Overview
Background
For most of the 20th century, from 1909 until the late 1990s, the premier treatment for symptomatic benign prostatic hypertrophy (BPH) was transurethral resection of the prostate (TURP). TURP was the first successful, minimally invasive surgical procedure of the modern era. To this day, it remains the criterion standard therapy for obstructive prostatic hypertrophy and is both the surgical treatment of choice and the standard of care when other methods fail.
Since the advent of medical therapy for symptomatic prostatic hypertrophy with 5-alpha reductase inhibitors and alpha-adrenergic blockers, the need for immediate surgical intervention in symptomatic prostatic obstruction has been reduced substantially. However, alpha-blockers do not modify prostate growth, and even the use of prostatic growth inhibitors such as finasteride or dutasteride often fails to prevent recurrent urinary symptoms of BPH and retention. In the past, these patients would almost certainly have undergone TURP years earlier.
The criteria for performing TURP surgery are now more stringent than before. In general, TURP surgery is reserved for patients with symptomatic BPH who have acute, recurrent, or chronic urinary retention; in whom medical management and less-invasive prostatic surgical procedures failed; who have prostates of an unusual size or shape (eg, a markedly enlarged median lobe, significant intravesical prostatic encroachment); who have azotemia or renal insufficiency due to prostatic obstruction; or who have the most severe symptoms of prostatism.
Less common uses of TURP include intractable prostatitis or for tissue sampling when standard biopsy techniques cannot be used.
The relative frequency of TURP compared to open prostatectomy in surgical patients varies from country to country. In 1990, the relative frequency rate of TURPs in surgical patients with BPH in the United States was 97%, with similar rates in Denmark and Sweden. The lowest rates of TURP were noted in Japan (70%) and France (69%).
The average age of patients currently undergoing TURP is approximately 69 years, and the average amount of prostate tissue resected is 22 g. Risk factors associated with increased morbidity include prostate glands larger than 45 g, operative time longer than 90 minutes, and acute urinary retention as the presenting symptom. The 5-year risk rate for a reoperation following TURP is approximately 5%. Overall mortality rates following TURP by a skilled surgeon are virtually 0%.
African Americans more typically present for TURP surgery with urinary retention or urinary infections and have a higher incidence of preexisting medical problems compared to the general population. According to Kang et al (2004), reports from the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial indicate that Asian and Asian American men have the lowest overall risk of clinical BPH and eventual TURP. [1]
Go to Prostate Cancer, Prostate-Specific Antigen, and Benign Prostatic Hypertrophy for complete information on these topics.
Indications
According to the Agency for Health Care Policy and Research guidelines for the diagnosis and treatment of BPH and the recommendations of the Second International Consultation on Benign Prostatic Hypertrophy, the absolute indications for primary surgical management of BPH are as follows: [2]
-
Refractory urinary retention
-
Recurrent urinary tract infections due to prostatic hypertrophy
-
Recurrent gross hematuria
-
Renal insufficiency secondary to bladder outlet obstruction
-
Bladder calculi
-
Permanently damaged or weakened bladders
-
Large bladder diverticula that do not empty well secondary to an enlarged prostate
Most men who present for surgical correction of their urinary outlet obstruction are those in whom medical therapy or alternative procedures have failed or are inappropriate for some reason. In general, patients with moderate-to-severe lower urinary tract obstructive symptoms (American Urological Association [AUA] symptom index >8) who have not responded to alpha-adrenergic blockers and/or 5-alpha reductase inhibitors are also candidates for surgical intervention.
A study by Blanchard et al showed that patients in whom alpha-blocker therapy is ineffective or those in whom it has failed tend to have poorer outcomes after TURP than men who proceed directly to a transurethral resection. [3] This is presumably from preoperative bladder damage and other risk factors that affect voiding rather than the size of the prostate. Operating time and weight of resected tissue have been documented as the same between the 2 groups; therefore, prostatic size alone does not account for the difference in outcomes.
Although persistent, progressive, or bothersome symptoms of urinary obstruction due to prostatic hypertrophy that are refractory to medical therapy constitute the most common indication for TURP, 70% of men undergoing the procedure have multiple indications. Patients with prostates larger than 45 g, who present with acute urinary retention, or who require operating times in excess of 90 minutes, are at increased risk for postoperative complications.
Surgical treatment of BPH is also indicated in cases of renal failure or insufficiency secondary to prostatic obstruction. Catheter drainage is usually recommended in such cases until the renal failure resolves. As many as 10% of men with BPH present with some degree of renal insufficiency.
Contraindications
The only absolute indication for an open prostatectomy over a TURP is the need for an additional open procedure on the bladder that must be performed at the same time as the prostatectomy. Such indications include open surgical resection of a large bladder diverticulum or removal of a bladder stone that cannot be easily fragmented by intracorporeal lithotripsy.
A relative indication for the selection of an open prostate surgery over a TURP is generally based on prostatic volume and the ability of the surgeon to complete the TURP in less than 90 minutes of actual operating time (although < 60 min is considered optimal).
In general, open prostatectomy can be justified in a patient with a prostate of 45 g or larger, but this is totally dependent on the skill and experience of the endoscopic urological surgeon. Most experienced urologists use a prostatic volume of 60-100 g as the upper limit amenable to endoscopic removal, but some highly skilled resectionists are capable of safely treating a 200-g prostate with TURP in less than 90 minutes.
Declining Frequency of TURP
The new availability of reasonable alternative medical and surgical treatment options means that TURP, once one of the most commonly practiced urological procedures, is now performed much less frequently. In 1962, TURP operations accounted for more than 50% of all major surgical procedures performed by urologists in the United States. By 1986, this had declined to 38%.
The 1985 Veterans Administration Normative Aging Study estimated the lifetime probability of surgical intervention for prostatic enlargement at 29%, and the 1986 National Health Survey estimated that 350,000 patients in the Medicare age group had a TURP that year, compared to fewer than 200,000 in that same age group by 1998.
These numbers should be considered within the context that the median age of the typical patient is rising (the number of older men with BPH-related symptoms in the United States is expected to increase from 5 million to 9 million persons by 2025), the size of the average resected prostate gland is increasing, and the typical patient has more comorbidities and is generally less healthy than surgical patients of the past.
A comprehensive review of transurethral prostatectomy in the Medicare beneficiaries by Wasson et al compared a national sample from 1991-1997 to a similar group for the period 1984-1990 found that the more recent group demonstrated a substantial decline in the number of TURP surgeries: 50% for white men and 40% for black men. [4] Compared to the peak period of TURP use in the 1980s, a higher proportion of the men undergoing the procedure were older in the more recent period, with 53% aged 75 years or older.
Another factor that must be considered when evaluating the general decline in the number of TURP procedures performed is the significant reduction in financial reimbursement to urologists for TURP surgeries in the United States.
Physician reimbursement from Medicare for a TURP has dropped from a high of $2000-$3000 in the past to approximately $650 today, with a 90-day global period that covers all postoperative care by the surgeon for 3 months. In many instances, performing a TURP is simply not profitable for the urologist when office overhead, billing and malpractice costs are considered, especially when complications occur.
Alternative surgical procedures, such as microwave therapy and prostatic laser surgery, are reimbursed at much higher levels, even though they may not be as durable or effective. This creates a strong financial disincentive for urologists to perform TURP procedures, except when no reasonable alternatives exist. A 2002 article by Donnell examined the history of Medicare policies and the effect of changes in Medicare reimbursement on TURP. [5]
In a large Canadian series reported by Borth et al, the number of TURP procedures dropped by 60% between 1988 and 1998, presumably because of medical therapy, despite an increase of 16% in the male population older than 50 years. [6] While the number of patients presenting with urinary retention was significantly higher in the 1998 group than in the 1988 cohort (55% vs 23%), no significant difference was noted in their average age, medical comorbidities, operative parameters, average size of prostate tissue resected, or complication rates.
