Urethral Anatomy
Female urethral anatomy
The urethra is approximately 4 cm long in the female. It is imbedded in the connective tissue supporting the anterior vagina. The urethra is composed of an inner epithelial lining, a spongy submucosa, a middle smooth muscle layer, and an outer fibroelastic connective-tissue layer.
The spongy submucosa contains rich vascular plexus that is responsible, in part, for providing adequate urethral occlusive pressure. Urethral smooth muscle and fibroelastic connective tissues circumferentially augment the occlusive pressure generated by the submucosa. Thus, all structural components of the urethra, including the striated sphincter muscle (discussed later), contribute to its ability to coapt and prevent urine leakage. See the image below.
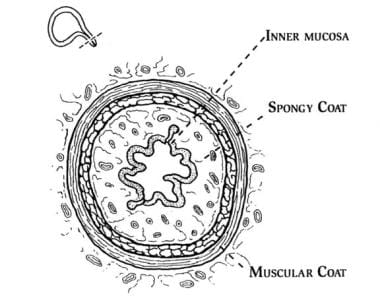 The female urethra is composed of 4 separate tissue layers that keep it closed. The inner mucosal lining keeps the urothelium moist and the urethra supple. The vascular spongy coat produces the mucus important in the mucosal seal mechanism. Compression from the middle muscular coat helps to maintain the resting urethral closure mechanism. The outer seromuscular layer augments the closure pressure provided by the muscular layer.
The female urethra is composed of 4 separate tissue layers that keep it closed. The inner mucosal lining keeps the urothelium moist and the urethra supple. The vascular spongy coat produces the mucus important in the mucosal seal mechanism. Compression from the middle muscular coat helps to maintain the resting urethral closure mechanism. The outer seromuscular layer augments the closure pressure provided by the muscular layer.
The urethral epithelium is composed of stratified squamous cells, which variably becomes transitional as the bladder is approached. The epithelium is arranged in longitudinal folds. At the base of the folds are scattered gland openings along the entire urethral length. The epithelium is supported by a loose lamina propria consisting of collagen fibrils and elastic fibers, arranged both circumferentially and longitudinally. A rich network of blood vessels is in the subepithelial layer.
The smooth muscle of the urethra is arranged longitudinally and obliquely with only a few circular fibers. The nerve supply is cholinergic and alpha-adrenergic. The longitudinal muscles may contribute to shortening and opening of the urethra during voiding. The oblique and circular fibers contribute to urethral closure at rest. Some research suggests smooth muscle contributions to urethral closure pressure may persist after urethral denervation, as inferred by the effects of systemic factors such as nitric oxide. [1]
The striated urethral musculature is complex. Its components and their orientation are not agreed upon universally. The voluntary urethral sphincter actually is a group of circular muscle fibers and muscular loops within the pelvic floor. The innermost layer, which is prominent in the proximal two thirds of the urethra, is the sphincter urethrae. More distally, the compressor urethrae and urethrovaginal sphincter are predominant. The voluntarily controlled urethral sphincter has been the target of injectable stem cell therapy in recent and ongoing trials. [2, 3]
These two muscles emanate from the anterolateral aspect of the distal half to distal third of the urethra and arch over its anterior or ventral surface. These striated muscles function as a unit. Because they are composed primarily of slow-twitch muscle fibers, these muscles serve ideally to maintain resting urethral closure. The muscles probably do maintain resting urethral closure, but they are known specifically to contribute to voluntary closure and reflex closure of the urethra during acute instances (eg, coughing, sneezing, laughing) of increased intra-abdominal pressure. The medial pubovisceral portion of the levator ani complex also is a major contributor to active bladder neck and urethral closure in similar situations.
