgical procedures (eg, herniorrhaphies).
An epididymal obstruction can be congenital or can result from an epididymal infection, trauma, or prior vasectomy. Congenital epididymal obstruction may occur in conjunction with atresia of the vas deferens, rendering surgical reconstruction impossible. This is usually associated with a cystic fibrosis genetic mutation, and these men may have no other phenotypic manifestations of cystic fibrosis. Some of these patients have a normal vas deferens and dysjunction of the vas deferens with the epididymis, and they may benefit from vasoepididymostomy.
Inflammatory obstruction of the epididymis can result from bacterial epididymitis. Neisseria gonorrhoeae usually affects only the distal epididymis, allowing a surgical bypass of the vas to the more proximal epididymis using a vasoepididymostomy.
Trauma to the epididymis is a relatively uncommon cause of an epididymal obstruction but may result from epididymal injury during scrotal surgeries (eg, spermatocelectomy, hydrocelectomy, testis biopsy).
An epididymal obstruction following vasectomy is the most likely cause of an epididymal obstruction. The buildup of high intraluminal pressures within the epididymis after a vasectomy can result in rupture of the delicate epididymal tubule, resulting in obstruction (eg, epididymal blowout). This phenomenon is more common in men who desire a reversal more than 10 years after their vasectomy and in patients in whom vasovasostomy has previously failed.
Presentation
Patients who desire vasovasostomy for vasectomy reversal self-refer for evaluation. All other patients present for an evaluation of infertility after a trial of unprotected intercourse. A careful physical examination suggests the diagnosis of vasal or epididymal obstruction that is amenable to a vasovasostomy or vasoepididymostomy, respectively. Men with a genital tract obstruction have testes of normal size (>20 mL volume or 4 cm length) and consistency. The epididymis feels prominent proximal to a site of obstruction and feels flat (empty) distal to an obstructed tubule. Dilatation of the entire epididymis suggests an obstruction at either the junction of the epididymis with the vas deferens or within the vas deferens itself.
Indications
The indications for a vasovasostomy include vasectomy reversal and relief of postvasectomy pain syndrome. The latter indication is uncommon and remains of controversial efficacy. Prior to undertaking a vasovasostomy for vasectomy reversal, the female partner should be evaluated by a gynecologist to exclude concurrent female causes of infertility. A vasoepididymostomy is performed for the treatment of a genital tract obstruction at the level of the epididymis.
Both vasal and epididymal obstruction are suggested by azoospermia in the presence of a normal semen volume. Low-volume azoospermia (< 1.5 mL) is more suggestive of ejaculatory duct obstruction than vasal or epididymal obstruction. Patients must have active sperm production in the testes to be considered a candidate for a vasoepididymostomy. For this reason, a testis biopsy is usually performed at the time of or prior to planned reconstruction to document active spermatogenesis. The authors prefer to conduct a biopsy at the time of reconstruction, as this avoids the inevitable scarring that can further complicate reconstruction at a later date.
Relevant Anatomy
To understand the surgical bypass procedures needed to restore sperm flow, it is important to understand the basic anatomy and physiology of the seminal tract. Sperm is produced and then released into the seminiferous tubules. The sperm transits through the rete testis, efferent ductules, and into the epididymal tubule. See image below.
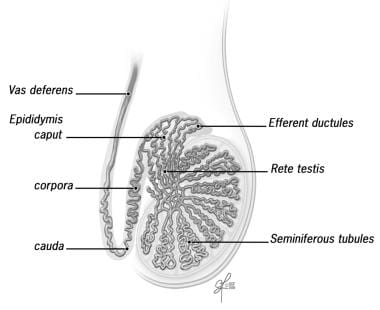 Sperm is produced in the seminiferous tubules and then transits through the rete testis, through the efferent duct, and into the epididymal tubule. Image reprinted with permission from Cleveland Clinic.
Sperm is produced in the seminiferous tubules and then transits through the rete testis, through the efferent duct, and into the epididymal tubule. Image reprinted with permission from Cleveland Clinic.
Epididymis
The epididymis consists of a single, highly convoluted tubule that is covered with tunica vaginalis. By convention, the epididymis is divided into the following anatomic segments: (1) the caput (head), (2) the corpora (body), and (3) the cauda (tail).
The proximal epididymis is involved in sperm maturation, whereas the distal region is the area of sperm storage. Vasoepididymal anastomosis to the more proximal epididymal tubule results in lower pregnancy rates because this bypasses a region of vital importance for sperm development.
Vas deferens
At the terminal end of the epididymis, a thick muscle wall that forms the proximal end of the vas deferens surrounds the tubule. The vas deferens follows the spermatic cord, courses through the inguinal canal, and enters the pelvis via the internal inguinal ring. From the pelvis, the vas travels behind the bladder and joins with the ipsilateral seminal vesicle to form an ejaculatory duct, which enters the prostate posteriorly. Contraction of the muscular wall of the vas deferens serves to propel sperm from the epididymis into the prostatic urethra via the ejaculatory ducts.
For more information about the relevant anatomy, see Male Reproductive Organ Anatomy and Ductus Deferens (Vas Deferens) and Ejaculatory Duct Anatomy.
-
Sperm is produced in the seminiferous tubules and then transits through the rete testis, through the efferent duct, and into the epididymal tubule. Image reprinted with permission from Cleveland Clinic.
-
Vasovasostomy modified one-layer technique: (A) A 9-0 nylon suture is passed through the entire vas wall, traveling full thickness through both ends. (B) Two 8-0 nylon seromuscular sutures are placed on either side of the 9-0 suture. (C) This pattern is repeated in each quadrant of the anastomosis, resulting in a total of 4 luminal sutures and 8 seromuscular sutures.
-
Vasovasostomy formal two-layer technique: (A) 9-0 nylon seromuscular sutures are placed in the posterior end of the vas at the 5- and 7-o'clock positions. (B) Six interrupted 10-0 nylon mucosal sutures are then placed to approximate the luminal ends of the deferens. (C) Finally, 4 additional 9-0 nylon seromuscular sutures complete the second layer of the anastomosis.
-
Vasoepididymostomy end-to-side technique: (A) Two 9-0 nylon sutures are used to secure the seromuscular layer of the vas to the epididymal tunic. (B) Four 10-0 nylon sutures are then placed to secure the mucosa of the vas to the epididymal tubule. (C) Finally, six to eight 9-0 nylon sutures are used to secure the seromuscular layer of the vas to the epididymal tunic.
-
Table 1. Surgically Correctable Causes of Ductal Obstruction
-
Table 2. Microsurgical Vasovasostomy
-
Table 3. Microsurgical Vasoepididymostomy
-
J stent insertion for the treatment of malignant obstruction. Video courtesy of Dennis G Lusaya, MD, and Edgar V Lerma, MD.
-
Vasectomy reversal. Video courtesy of Dennis G Lusaya, MD, and Edgar V Lerma, MD.