Anatomic Considerations
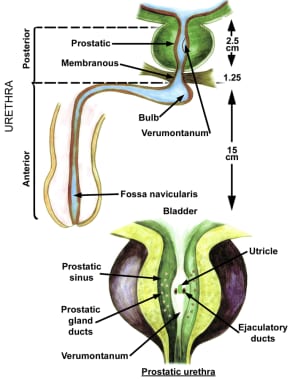
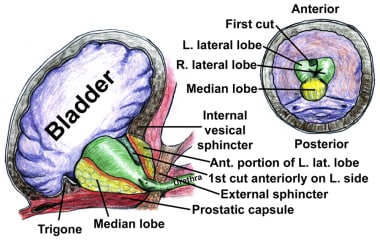
The prostate is divided into 3 zones: peripheral, central, and transition. The peripheral zone is the largest of the zones, encompassing approximately 75% of the total prostate glandular tissue in men without BPH. Most prostate cancers originate in the peripheral zone. It is located posteriorly and extends laterally on either side of the urethra.
The central zone is smaller and extends primarily around the ejaculatory ducts. It differs from the peripheral zone primarily in cytologic details and architecture.
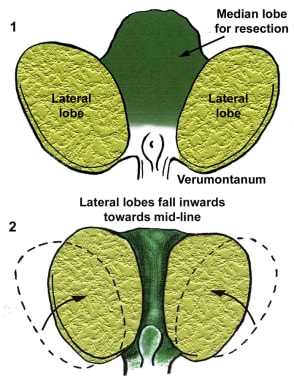
The transition zone is usually the smallest of the 3: it occupies only 5% of the prostate volume in men younger than 30 years. This is the zone thought to be the origin of BPH. The transition zone consists of two separate lobes on either side of the urethra and usually involves a small grouping of ductal tissue near the central portion of the prostatic urethra near the internal sphincter.
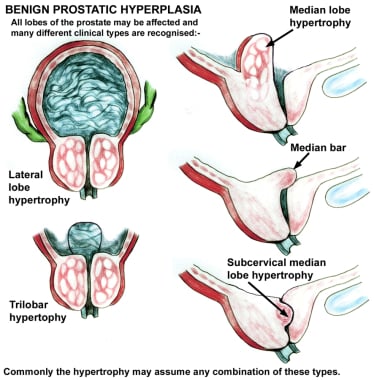
As the transition zone expands, it can comprise up to 95% of the prostate volume, compressing the other zones. Intraoperatively, the 2 enlarged lobes of the transition zone can be seen obstructing the prostatic urethra on either side. Thus, the term lateral lobes is often used intraoperatively for this tissue to distinguish it from any hyperplastic periurethral gland tissue.
The periurethral glands are less commonly involved with BPH, but when they do become enlarged, they can form what is termed a median lobe, which appears as a teardrop-shaped midline structure at the posterior bladder neck. This can ball-valve into the urethra, creating severe obstructive voiding symptoms. Any significant intravesical extension of prostatic tissue can act as a valve when the detrusor pressure increases and presses this tissue against the bladder neck or across the outlet to the urethra, creating a functional obstruction (see the image below).
In some earlier jargon, the transition zone and periurethral region were called the central gland or inner gland, and the peripheral and central zones were called the outer gland. This terminology should be avoided both because it is vague and because it creates confusion with the now-standard anatomical label of the central zone.
Prostatic calculi are formed from calcification of the corpora amylacea and precipitation of prostatic secretions. They occur between the transition zone and the compressed peripheral zone; in fact, they can be used as a marker for this border. Prostatic calculi are generally composed of calcium phosphate and are not considered clinically significant. Chemical analysis is unnecessary.
Although prostatic calculi may arise spontaneously, they also may be formed in response to an inflammatory reaction or as a consequence of another pathological process that produces acinar obstruction. Some practitioners believe that calcifications that form in response to bacterial prostatitis may harbor bacteria that periodically flourish, causing recurrent prostatitis. Proponents of this theory advocate TURP to liberalize these calcifications as a treatment for recurrent prostatitis.
If a channel is opened during surgery that allows these calculi to be expressed, they often flow out by themselves if the opening is large enough. They can be milked out by using the end of the cutting loop without current to gently press around the opening where the prostatic stones are seen and can be pushed into the opened prostatic fossa. They can be rinsed into the bladder and evacuated with the rest of the resected prostatic chips.
The prostate is thinnest and most narrow anteriorly (the 12-o’clock position when viewed through a cystoscope). Care should be taken when operating in this area to avoid perforating the prostatic capsule, especially if this portion of the prostate is resected early in the operation. Abundant venous blood vessels are located in the area just anterior to the prostatic capsule, which can cause significant bleeding that cannot be easily controlled if the vessels are damaged during resection.
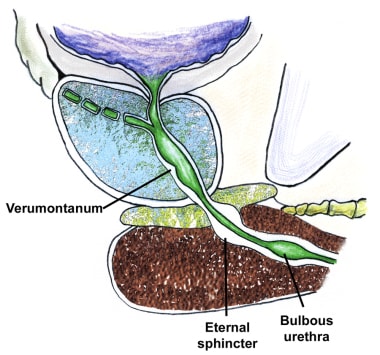
The external sphincter muscle tends to be slightly tilted, with the most proximal portion located anteriorly, opposite the verumontanum. The external sphincter can be identified cystoscopically by its wrinkling and constricting action as the resectoscope is withdrawn. Upon reinsertion, the superficial mucosa in front of the telescope tends to bunch up. This is because the external sphincter muscle is imbedded within the urogenital diaphragm, which is relatively fixed in position, while the prostate has some limited mobility.
The verumontanum is the single most important anatomical landmark in TURP (see the image below). It is a midline structure located on the floor of the distal prostatic urethra just proximal to the external sphincter muscle. It appears as a small, rounded hump that is best seen when withdrawing the telescope through the prostate while visualizing the prostatic floor at the 6-o’clock position.
The orifices to the ejaculatory ducts emerge in the verumontanum (see the first image below). Its importance lies in its position immediately proximal to the external sphincter muscle (see the second image below), which allows it to be used as the distal landmark for prostate resection. The precise distance between the verumontanum and the external sphincter demonstrates some slight individual variation and should be verified visually before starting the resection and periodically during the surgery.
The proximity of the ureteral orifices to the cephalad margin of the hypertrophied prostate varies, particularly in patients with an enlarged median lobe. This distance should be frequently assessed throughout surgery.
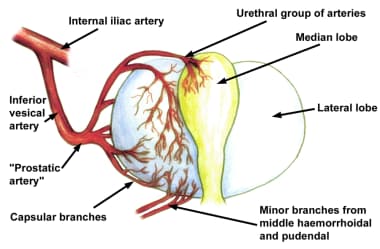
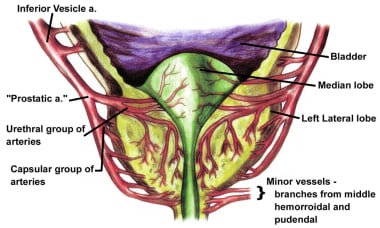
The vascular anatomy of the prostate was accurately described in detail by Rubin Flocks in 1937. [7] The blood supply of the prostate comes primarily from branches of the inferior vesical artery, which is a branch of the internal iliac artery (see the image below).
When the inferior vesical artery reaches the prostate just at the vesicoprostatic border, it branches into 2 groups of arteries (see the image below). One penetrating group passes directly into the prostate toward the interior of the bladder neck. Upon reaching the prostatic interior near the urethra, most of these branches turn distally and parallel the prostatic urethra, while others supply the median lobe.
Vessels that parallel the prostatic urethra supply most of the blood to the hypertrophied lateral lobes. The second large group of arteries follows the exterior of the prostatic capsule posterolaterally, periodically giving rise to perforating vessels, and supplies the area around the verumontanum.
See Prostate Anatomy and Male Urethra Anatomy for more information.
Development of Benign Prostatic Hyperplasia
Mechanisms of disease
The prostate has been described as the organ of the body most likely to be involved with disease of some sort in men older than 60 years. This statement characterizes any histological evidence of BPH as a disease, which is certainly debatable, but there is no argument that BPH is an extremely common clinical entity.
As the hyperplastic process increases the volume of the prostate, the urethral lumen is compressed, causing outlet obstruction. An enlarged median lobe may cause relatively more severe symptoms than lateral lobe hyperplasia of similar magnitude because it can act as a valve at which increased bladder pressure may actually cause further obstruction. Intravesical extension of the lateral lobes may act in a similar fashion.