Histologic examination of the striated urethral sphincter indicates the muscle complex largely surrounds the urethra in an incomplete fashion. Fibers have been observed to be deficient along the posterior aspect of the urethra. Thus, the shape of the muscle complex can be described as resembling a horseshoe or an omega symbol. Investigations using ultrasonographic imaging of the urethra also have confirmed a paucity of muscle bulk along the posterior urethra. [4]
The urethral meatus empties into the vaginal vestibule after the distal urethra pierces the perineal membrane. The mucosa of the meatus is continuous with that of the vulva. Support of the urethra and bladder neck is thought to be important in the maintenance of continence during sudden increases in intra-abdominal pressure. The support mechanism is complex and incompletely understood, but computer models based on 3-dimensional anatomical images are providing more insight into this. [5]
The posterior wall of the urethra is embedded in and supported by the endopelvic connective tissue. This sheet of connective tissue consists of collagen, elastin, and a small amount of smooth muscle. The connective tissue envelops the anterior vagina. This supportive tissue has been likened to a sling or a hammock around the urethra and bladder neck. Recent research has highlighted the importance of hormones and genetic factors as determinants of connective-tissue integrity in stress urinary incontinence and pelvic organ prolapse. [6]
The endopelvic connective tissue in this area is attached to the perineal membrane ventrally and laterally to the levator ani muscles by way of the arcus tendinous fascia pelvis. The arcus tendinous fascia pelvis is a condensation of connective tissue, which extends bilaterally from the inferior part of the pubic bone along the junction of the fascia of the obturator internus and levator ani muscle group to near the ischial spine. This tissue provides secondary support to the urethra, bladder neck, and bladder base.
Defects in this tissue are believed to result in cystocele development and urethral hypermobility. The primary support to this area and the entire pelvic floor is believed to be the levator ani muscle complex. At rest, the constant tone mediated by slow-twitch muscle fibers is thought to constitute the major supportive mechanism. Similar to the urethral sphincter muscle groups, the fast-twitch fibers of the levator ani complex aid in suddenly stopping the urinary stream during the voluntary guarding reflex. With acute increases in intra-abdominal pressure, forceful contraction of these fast-twitch levator fibers elevates the pelvic floor and tightens connective-tissue planes, thereby supporting the pelvic viscera. [7]
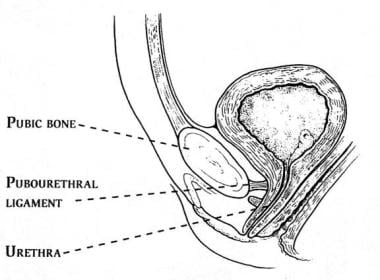
The anterior distal wall of the urethra is attached to the pubic bone by the pubourethral ligaments. These ligaments consist of extensions of the perineal membrane, as well as the caudal and ventral portions of the arcus tendinous fascia pelvis. The ligaments may limit movement of the anterior wall of the urethra during increases in intra-abdominal pressure but probably exert a lesser degree of support to the posterior wall. See the image below.
The previously described endopelvic connective tissue, when intact, provides support to the urethra as a whole. With increases in intra-abdominal pressure, some believe that the urethra is compressed shut against this firm support. Stress incontinence may be associated with a deficiency in the hammocklike support of the endopelvic connective tissue coupled with relative preservation of pubourethral ligament anterior urethral support. This may partially explain the commonly observed complex rotational descending motion of the bladder neck associated with stress incontinence.
As the pubourethral ligaments limit downward motion of the anterior urethral wall, they may provide a pivot point for rotational motion around the pubic bone. Furthermore, some theorize anterior wall support may also serve to pull the anterior and posterior urethral walls apart during straining, thereby directly contributing to bladder neck incompetency and stress incontinence.
Unlike male anatomy, in which the bladder neck and prostate comprise the internal urinary sphincter, the internal sphincter in females is functional rather than anatomic. The bladder neck and proximal urethra constitute the female internal sphincter. However, female external sphincter (ie, rhabdosphincter) has the most prominent effect on the female urethra. This occurs at the urogenital triangle, located approximately 1.8 cm distal to the bladder neck, and affects approximately 1.5 cm of urethral length. See the image below.
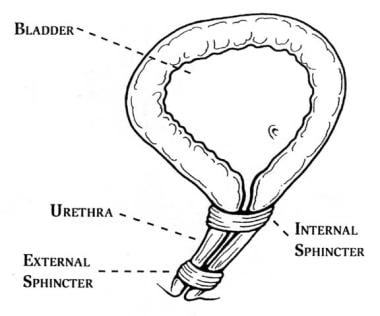 The female urethra contains an internal sphincter and an external sphincter. The internal sphincter is more of a functional concept than a distinct anatomic entity. The external sphincter is the muscle strengthened by Kegel exercises.
The female urethra contains an internal sphincter and an external sphincter. The internal sphincter is more of a functional concept than a distinct anatomic entity. The external sphincter is the muscle strengthened by Kegel exercises.
Male urethral anatomy
In men, the urethra averages 20-22 cm in length, and its anatomy is somewhat more complex than the female urethra. The male urethra is divided into anterior and posterior portions. The anterior urethra contains the penile (or pendulous) urethra and the bulbous urethra. The urethra is surrounded by the corpus spongiosum within the penis. The posterior urethra comprises the membranous urethra and the prostatic urethra.
Starting at the bladder neck, smooth muscle fibers extend from the bladder neck into the preprostatic urethra. These form the internal sphincter, which preserves continence and prevents retrograde flow of semen into the bladder during ejaculation. Puboprostatic ligaments are paired fibrous streaks that originate from the endopelvic fascia. They attach to the lower fifth of the pubic bone, lateral to the symphysis and the junction of the external urethral sphincter and prostate. They support the external sphincter and sustain the urethra in its position in the pelvic floor. [8]
Anatomic support and pelvic innervation are important factors in the etiology of postprostatectomy incontinence. The intactness of the urethral suspensory mechanism appears to have a relevant role in the preservation of urinary continence following radical prostatectomy. [9] A puboprostatic ligament-sparing approach allows the preservation of the maximal urethral length and the anterior urethral support remains intact. [8]
In the prostatic urethra, which is just distal to the bladder neck, the epithelium is transitional. The orifices of the prostatic glands can be found here. On the floor of the prostatic urethra is an elevation termed the verumontanum. This area contains a small pocket called the utricle. Distal to the utricle are the orifices of the two ejaculatory ducts.
Continuing distally, the membranous urethra is encountered. This short segment traverses the urogenital diaphragm, which contains the external urethral sphincter. Cowper glands are adjacent to the urethra. The epithelial cells become more elongated and appear as stratified columnar cells.
The next portion distally is the bulbous urethra. It derives its name from the bulb of the corpus spongiosum, which it traverses. The ducts of the Cowper glands empty in this location. The epithelium is composed of pseudostratified columnar cells.
Thereafter, the penile urethra passes through the corpus cavernosum urethrae and makes up more than half of the total anatomic urethral length. The epithelium is comprised of pseudostratified columnar cells, except in the fossa navicularis, the slightly widened distal part of the urethra that passes through the glans penis. In this distal-most segment, stratified squamous epithelium is present.
Throughout the penile urethra, the periurethral tissue contains many small mucus-secreting glands called the glands of Littre. These are more numerous along the roof of the urethra, and they empty into small recesses called the lacunae of Morgagni. Of note, the bulbous and penile urethra together sometimes are referred to as the cavernous urethra.
Bladder Anatomy
The bladder wall is made up of muscle fibers extending in all directions. This configuration is well suited to uniformly decreasing the bladder size in a grossly spherical manner when contracting.
At the bladder neck, the muscular bladder wall is more organized, and 3 relatively distinct layers become apparent. The inner longitudinal layer of the bladder fuses with the inner longitudinal layer of the urethra. The middle circular layer of the bladder is most prominent in the proximity of the bladder neck, and it fuses with the deep trigonal muscle. The outer longitudinal layer contributes a portion of anterior fibers to the pubovesical muscles, which terminate on the posterior surface of the pubic bone. Posteriorly, the outer longitudinal fibers interdigitate with deep trigonal fibers and the detrusor muscle.