It has been known for many years, however, that prostate size alone is not a reliable or accurate predictor of the presence or degree of urinary outlet obstruction. The failure of several purely obstructive therapies, such as prostatic balloon dilatation, and the obvious success of alpha-adrenergic blockers eventually led to the description of BPH as having both a dynamic (neurogenic) and a mechanical (obstructive) component.
Thus, at the same time as the occurrence of mechanical obstruction, a dynamic component involving the stromal prostatic tissue and bladder is present, which is often more significant in causing urinary symptoms than simple mechanical obstruction from an enlarged prostate. The precise interaction of these two mechanisms, mechanical and dynamic, is not well understood.
When a bladder is trying to empty through a blocked outlet from an obstructing prostate gland, the intravesical pressure required to open the bladder neck is increased. The bladder is initially able to produce a higher transitory voiding pressure when required, but loses muscle tone over time.
Isolated muscle bundles hypertrophy in response to the need for a higher intravesical pressure to overcome the increased resistance to voiding, and bladder trabeculation often follows. The spaces between these hypertrophied bundles tend to become thinner, with less functional muscle. Eventually, this can progress to the point at which the bladder becomes almost nonfunctional.
Bladder trabeculation is usually graded on a scale of I-IV. When seen on cystoscopy images, it is a relative indicator of the degree and duration of any bladder outlet obstruction (eg, BPH), although any detrusor hyperactivity problem can possibly produce bladder trabeculations, even without an identifiable obstruction. Initial symptomatic changes include increased bladder instability and irritability, which can eventually progress to muscular decompensation with permanent loss of detrusor contractile ability.
Evidence indicates that obstruction causes partial denervation of bladder smooth muscle, which results in further bladder irritability and involuntary detrusor contractions. Fortunately, most of these hyperactive symptoms resolve over time with removal of the prostatic obstruction or with a response to appropriate medications. The detrusor becomes less able to maintain a constant voiding pressure over time, which leads to early termination of voiding, intermittency of the urinary stream, and higher residual urine volume, accompanied by loss of bladder compliance.
Overall bladder mass increases because of detrusor muscle hypertrophy, but collagen deposition is also increased, which eventually contributes to decompensation, urinary retention, and permanent loss of detrusor contractile ability.
Proposed causes
BPH is thought to be caused by aging and by long-term testosterone and dihydrotestosterone (DHT) production, although their precise roles are not completely clear.
Histopathologic evidence of BPH is present in approximately 8% of men in their fourth decade and in 90% of men by their ninth decade. Loss of testosterone early in life prevents the development of BPH. The similarities in presentation, pathological examination findings, and symptoms of BPH among identical twins suggest a hereditary influence.
Once BPH has developed, it tends to progress. Cross-sectional studies based on cadaver autopsies or consecutive patients seen in urology clinics suggest that the growth rate decreases with age. In patients aged 31-50 years, the prostate doubling time averages 4.5 years. In men aged 51-70 years, the prostatic doubling time is approximately 10 years, while in men older than 70 years, the doubling time increases to more than 100 years. Note that these findings may only reflect a selection bias in the sample group.
A 5-year longitudinal study by Rhodes and colleagues of 631 community men aged 40-79 years from Olmsted County, Minnesota demonstrated an average annual prostate growth rate of 1.6%. This remained essentially constant regardless of age, although men with larger prostates tended to have higher growth rates.
The average prostate weighs approximately 20 g by the third decade and remains relatively constant in size and weight unless BPH develops. The typical patient with BPH has a prostate that averages 33 g. Only 4% of the male population ever develops prostates of 100 g or larger. (The largest recorded prostatectomy specimen weighed 820 g. This prostate was removed by open suprapubic prostatectomy. Unfortunately, the patient ultimately died of hemorrhage.)
Symptoms of BPH tend to progress slowly over time in most individuals, with an average annual increase of 0.14-0.44 points per year in the AUA symptom index for men aged 60 years and older. Once BPH has begun, the prostate grows an average of 0.6 mL in volume annually, with a mean decrease in average urinary peak flow rate of 0.2 mL per second each year. Men older than 70 years and those with a baseline peak flow rate less than 10 mL/s tend to have a more rapid and dramatic decline in their peak flow rates over time.
DHT has an affinity for prostate cell androgen receptors that is 5 times greater than that of testosterone. The levels of 5-alpha reductase are increased in the stromal tissue of men with BPH compared to controls. This and other data indicate that DHT is much more important in the development of prostatic hypertrophy than testosterone is.
The success of 5-alpha reductase blockers, such as finasteride and dutasteride, in reducing prostatic size and relieving symptoms seems to confirm this, although it does not explain the relative lack of symptom relief in those with smaller prostate glands treated with these agents.
Clinical manifestations and medical treatment
Classic symptoms of BPH include a slow, intermittent, or weak urinary stream; the sensation of incomplete bladder emptying; double voiding (the need to void within a few seconds or minutes of urinating); postvoid dribbling; urinary frequency; and nocturia. Patients may also present with acute or chronic urinary retention, urinary tract infections, gross hematuria, renal insufficiency, bladder pain, a palpable abdominal mass, or overflow incontinence.
Upon physical examination, the bladder may be palpable during the abdominal examination and the prostate may be enlarged during the digital rectal examination. Symptoms are not necessarily proportional to the size of the prostate on digital rectal examination or transrectal ultrasound findings.
Alpha-adrenergic receptors are present and functional in the stromal smooth muscle of the prostate and especially at the bladder neck. Many studies have documented the success of various alpha-adrenergic blockers in relieving symptoms of BPH. Evidence from the Medical Therapy of Prostate Symptoms Trial indicates that combination therapy with both an alpha-blocker and a 5-alpha reductase inhibitor can delay the progression of symptoms and is more effective over time than either medication alone for reducing symptom scores and improving peak urinary flow rates.
Treatment & Management
Anesthesia for TURP
Spinal anesthesia is generally preferred for transurethral resections for a number of reasons, not the least of which is the ability to converse with the patient and to evaluate him for symptoms of an early dilutional hyponatremia (ie, transurethral resection [TUR] syndrome) during surgery.
Spinal anesthesia may make recovery a little easier with better pulmonary toilet. Patients recovering from a general anesthetic often cough heavily, which tends to increase hematuria. Laryngeal mask anesthesia (LMA) tends to minimize many of the negative aspects of general anesthetic and is now used for many TURP cases.
A large national survey and cooperative study of 13 institutions by Mebust et al confirmed that up to 79% of transurethral prostate resections are performed with the patient under spinal or epidural anesthesia. [8]
A 1998 study by Fredman et al compared general versus spinal anesthesia in patients older than 60 years undergoing short transurethral prostate surgery. The study demonstrated that general anesthesia with propofol and desflurane facilitated shorter induction and recovery times without adversely affecting patient comfort. In this study, these general anesthetic agents appeared to be preferable to spinal anesthesia for TURP, at least in this particular age group. [9]
Several studies have failed to show any significant differences in complication rates, operative mortality and morbidity, or blood loss between regional and general anesthesia. Transurethral resections have even been performed with local anesthesia and sedation, although these have only been performed on relatively small prostates averaging just 11 g of resected tissue.
The obturator nerve runs near the prostate and can be electrically stimulated during transurethral prostate surgery, causing a violent thrusting of the leg, which is called the obturator nerve reflex. This reflex can possibly lead to inadvertent intraoperative surgical complications. The problem of unintentional obturator nerve stimulation can be corrected under general anesthesia by paralyzing the patient.
The obturator reflex most often occurs while resecting bladder tumors on the lateral walls of the bladder. In these cases, the reflex may be prevented by injecting a local anesthetic into the sensitive area through a special needle passed through the resectoscope.
Equipment for TURP
Irrigating solutions
A number of solutions may be used for irrigation during TURP. Saline cannot be used because it conducts electricity, which diffuses the current and prevents it from cutting or cauterizing tissue. In addition, the current can possibly be transmitted down the shaft of the resectoscope and affect the surgeon.
If the electrosurgery (cautery) unit does not appear to be functional, inadvertent use of normal saline (isotonic sodium chloride) irrigation is one of the first things to check besides the grounding pad, power switch, and cord connections. If normal saline is accidentally used, no cutting or coagulating will occur. It will appear as though the electrosurgical unit is not working.
Accordingly, solutions that do not conduct electricity, such as sterile water, glycine, and sorbitol/mannitol, must be used instead of isotonic sodium chloride solution during TURP.
Sterile water is rarely used because, when absorbed in large quantities during the procedure, it causes hyponatremia, intravascular hemolysis, and hyperkalemia. Therefore, nonhemolyzing solutions of sorbitol/mannitol or glycine are used most often. These relatively isotonic agents protect against hemolysis but cannot prevent dilutional hyponatremia because their intravascular absorption increases fluid volume without adding any sodium.
The original nonhemolyzing irrigating solution recommended by Creevy and Webb in 1947 was 4% glucose, which is no longer used in modern transurethral surgery. [10] However, as noted by Collins et al, a 5% glucose solution may be a reasonable and economical substitute for the much more expensive glycine irrigation fluid in developing countries, where it would be less hemolyzing and safer than sterile water. [11]
Currently, glycine is probably the most popular irrigation media used for TURP surgery, with an osmolality of approximately 200 mOsm/kg (compared to 290 mOsm/kg for normal serum). Though not truly isotonic, it is close enough to be essentially nonhemolyzing. The metabolism of glycine into glycolic acid and ammonia has been postulated as a contributing factor to TUR syndrome. Studies performed on rats given intravenous (IV) and retroperitoneal glycine found toxic effects from the glycine on the liver, kidneys, and pancreas.
Glycine inhibits neurotransmission and may rarely cause visual disturbances if absorbed in large amounts. Complaints of prickling sensations, increased nausea, hypotension, bradycardia, and confusion have been reported when more than 1000 mL of glycine has been absorbed. Progressively increasing adverse effects occur as the amount of absorbed glycine increases. This becomes potentially serious when 3000 mL or more is absorbed.
Most experts, however, believe that these effects are relatively insignificant during routine TURP procedures. Using a sorbitol/mannitol solution helps avoid these problems. Glycine toxicity has been linked to the rare fatality, but this is considered an extremely rare event.
A new bipolar resectoscope, with a redesigned generator that can operate safely with normal saline (isotonic sodium chloride solution) irrigation, is now available (see below). The advantages of such a system include the total elimination of hyponatremia (TUR syndrome) because normal saline is used as the irrigant.
Coaxial continuous-flow rectoscopes
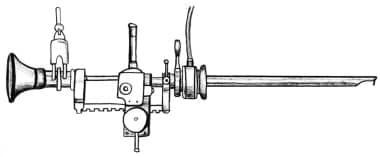
In 1975, Jose Iglesias de la Torre reported a reliable external spring-loaded continuous-flow rectoscope that is the most popular resectoscope working element style used today (see the image below). [12]
 Demonstration of the Iglesias resectoscope, with the free hand allowing a finger in the rectum to elevate the floor of the prostate.
Demonstration of the Iglesias resectoscope, with the free hand allowing a finger in the rectum to elevate the floor of the prostate.
The main advantage of a resectoscope that allows the resection to be performed with a single hand, as in the Iglesias design and the earlier Nesbit design, is that it leaves the second hand free to place a finger in the rectum to help raise the apex and floor of the prostate. The primary disadvantage is that some of the sensory perception from cutting the tissue is lost.
The Iglesias working element uses the thumb and the spring to do the actual cutting, while the older Stern-McCarthy model allows the resection to be controlled by the thumb and first two fingers using a rack-and-pinion mechanism, which provides finer motor control and excellent tactile sensory feedback. Most urologists today use the Iglesias model, but a few prefer the original Stern-McCarthy design for these reasons (see the image below).
Continuous-flow resectoscopes like the Iglesias model are designed to eliminate the need for intermittent bladder evacuations, which interrupt the resection and waste time while the surgeon needs to become reoriented. They also help maintain low fluid pressure in the prostatic fossa and bladder, which should reduce fluid absorption.
The main disadvantages are the tendency of the resected chips to sometimes flow toward the telescope and interfere with vision; the slightly reduced wire loop size available because of the coaxial nature of the instrument; and the lack of any definitive study that proves they actually save time, reduce blood loss, or decrease absorption of irrigation fluid intraoperatively. Nevertheless, most urologists find continuous-flow instrumentation convenient and beneficial.
Bipolar technology, which allows normal saline to be used as an irrigation fluid to reduce hyponatremia, is just the latest in a long line of technological innovations in TURP. In a bipolar system, instead of a grounding pad on the patient, the ground electrode is placed inside the sheath of a modified continuous-flow resectoscope, allowing the cutting current to pass directly between the wire loop and the sheath, or is built into the electrode itself, where the more proximal of the dual adjacent, parallel wire loops acts as the ground.
The current generated by a bipolar instrument tends to remain superficial, which generally prevents the potentially dangerous obturator reflex often associated with transurethral resection of bladder tumors. The wire loop may be slightly smaller than a conventional resectoscope, but it otherwise looks and operates the same. No special or additional surgical skills are required.
This type of technological improvement permits transurethral endoscopic surgeries to be performed more safely, especially in high-risk patients and those at particular risk for dilutional hyponatremia or iatrogenic trauma from an inadvertent obturator reflex. Overall surgical time and blood loss are comparable with those of the standard TURP. The only real disadvantage is the relatively high cost of the new instrumentation.
Modern coaxial continuous-flow bipolar resectoscopes are currently the overwhelming first choice of urologists for TURP instrumentation.
Suprapubic trocars
Suprapubic trocars can also be used to establish continuous-flow irrigation. These instruments require a small skin incision and create a small cystostomy wound in the bladder, but they offer several distinct advantages over the more popular single coaxial continuous-flow instruments. One advantage is that chips and irrigation flow away from the telescope toward the drainage tube in the bladder, which improves visualization. Another is that a larger wire loop can be used with the same caliber resectoscope sheath.
A third advantage is that the suprapubic trocar can keep the bladder fluid pressure at or below only 8 cm water, which is well below the 10-15 cm water pressure of the pelvic veins and periprostatic venous system; this keeps fluid absorption down. When compared directly to a coaxial continuous-flow system, the suprapubic trocar technique has been found to allow shorter operating times with lower intravesical pressures and less fluid absorption.
We prefer to use a suprapubic trocar for establishing continuous flow when trying to resect larger prostates (>80 g), following the technique of Dr. Paul O. Madsen of Madison, Wisconsin, who extensively studied fluid absorption during TURP and routinely used temporary suprapubic trocar cystostomies for continuous flow in his TURP procedures. [13] The technique proved especially useful in larger prostates, or even in moderate-sized prostates when only a 24F sheath can be used, allowing a full-thickness cut of tissue to be made without compromising irrigant flow.
We use the Wolf 14F suprapubic trocar because it rarely clogs and can be placed easily. The placement technique is to fill the bladder to capacity (or with at least 200-300 mL) while the resectoscope is in place and then make a small suprapubic stab wound approximately 1 cm above the pubic symphysis.
The trocar is placed into the stab wound, with the sharpened obturator tip angling slightly superiorly toward the patient’s head. This is performed in such a manner that the final intravesical trocar position is angled toward the posterior bladder wall and away from the resection site. Pressure is applied to the trocar to allow it to penetrate into the bladder.
The sharpened obturator tip is then replaced with the fenestrated drainage insert attached to suction tubing. The other end of the suction tubing is in a floor drain. No mechanical suction is needed because gravity provides an adequate flow rate.
The key to placement of the suprapubic trocar is to have the bladder completely filled before attempting to place the trocar. This can be achieved safely even in patients with previous abdominal surgery. At the end of the case, when the Foley catheter is in place and the irrigation fluid is clear, the suprapubic trocar is removed and the stab wound site is covered with a small compression dressing using gauze pads and an elastic adhesive bandage (eg, a large Band-Aid). No sutures are required.
No significant extravasation occurs unless the trocar is removed too early and the resectoscope must be reintroduced. Therefore, wait until just before the patient is ready to be transferred from the cystoscopy table before removing the suprapubic trocar. Only very rarely does any significant intravesical bleeding occur from the trocar site. This can be cauterized with the resectoscope before the case is completed and the trocar is removed.