The trigone is a triangular structure formed by the bladder neck or urethral opening and the orifices of the right ureter and left ureter. The superior border of the trigone is a raised area called the interureteric ridge. Deep to the mucosa at this point are two muscular layers. The superficial layer connects to the longitudinal urethral musculature, while the deep muscle layer fuses with the detrusor.
The deep layer in the trigone also fuses with the Waldeyer sheath, which is the fibromuscular covering of the intramural ureter. The ureter enters the bladder wall obliquely, and the muscle fibers are longitudinal in orientation at this point. Overall, the intermural segment of the ureter is roughly 1.5 cm in length.
The bladder mucosa is transitional epithelium. It is loosely connected to the muscular wall by way of a connective-tissue layer termed the lamina propria. At the trigone, the epithelium is more densely adherent to the underlying muscle. Current areas of research are investigating the role of the urothelium or bladder mucosa in various conditions, such as interstitial cystitis and overactive bladder, that present as voiding dysfunction. The bladder wall with its various layers is now coming to be known as an increasingly complex neuromuscular network under the influence of a number of local, regional, and systemic chemical and hormonal mediators.
Retropubic Space Anatomy
Ventrally, the retropubic space is bounded by the pubic bones and the midline fibrocartilage of the pubic symphysis. The floor and deep dorsal aspect of the retropubic space consist of the anterior or ventral surface of the bladder and proximal urethra. The endopelvic connective tissues, which lie between the bladder and muscular wall of the vagina, extend laterally to the pelvic side walls at the arcus tendineus fasciae pelvis and also contribute.
The remainder of the dorsal border consists of the pelvic parietal perineum and the transversalis fascia. As originally described by Retzius, this potential space extends in a cephalad direction to the level of the umbilicus. [10] The portions of the cephalad and dorsal borders are covered with peritoneum and can be accessed from within the peritoneal cavity. Above the arcuate line, the posterior rectus sheath is present.
Important structures in the region include the pectineal ligament, or Cooper ligament, which lies on the superoposterior or superior dorsal surface of the pubic ramus. This ligament was the basis of the Burch retropubic urethropexy procedure for stress incontinence. A flat triangular extension of the Cooper ligament, the lacunar ligament, widens as it travels medially and joins the inguinal ligament at the pubic tubercle. The pubovesical ligaments, pubourethral ligaments, and extrinsic muscles of the urethra also lie in the retropubic space. Otherwise, the periosteum of the dorsal pubis is relevant in that it was used as the anchor for the Marshall-Marchetti-Krantz retropubic urethropexy, also for stress incontinence.
Dorsally, the inferior aspect of the bladder lies on the anterior vagina, cervix, and lower uterine segment. Lateral to the bladder and bladder neck, within the endopelvic connective tissue abutting the reproductive organs, lies a venous plexus. These prominent veins are a frequent source of bleeding during retropubic urethropexy.
Neuroanatomy of the Lower Urinary Tract
Central nervous system
Intact neuroanatomy and neurophysiologic function are essential to both the storage and micturition phases of lower urinary tract function. These phases are controlled largely by the peripheral autonomic nervous system, with important reflex information contributed by sensory nerves from the bladder and urethra. Modulation is provided by higher central nervous system centers that facilitate the conscious control of lower urinary tract function. Lesions anywhere along these neuroanatomic pathways can contribute to or cause incontinence and voiding dysfunction.
Voluntary control of detrusor activity is thought to arise in the frontal cerebral cortex. This area is in communication with the pontine mesencephalic reticular formation, which serves as the brainstem micturition center. Maturation of these and higher centers are essential in childhood acquisition of the ability to voluntarily suppress micturition. Diseases that involve this area of the brain (eg, stroke, multiple sclerosis, Parkinson disease, brain tumors) may cause or contribute to incontinence.