Coagulating intermittent cutting device
A modified cutting system designed to decrease blood loss and hematuria has been developed for TURP. This new coagulating intermittent cutting (CIC) device uses a constant voltage pulse current with controlled pulse intervals to help reduce bleeding.
Positioning for TURP
Make sure the patient is positioned with the buttocks flush with the end of the cystoscopy table. Resection of the anterior prostate opposite the bladder neck can be impaired when deflection of the resectoscope is restricted by the edge of the cystoscopy table. Varying patient height to a comfortable level and using the Trendelenburg position appropriately should make the resection easier and more comfortable and can facilitate visualization.
Complication Prevention
Avoidance of hypothermia
Hypothermia, defined as a core body temperature of 36°C or less, induces shivering, which has been shown to increase oxygen demands by as much as 500%. Aside from chilling and shivering, bodily cooling produces a number of cardiovascular changes such as bradycardia, reduced cardiac output, higher mean arterial pressure, increased cardiac stress, and greater vascular resistance. Hypothermia is particularly worrisome as core body temperature approaches 35°C or less, which is sufficient to induce angina, cardiac arrhythmias, and myocardial infarctions.
Room-temperature irrigation can result in a substantial decrease in the patient’s core body temperature, particularly if continuous-flow irrigation is used. Other factors that may increase the risk of hypothermia include longer resection times, larger amounts of irrigating fluid absorbed, increased prostate size, small body habitus, and low body weight. Ambient temperature in the operating room is another important factor.
Consequently, irrigating fluid warmed to body temperature is strongly recommended, along with the use of warming blankets and other appropriate thermal modalities. No significant increase in blood loss has been found with the use of irrigating fluid warmed to body temperature.
Warmed saline may also improve the performance of bipolar TURP instrumentation. A high-flow, low-pressure fluid warmer specifically designed for TURP irrigation is commercially available from Smiths Medical. (For information on their level 1 “NormoFlo” model H-1100 fluid warmer, call 1-800-553-8351 or go to www.smiths-level1com/fluid_warming_normo.htm.)
Discontinuance of anticoagulants
Patients should be taken off anticoagulants at an appropriate time prior to surgery. For most patients on warfarin, this is 3-4 days preoperatively. The prothrombin time (PT) and the activated partial thromboplastin time (aPTT) should be checked just prior to the surgical procedure.
For patients on clopidogrel, 14 days off the medication prior to TURP surgery is recommended, but 10 days may be sufficient. Unlike other surgeries, TURP relies more heavily on normal coagulation to help control postoperative bleeding. Only a bleeding time can help determine if any lingering anticoagulant effects from the clopidogrel are present. Platelet transfusion is the only way to correct the anticoagulant effects of clopidogrel but should be used only as a last resort when absolutely necessary.
In selected patients subject to an unusually high medical risk, short-term heparinization can be used while other anticoagulants are discontinued. The heparin can then be reversed just before surgery. This technique requires careful monitoring and usually hospitalization. In such situations, careful consideration should be given to alternative treatments for BPH, such as medical therapy, photoselective vaporization of the prostate (PVP), intermittent catheterization, suprapubic tube placement, permanent Foley catheterization, or a urethral stent.
A 2002 study by Dotan et al comparing patients on warfarin who received either a standard protocol of anticoagulant discontinuation or low-molecular-weight heparin (LMWH) substitution found that the surgical blood loss was approximately equal between the 2 study groups and that neither received any additional blood transfusions. [14]
However, the heparinized group required more inpatient hospital days because of prolonged hematuria and a longer period of catheterization that averaged approximately 2 days more than the group who received the standard therapy. This study suggests that low molecular weight heparin substitution may be a reasonable alternative to oral anticoagulant discontinuation for selected patients.
Even low-dose aspirin has been shown to increase bleeding after TURP. Nielsen et al, in a prospective, randomized, double-blind, placebo-controlled study of patients who received either a single-dose placebo or a 150-mg aspirin tablet 10 days before surgery, found no significant difference in resected tissue weight, operative time, complications, or intraoperative blood loss, though postoperative bleeding was significantly higher in the aspirin group. [15]
Therefore, we recommend that aspirin be stopped at least 10 days prior to surgery and, preferably, 14 days before.
The issue of when to restart anticoagulants after TURP remains under debate. The timing is highly variable and depends on many factors, such as the original reason for anticoagulation, the patient’s overall clinical situation, the size and difficulty of the transurethral surgery, and the degree and length of postoperative bleeding, among others.
We generally wait until the urine is grossly clear for 24 hours before resuming warfarin, but we recommend confirming that the urine is grossly clear for at least 48 hours before restarting clopidogrel or aspirin because of the longer half-life of these agents and the inability to easily reverse them, if needed.
Preoperative antibiotic prophylaxis
Some controversy exists regarding the use of systemic antibiotics prior to the initiation of prostate surgery. The majority of studies have demonstrated a benefit, and most urologists use them routinely. A broad-spectrum cephalosporin or fluoroquinolone is usually preferred.
Elderly patients with BPH often have moderate postvoid residual values, bacteriuria, and/or frank urinary infections. Preoperatively, up to 25% of patients with BPH have a documented urinary tract infection. With these issues in mind, administering a preoperative systemic antibiotic seems reasonable and prudent.
Patients with indwelling Foley catheters are presumed to be infected regardless of culture results and should be routinely given broad-spectrum antibiotic coverage before surgery. Culture-specific antibiotics are preferred.
The issue of how long to maintain the antibiotics and whether to use them postoperatively is even less clear, although some evidence indicates that 2 weeks of postoperative antibiotic coverage can help reduce urethral stricture formation.
A 2002 meta-analysis by Berry and Barratt suggested that the type of antibiotic was relatively unimportant when used prophylactically in low-risk individuals. [16] They found that prophylaxis significantly decreased bacteriuria and septicemia, even in men with sterile urine preoperatively. Effective agents included quinolones, aminoglycoside, trimethoprim-sulfamethoxazole (TMP-SMZ), and cephalosporins. Short-course therapy was found to be more effective than single-dose regimens, regardless of the agent chosen.
After postoperative catheter removal, we generally use doxycycline, TMP-SMZ, or a nonsystemic urinary antiseptic such as nitrofurantoin. The partially devascularized and necrotic prostatic tissue tags and any remaining tissue remnants are perfect breeding grounds for bacteria; therefore, some antibacterial prophylaxis should probably be maintained for at least the first 15 days after a TURP. This may also help reduce urethral stricture formation.
Preoperative Studies
Laboratory tests
Perform a urinalysis with culture and sensitivity to evaluate for acute infection.
A complete blood count (CBC) is needed to establish preoperative hemoglobin and hematocrit levels and platelet counts. These findings are helpful in determining blood loss and deciding on possible transfusions postoperatively. The overall risk rate of blood loss following TURP sufficient to justify transfusion is approximately 2.5%.
Creatinine, blood urea nitrogen (BUN), and electrolyte levels are used to establish the presence of a new azotemia, which may prompt the physician to request appropriate studies, such as renal ultrasound examinations, to determine if bilateral hydronephrosis is present. Baseline electrolyte levels are needed to help identify hyponatremia.
In regard to coagulation studies, several studies have indicated that PT and aPTT are not generally necessary or cost effective without a history of unusual bleeding or use of an anticoagulant medication. However, because of the substantial risk of hemorrhage during TURP, many surgeons assess coagulation status preoperatively in higher-risk patients, patients on anticoagulants, and those with larger prostate glands.
Performing bleeding time studies should be considered in patients with renal insufficiency or those taking platelet-inactivating drugs such as aspirin, clopidogrel, or ibuprofen. Obtaining information about bleeding time just prior to surgery is particularly useful and recommended in patients who normally take clopidogrel because PT and aPTT are not affected by this particular anticoagulant medication.
A high prostate-specific antigen (PSA) level suggests an active urinary or prostatic infection, a markedly enlarged prostate, or possible prostatic carcinoma.
Diagnostic imaging
The increase in intravascular fluid absorption that happens during a TURP can quickly cause congestive heart failure (CHF) in susceptible patients; therefore, a preoperative chest radiograph is useful for comparison.
Early use of furosemide (Lasix) intraoperatively should be considered in patients at increased risk for CHF or hyponatremia and in patients in whom blood loss is excessive. Beta-blockers and/or nitrate vasodilators are frequently recommended on an individual basis in patients with a significant cardiac history undergoing TURP to minimize heart-related problems during and immediately following surgery.
Intravenous pyelograms and computed tomography (CT) scans also may be used selectively to evaluate associated conditions, such as hematuria, but they are not part of the routine evaluation for prostatic obstruction or hyperplasia.
Ultrasonographic examinations of the kidneys, bladder, and prostate, although not routinely recommended, are performed in some centers to help exclude other pathologies in the urinary system and to help estimate prostatic volume.
Renal ultrasonography is not recommended as a routine preoperative study, although it can be useful for helping detect hydronephrosis in cases of azotemia or pathological sources of hematuria not related to prostatic disease. It should also be considered in patients with a history of chronic or recurrent urinary tract infections, previous urinary tract surgery, or urolithiasis.
Electrocardiography
Electrocardiograms are usually performed because most patients are elderly and occasionally a patient demonstrates a silent myocardial infarct or other cardiac problem that affects the timing of the surgery.
Urodynamic studies
Preoperative urodynamic studies are indicated in patients who may have underlying neurologic disease or potentially nonfunctioning bladders. Poor postoperative results in terms of bladder emptying can occur in patients with associated neurological abnormalities affecting the urinary bladder.
Some authorities, such as the International Scientific Committee (ISC) of the International Consultation on BPH, recommend pressure-flow studies for all patients before any invasive procedures or when a more precise diagnosis of bladder outlet obstruction is required. [17]
The Urodynamics Subcommittee of the ISC recommends filling cystometry in addition to pressure-flow studies. They argue that patients who clearly demonstrate obstruction tend to fare better with surgery. This would allow physicians to direct invasive therapy only to those patients most likely to benefit from it. Patients who do not demonstrate significant obstruction based on findings from these studies or those with equivocal results may only need less-invasive and less-expensive therapies.
A study by Van Venrooij et al that evaluated patients urodynamically before and after TURP found that 50% of patients with a urodynamically unstable bladder preoperatively developed stable bladder function by 6 months after their TURP procedures. [18] This suggests that urodynamics may not always be highly predictive of outcome success after TURP, particularly when dealing with unstable bladders.
Uroflowmetry provides information about the force of the patient’s urinary stream and the volume of urine voided. Although it is not necessary in all patients planning to undergo TURP, many surgeons find the information useful. This modality also allows for objectively tracking response to medical or surgical therapies for BPH. Generally, a peak flow rate of at least 13 mL/s with a voided volume of at least 150 mL is considered adequate, whereas a peak flow rate of less than 10 mL/s indicates probable obstruction.
Sonographic measurement of postvoid residual urine volume is useful for helping differentiate patients with overflow incontinence from those with purely irritative problems. Although this test is not specifically required before BPH therapy, postvoid residual volume findings can be helpful for assessing a patient’s ability to completely empty his bladder and his response to both medical and surgical interventions.
Whereas a postvoid residual urine volume of less than 50 mL is generally recognized as normal, no absolute quantity exists that represents an abnormal postvoid residual urine volume. For most practical purposes, an upper limit of 150-200 mL is reasonable.
Cystoscopy
Cystoscopy is another optional test that is not specifically required or recommended as part of the routine TURP workup. However, it is useful for helping establish the size and shape of the prostate and for checking for intravesical prostatic extension, bladder tumors, and other pathology.
Cystoscopy results can be very helpful in the selection of therapy once surgical intervention has been chosen because some patients may not be candidates for various surgical interventions depending on the size and/or shape of their prostate glands. Transurethral resection can be performed on a prostate gland of any size or shape, depending on the skill and experience of the operating surgeon (see image below).
Biopsy and histologic evaluation
Although histologic confirmation of BPH is not necessary prior to TURP, patients with an elevated PSA level and/or abnormal digital rectal examination findings should be considered for prostate needle biopsy to help rule out carcinoma prior to transurethral resection. TURP may limit the treatment options and timing for definitive prostate cancer therapy and may be unnecessary once a definitive prostate cancer treatment is initiated.
BPH occurs primarily as two histological types: stromal hyperplasia (fibromuscular) and glandular hyperplasia (nodular, epithelial). The normal prostate consists of approximately 50% stroma. The rest is divided among gland elements, acinar lumens, and epithelial elements as determined by morphometric studies. The growth rate of the epithelium, and particularly the stroma, in BPH is much faster than the growth rate of these same tissue types in normal prostates.
As the name implies, stromal hyperplasia results from proliferation of the fibromuscular stromal fibers that separate the acini of prostate glands. Histologically, the appearance is identical to the stromal hyperplasia observed in uterine fibroids. This type of hyperplasia is theorized to respond better to medical management with alpha-blockers, which relax the tone of the muscle fibers. Hyperplastic prostate tissue that arises primarily from the periurethral zone tends to be mainly stromal and fibromuscular. Median lobe enlargement is primarily stromal fibromuscular tissue.
Glandular hyperplasia appears as nodules of redundant glandular acini, which are mainly epithelial in nature. The cells have clear cytoplasm, and the prostatic ducts or lumens may be dilated or characterized by complexed folds. BPH that is primarily nodules of glandular hyperplasia is likely to be larger and more likely to respond to hormonal therapy such as finasteride and dutasteride than BPH that is primarily stromal and fibromuscular.
Prostate resections of 50 g or less are predominantly stromal, with the glandular (nodular) component averaging only approximately 22% of the total. The average size of the nodules in these small- to medium-sized prostates is 4 mm or less in diameter. Prostates heavier than 50 g in resected weight typically demonstrate glandular or nodular tissue constituting more than 50% of the total prostate, with the average nodule measuring 8 mm or more in diameter.
Prostatic tissue growth from the relatively small transitional zone, which is located just lateral and distal to the internal sphincter, is the primary site of origin of the majority of BPH glandular tissue.
Overview of Technique
The goal of prostate surgery for BPH is to remove the obstructing tissue while minimizing damage to surrounding structures, with as little discomfort to the patient as possible. The accessibility of the obstructing prostate via transurethral endoscopy affords the opportunity to remove the obstruction without open surgery. It also protects the surrounding organs from injury by removing the tissue from the intraluminal surface of the prostate.
Electrosurgical TURP remains the standard for endoscopic removal of obstructive BPH tissue and is the primary focus of this review.
TURP is a surprisingly challenging procedure technically, with a protracted learning curve. The procedure tends to be required in older, less healthy men. Continuing improvements in instrumentation and technique allow accomplishment of this procedure more easily for the surgeon and less dangerously for the patient.
Open prostatectomy is more appropriate for larger prostates, in which endoscopic resection would be so lengthy that dangerous fluid shifts and other complications are more likely to occur. This is entirely dependent on the skill and experience of the operating surgeon. Surgeons need to know their own resecting capability and should not attempt a TURP in a prostate that is clearly too large.
If the transurethral resection will take longer than 90 minutes of operating time to complete, then an open prostatectomy, referral to a more experienced colleague, or some alternative therapy is recommended. If in the middle of the case and unable to complete the entire resection in 90 minutes, at least finish one lateral lobe, the median lobe (if enlarged), and the bladder neck. This often results in very good clinical outcomes and offers essentially the same symptom relief as the completed TURP.
Electrosurgical Transurethral Resection of Prostate
Many variations are described for the basic operative approach to TURP. Various regions of the country and specific teaching institutions use slightly different techniques to perform the procedure. However, a number of general principles are applicable to all variations of TURP and are outlined below. Aspects of particular technical approaches (eg, those of Nesbit and Milner) will be noted where appropriate.
Initial steps
Instruments should be carefully checked before starting. In particular, check that any insulating pieces, attachments, parts, or tips are firmly secured. Some continuous flow systems use a plastic insulating beak at the end of the inner sheath, which can come loose and break off in the bladder during surgery. If this occurs, removal can be challenging. Meanwhile, the patient is most likely actively bleeding, absorbing additional irrigating fluid, and delaying the completion of the surgery.