Efferent connections begin in the pons and terminate in the sacral micturition center at the S2 to S4 level. These pathways help facilitate appropriate and efficient micturition, as damage to these tracts (ie, spinal cord injury) may result in involuntary detrusor contractions (suprasacral interruption) or possibly detrusor paralysis (sacral interruption) depending on the level of the lesion. Elements of sphincteric dyssynergia may also present, placing the upper urinary tract at risk of injury if elevated intravesical pressures develop. Alternatively, an incompetent fixed, open sphincter may develop, leading to continual incontinence.
A neural loop involving the bladder, sacral micturition center, and urethral sphincter mechanism via the pudendal nerve has been described. This pathway enables coordination of urethral sphincter relaxation and detrusor contraction. Simultaneous urethral relaxation and detrusor contraction depend on this neural pathway being intact. Dysfunction in this loop may result in a number of scenarios, commonly presenting as continual incontinence with detrusor paralysis and an incompetent fixed, open sphincter. However, the development of sphincteric dyssynergia and involuntary detrusor contractions also may occur.
Nervous system divisions
Healthy functioning of the lower urinary tract is partly dependent upon the interplay of sympathetic (ie, adrenergic) and parasympathetic (i.e. cholinergic) input to the urethra, urethral sphincter, and bladder. Bladder filling normally takes place with little to no increase in intravesical pressure. This phenomenon is largely due to the predominance of sympathetic activity during the filling and storage phase.
Stimulation of beta-adrenergic receptors in the detrusor muscle promotes bladder relaxation, as evidenced by the effect of the drug Mirabegron. Stimulation of alpha-adrenergic receptors in the pelvic ganglia supplying the bladder inhibits the parasympathetic activity that would otherwise produce bladder contractions. Furthermore, alpha-adrenergic receptors predominate in the smooth muscle of the bladder neck and urethra and subsequently cause these structures to contract with stimulation, further promoting urine storage.
Micturition is largely due to parasympathetic activity or cholinergic stimulation of the bladder, urethral sphincter, and urethra. Muscarinic receptors in detrusor muscle promote bladder contraction when stimulated. Stimulation of muscarinic receptors on the alpha-adrenergic nerves to the bladder neck and urethra prevent activation of smooth muscle there and thus cause urethral and bladder neck relaxation. However, despite cholinergic agents generally being thought of as promoters of micturition, they can also stimulate nicotinic receptors on preganglionic fibers, providing sympathetic signaling to the urethra and bladder neck, resulting in contraction of these structures.
Nonadrenergic noncholinergic nerves stimulated via adenosine triphosphate purinergic receptors have been discovered and may be very important in bladder contractility. Other research suggests prostaglandins may also activate these receptors. Estrogen receptors have also been noted in the pelvic floor, bladder, bladder neck, and urethral musculature. While estrogen stimulation increases the density of alpha-adrenergic receptors in the urethral smooth muscle, progesterone enhances beta-adrenergic activity. At present, the complex neuromuscular network underlying urothelium and detrusor signaling is an active area of active scientific work.
Bladder and urethral sensory innervation
Afferent sensory innervation of the bladder originates with stretch and pain receptors in the bladder. Stretch receptors, which are responsible for bladder proprioception and the sensation of fullness, are the origin of impulses traveling via the pelvic nerves. These signals traverse the posterior columns ipsilaterally and eventually stimulate the brain stem micturition center. Connections from the brain stem to the cerebral cortex then provide for conscious awareness of bladder distention so the decision to void can be made.
Pain receptors are also present in the bladder but not as densely as stretch receptors. These receptors are responsible for sensing temperature, tactile stimulus, and chemical irritants. The generated impulses travel by way of the hypogastric nerves and synapses in the posterior root ganglia. These impulses then cross to the contralateral side before ascending in the spinothalamic tract and the thalamic nuclei to eventually reach the cerebral cortex and convey a feeling of discomfort.