Confirm that the cutting loop of the resectoscope fits perfectly into the sheath without any gaps. This helps avoid tags of tissue that interfere with vision and would have to be cut again, wasting valuable time.
A spare telescope and sheath, extra cutting loops, a backup electrosurgical unit, instruments for a perineal urethrostomy, and a small (24F) resectoscope set should be immediately available in case they are needed. The smaller resectoscope set should be used if the urethra proves to be narrower than expected.
Use of a plastic barrier sheath (eg, Lingeman sheath, O’Connor sheath) helps maintain sterility and protects the operative field, while allowing digital manipulation of the prostate through the rectum.
If the patient has been catheterized, gentle irrigation of the urethra with a Toomey or bulb syringe rinses mucus, blood clots, and other debris into the bladder, where these materials will not interfere with vision. This debris can then be easily rinsed out when the bladder is drained through the cystoscope.
As noted (see Preparation), an isotonic solution should be used for intraoperative irrigation. Isotonic irrigating solutions of glycine, sorbitol, or mannitol are used most often. Although they prevent hemolysis, these solutions can still cause hyponatremia, confusion, and visual disturbances if large volumes are absorbed.
Irrigating fluid should be kept at the lowest height (pressure) level possible to maintain an adequate flow. Raising the irrigating fluid level from 60 cm to 70 cm height has been shown to dramatically affect fluid absorption. A starting fluid height of 60 cm is suggested and is usually sufficient. The fluid should be warmed to body temperature to avoid chilling the patient, and adequate fluid should be available. Surprisingly large volumes of fluid may be needed if continuous-flow instrumentation is used.
Cystoscopic inspection and visualization of anatomy
The procedure always begins with a careful cystoscopic inspection of the anterior urethra, external urinary sphincter, verumontanum, prostatic urethra, bladder neck, prostatic median lobe, trigone, ureteral orifices, and the rest of the bladder using a small-caliber cystoscope (see the image below). This inspection is important not only to verify the absence of associated pathologies (eg, bladder tumors, urethral strictures, vesical stones) but also to help the surgeon obtain a clear 3-dimensional mental image of the patient’s specific anatomical features and relationships.
Preparation, positioning, instruments, and cystoscopy are shown in in the video below.
The initial cystoscopy should be performed gently, avoiding contact with the superficial surface of the enlarged lateral and medial lobes of the prostatic urethra as much as possible. Their surface is often hyperemic and they bleed easily, which interferes with vision during the cystoscopy and resection.
After careful inspection and orientation, the bladder is distended with approximately 100 mL of fluid, which helps to improve anatomical identification and better visualize the prostate, bladder neck, median lobe, and bladder wall. The external urinary sphincter is located just distal to the verumontanum, which is specifically used as the distal resection border and landmark to prevent any injury to the sphincter.
The relative distance between the ejaculatory ducts of the verumontanum and the proximal edge of the external sphincter muscle should be carefully noted in order to better judge the absolute distal limits of resection. Despite the fact that 10-20% of the prostate may extend distally beyond the verumontanum, especially in larger prostate glands, the verumontanum remains the distal margin of resection in most circumstances.
In very large prostates, some expert and experienced resectionists remove apical and lateral lobe tissue located adjacent or slightly distal to the verumontanum, arguing that failure to remove this tissue results in an incomplete resection and postoperative voiding difficulties in some patients. However, the risk of inadvertent and permanent injury to the external sphincter muscle is quite high when resecting in this area, so caution is advised.
Always know exactly where the verumontanum is located, and do not resect or cauterize it. Besides removing an important landmark, this may also lead to painful ejaculation later. Without this landmark, one can easily lose orientation and risk damaging the external sphincter muscle, causing permanent incontinence. If, during the case you are not absolutely certain of your exact location, orientation, or position relative to the verumontanum, stop resecting immediately and reorient by finding a stable landmark (eg, bladder neck or verumontanum).
The external sphincter muscle is identified by (1) its wrinkling and constricting action as the resectoscope is withdrawn and (2) the bunching-up of the superficial mucosa just in front of the telescope as it is reinserted. The fibers of the external sphincter are embedded within the urogenital diaphragm, which is relatively fixed in position, while the prostate has some limited mobility.
Placement of resectoscope
The resectoscope sheath should be well-lubricated and placed with the aid of an obturator to prevent trauma to the urethral mucosa or false passage formation.
If there is any doubt as to whether the urethra is of adequate size to easily accommodate the resectoscope sheath that will be used, the urethra should first be calibrated. Most adult male urethras allow a well-lubricated 28F sheath to pass easily. To prevent postoperative urethral strictures, do not to use a resectoscope that is much larger than the patient’s urethra, fossa navicularis, or urethral meatus.
A smaller-sized resectoscope sheath (24F) should be used if the urethra appears too narrow for easy access with larger instruments; therefore, a 24F resectoscope sheath and appropriately sized cutting loops should always be immediately available. We use a 28F resectoscope sheath only on the largest prostates and prefer a 24-26F sheath for routine use.
If the patient has an extremely small urethra, an internal urethrotomy (preferred) or gentle dilation of the urethra with sequential Van Buren sounds may be needed. A meatotomy can also be performed if the urethral meatus is inadequate. While most surgeons normally perform a ventral meatotomy, Winston Mebust and others have suggested making a dorsal internal urethrotomy, arguing that large ventral meatotomies often result in a split or diverse stream with splattering and splaying.
It is essential not to force the resectoscope sheath into the urethra. This commonly results in a urethral stricture at the fossa navicularis and possibly elsewhere in the urethra (see the image below).
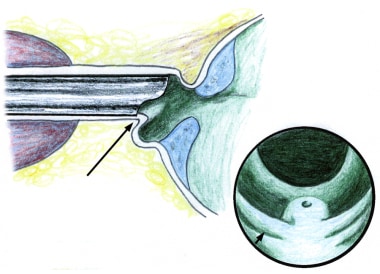 Beware of resecting folds of tissue that may build up in front of the edge of the beak of the resectoscope because this may cause a perforation. Additional urethral dilation or use of a smaller resectoscope sheath can help prevent this from occurring.
Beware of resecting folds of tissue that may build up in front of the edge of the beak of the resectoscope because this may cause a perforation. Additional urethral dilation or use of a smaller resectoscope sheath can help prevent this from occurring.
A perineal urethrostomy can be performed to create temporary access into the bulbous urethra to avoid any additional trauma to the rest of the urethra (see the image below). The perineal urethrostomy is especially important if obtaining easy passage of the resectoscope sheath proves difficult, if patients have contractures or penile prostheses, or if the urethral length proves to be too long for the standard instruments. One of the most common mistakes made in TURP surgery is the failure to use a perineal urethrostomy appropriately.
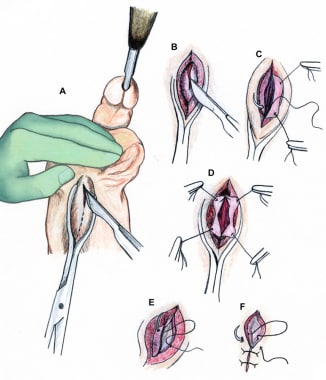 Technique of perineal urethrostomy. Incision is made over sound (A-B). Optional stay sutures used to help open urethra (C-D). Wound closed in layers with absorbable material (E-F).
Technique of perineal urethrostomy. Incision is made over sound (A-B). Optional stay sutures used to help open urethra (C-D). Wound closed in layers with absorbable material (E-F).
Resection of prostatic tissue
Always keep in mind and follow an orderly resection plan, regardless of which technique is used. As Winston Mebust noted, “The actual technique is probably not as important as planning it carefully and executing it precisely, so that the operation is complete and the surgeon remains oriented throughout the procedure.”
Once the resection has been started in a particular area, that portion of the resection should be finished completely before moving on to another location. Performing a partial or incomplete resection in several lobes at once is discouraged because of the resultant increased bleeding and fluid absorption. In addition, terminating the procedure quickly, if necessary, is more difficult.