Afferent impulses from pain receptors can trigger detrusor contractions via a normally suppressed reflex pathway. Conditions of severe mucosal irritation (eg, urinary tract infection, intravesical foreign body, chemical irritant) can unmask this reflex. In addition, disorders resulting in the loss of conscious cerebral cortical input may be responsible for the emergence of this reflex.
Somatic innervation
The striated muscle of the urethral sphincter complex receives innervation from the second through fourth sacral segments via the pudendal nerve. The precise source of these fibers is controversial, but they are somatic and largely under voluntary control, yet they also can be engaged by an involuntary guarding reflex during intra-abdominal pressure increases (eg, sneezing, coughing, laughing). Similarly, the levator ani complex is largely under voluntary control via the nerve to the levator ani but also has involuntary activity and receives innervation from variable sources, as noted in anatomical studies. [11]
The role of the pudendal nerve in stimulating the striated muscles of the external urethral sphincter mechanisms is re-developing as an area of therapeutic interest. As regenerative medicine has focused on using stem cells, their secretome, or other means to increase muscle bulk in the striated sphincters, maintaining their functional capacity via intact innervation remains an important consideration. Methods for regenerating or preventing the loss of pudendal nerve function is an active area of research. [12]
Pelvic Diaphragm Anatomy
Levator ani
The pelvic diaphragm lines the floor of the bony pelvis and is composed of four sheets of muscles: the pubococcygeus, iliococcygeus, ischiococcygeus, and coccygeus. See the images below.
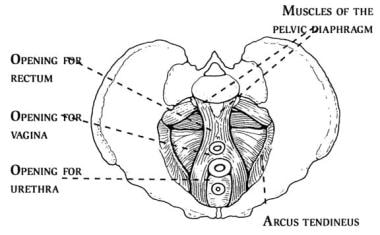 The pelvic diaphragm (ie, levator ani musculature) is composed of pubococcygeus, iliococcygeus, ischiococcygeus, and coccygeus muscles. It contains 3 openings through which the rectum, urethra, and cervix pass.
The pelvic diaphragm (ie, levator ani musculature) is composed of pubococcygeus, iliococcygeus, ischiococcygeus, and coccygeus muscles. It contains 3 openings through which the rectum, urethra, and cervix pass.
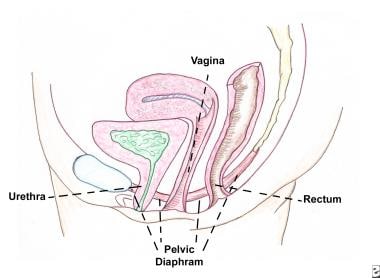 This is the side view of the pelvic diaphragm. The pelvic diaphragm supports the pelvic organs (eg, bladder, uterus, rectum).
This is the side view of the pelvic diaphragm. The pelvic diaphragm supports the pelvic organs (eg, bladder, uterus, rectum).
The pelvic diaphragm is often referred to as the levator ani complex. The levator ani musculature is attached to the inner sides of the bony pelvis by a condensation of pelvic fascia termed the arcus tendineus.
The levator ani is the most important component of the pelvic diaphragm because the integrity of the pelvic floor depends on its function. When the levator ani is damaged, stress urinary incontinence and herniation of the pelvic organs through its urogenital opening may develop. [13]
Literature reviews report the prevalence of levator ani defects range from 10% to 36% in women but only 5% to 13% of women complain of pelvic prolapse symptoms. Defects include partial or complete avulsion of the levator ani from its pubic attachments (right, left, or bilateral) and appreciable thinning in the pubovisceral region. [14]
Assessed by MRI, female high impact frequent intense training athletes had about a 20% greater cross-sectional area and width of the levator ani muscles, compared to age-matched nonathletic women. Research is needed to understand the role physical activity, a potentially modifiable risk factor, plays in women with risk factors for levator ani muscle injury. [15]
Supporting ligaments and fascia
The urethropelvic ligament is a fibrous band of connective tissue that lines the undersurface of the bladder neck and attaches laterally to the arcus tendineus. The urethropelvic ligament provides the major support to the bladder neck and proximal urethra. Laxity of the urethropelvic ligament results in stress urinary incontinence.
The pubocervical fascia is a fibrous sheet of connective tissue that lines the base of the urinary bladder and inserts laterally into the arcus tendineus. An intact pubocervical fascia prevents herniation of the bladder and the proximal urethra through the urogenital opening of the levator ani complex. Damage to the pubocervical fascia may cause the bladder to herniate through this opening, descending into the vagina, and resulting in cystocele formation and stress urinary incontinence. [13]
The term “paravaginal defect” describes the detachment of the pubocervical fascia from the arcus tendineus. This defect is associated with descent of the lateral part of the anterior wall, resulting in a cystourethrocele. [16]
The cardinal ligaments arise from the arcus tendineus and anchor to the uterine cervix. The cardinal ligaments stabilize and support the uterus, vagina, and bladder. Weakening of the cardinal ligaments may cause herniation of the pelvic organs through the urogenital opening of the levator ani, resulting in cystocele formation and uterine descensus or prolapse. See the image below.
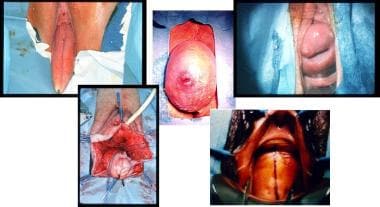 This photo illustrates a variety of pelvic organ prolapses, including grade-IV cystocele, uterine descensus, enterocele, and rectocele alone or in combination. In situations where a significant prolapse (eg, uterus, bladder) has occurred, evaluate for possible ureteral obstruction at the level of the pelvic inlet.
This photo illustrates a variety of pelvic organ prolapses, including grade-IV cystocele, uterine descensus, enterocele, and rectocele alone or in combination. In situations where a significant prolapse (eg, uterus, bladder) has occurred, evaluate for possible ureteral obstruction at the level of the pelvic inlet.
The uterosacral ligaments originate from a condensation of the fibrous connective tissue overlying the sacral promontory and insert into the uterine cervix. The uterosacral ligaments stabilize the uterus in the bony pelvis. They can be utilized for transvaginal surgery to address pelvic organ prolapse. However, weakening of the uterosacral ligaments may cause uterine prolapse or vaginal vault prolapse. [17]
-
The female urethra is composed of 4 separate tissue layers that keep it closed. The inner mucosal lining keeps the urothelium moist and the urethra supple. The vascular spongy coat produces the mucus important in the mucosal seal mechanism. Compression from the middle muscular coat helps to maintain the resting urethral closure mechanism. The outer seromuscular layer augments the closure pressure provided by the muscular layer.
-
The female urethra contains an internal sphincter and an external sphincter. The internal sphincter is more of a functional concept than a distinct anatomic entity. The external sphincter is the muscle strengthened by Kegel exercises.
-
The pubourethral ligaments suspend the female urethra under the pubic arch.
-
The pelvic diaphragm (ie, levator ani musculature) is composed of pubococcygeus, iliococcygeus, ischiococcygeus, and coccygeus muscles. It contains 3 openings through which the rectum, urethra, and cervix pass.
-
This is the side view of the pelvic diaphragm. The pelvic diaphragm supports the pelvic organs (eg, bladder, uterus, rectum).
-
This photo illustrates a variety of pelvic organ prolapses, including grade-IV cystocele, uterine descensus, enterocele, and rectocele alone or in combination. In situations where a significant prolapse (eg, uterus, bladder) has occurred, evaluate for possible ureteral obstruction at the level of the pelvic inlet.