The surgeon should always be prepared to terminate the procedure with relatively little notice if the patient develops complications. This is aided by finishing the resection completely in one area of the prostate at a time. In these cases, good results have generally been reported when at least one complete lobe has been resected. In some very high-risk patients, it may be reasonable to intentionally complete only the median lobe and one lateral lobe so as to reduce the total anesthesia and operating times.
Resect tissue only when pulling or withdrawing the cutting loop toward the resectoscope, never when pushing it forward. This cleanly separates the resected tissue from the rest of the prostate gland and prevents tunneling, perforation, bladder injury, and extravasation.
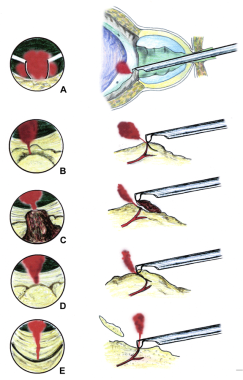
If a very large and obstructing median lobe is present, it should be resected first, regardless of the method chosen for the rest of the transurethral resection. An attempt should be made to identify the ureteral orifices, but this may be impossible in some cases until the markedly enlarged median lobe has been at least partially removed. Intravenous (IV) methylene blue dye can be used to help find the ureteral orifices and prevent their inadvertent resection.
A modification designed to reduce bleeding from an enlarged median lobe involves making several small, short cuts in the cleft between each lateral lobe and the median lobe. This resection cuts off much of the blood supply to the bulk of the median lobe, which comes from the penetrating periurethral prostatic branches of the inferior vesical artery at the bladder neck.
The bulk tissue of the median lobe is resected from the top down, while the end of the resectoscope is at the bladder neck to avoid subtrigonal tunneling and bladder neck injury, which can lead to extravasation and increased fluid absorption (see the image below).
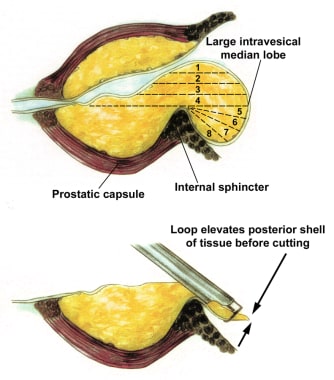 Median lobe resection. Loop without current is used to gently lift the posterior flap of the median lobe tissue now lying on the bladder surface. Resection can now be performed without risk of bladder injury.
Median lobe resection. Loop without current is used to gently lift the posterior flap of the median lobe tissue now lying on the bladder surface. Resection can now be performed without risk of bladder injury.
When the resection approaches the bladder floor, the cutting loop can be used, without any electrical current, to gently lift the lip of the remaining median lobe tissue up and away from the bladder floor and trigone (see the image below). Once lifted free, current can be applied to the cutting loop and the elevated median lobe tissue can be resected. This portion of the median lobe resection requires relatively thin superficial cuts of tissue. The process is repeated until the entire median lobe has been resected.
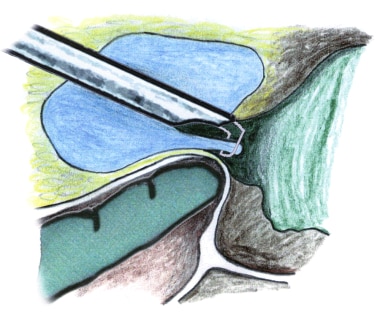 Resection of the prostate at the bladder neck. With a finger in the rectum for guidance, the loop without current can be used to lift a flap of prostatic tissue prior to cutting. This helps avoid perforation and subtrigonal tunneling.
Resection of the prostate at the bladder neck. With a finger in the rectum for guidance, the loop without current can be used to lift a flap of prostatic tissue prior to cutting. This helps avoid perforation and subtrigonal tunneling.
The bladder should be reinspected to identify the ureteral orifices, which should be visible. Resected prostatic chips and blood clots may need to be evacuated to allow for visualization of the ureteral orifices, trigone, and bladder floor. Great care should be taken to avoid inadvertent resection of the trigone, especially laterally where the ureteral orifices may be directly underneath the enlarged median lobe. Once the median lobe has been resected, the rest of the TURP procedure can be performed in a routine manner (see the image below).
When resecting at the bladder neck, make sure enough fluid is present in the bladder to keep the posterior bladder wall away from the surgical area. Approximately 100 mL prevents accidental posterior bladder wall injury and perforation. Before resecting at the 4- to 5-o’clock and 7- to 8-o’clock positions at the bladder neck, visually check the position of the ureteral orifices to prevent their inadvertent resection.
Once the inferior bladder neck area has been adequately resected, respect the margin as the proximal limit of resection. Resecting additional tissue from the bladder neck later during the procedure is often tempting, but this often results in undermining the trigone and inadvertent resection of one or both ureteral orifices.
If one or both ureters are inadvertently resected, avoid using cautery near the cut end to prevent scarification and possible hydronephrosis. This usually heals without problems.
When cutting the anterior tissue at the 12-o’clock position, making relatively shallow straight cuts is better, which leaves the surface fairly flat, rather than trying to make it concave or curved. The prostate is thinnest here; the external sphincter is at its most proximal position and the risk of perforation is relatively high, especially if this area is resected early in the case.
Try to resect using long smooth strokes (see the image below). This prevents chopping of the prostatic fossa, avoids capsular perforations, helps limit bleeding, and shortens the overall resection time.
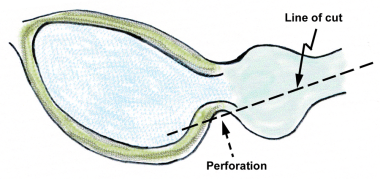 Perforation at the inferior bladder neck can easily occur if the line of resection is too straight or is not carved to fit the shape of the prostate in this area.
Perforation at the inferior bladder neck can easily occur if the line of resection is too straight or is not carved to fit the shape of the prostate in this area.
The adenomatous tissue of the hypertrophied prostatic adenoma is usually somewhat irregular, off-white to light-yellow in color, and appears slightly granular or nodular. This changes to the smooth, white, glistening, vertically striated surface of the compressed peripheral zone tissue when the surgical capsule is reached. This is the lateral limit of resection because going deeper causes perforations, with extravasation and increased bleeding.
Try to resect all the prostatic tissue possible without perforating the capsule or unduly extending the operating time. Residual tissue can regrow, foster infection, increase fluid absorption, and tends to bleed. An old urology axion states “It is not how much is taken out that causes the postoperative problems, it is how much is left in.”
Intraoperative priapism occurs in approximately 4% of patients undergoing TURP surgery. It is less common with a spinal block than with other types of anesthesia. Occurrence varies according to patient age, with younger patients (< 50 y) affected most often.
Treatment is usually with intracavernosal phenylephrine injections. We prefer to use 1 mL phenylephrine at 10 mg/mL diluted in 49 mL of isotonic sodium chloride solution, which results in a concentration of 0.2 mg/mL. One to 2 mL is injected with a 25-gauge needle directly into the corpora cavernosa every 10-15 minutes as needed.
In our experience, detumescence occurs 2-3 minutes after a single 1 mL injection in all patients receiving this therapy. Only a transitory mild increase in blood pressure has been noted, which occurs approximately 10 minutes after the injection and returns to normal within 30 minutes. We have not needed more than a single injection, but the process can be repeated every 10-15 minutes if necessary.
If resecting at an angle or in a location at which the verumontanum is not clearly visible, make sure the instrument is held very steady, without any distal migration of the resectoscope sheath. If unsure, check the relative position often by rotating or slowly withdrawing the resectoscope until the verumontanum is visible.
Avoid cutting off a large block of tissue that will float freely in the bladder. This becomes very difficult to extract from the bladder and requires chopping or resecting the tissue, which is mobile and unstable. This wastes valuable time that is better spent elsewhere and risks a bladder injury.
Avoid unnecessary use of cautery because it damages normal tissue, increases postoperative irritative symptoms, and promotes scarring, especially at the bladder neck. Cautery involving the verumontanum is discouraged because it can result in painful ejaculation.
Immediately cauterize larger bleeding vessels that interfere with vision because another chance may not be available later. Do not cauterize vessels in areas where additional resecting will be performed, unless the tissue cannot otherwise be visualized. This prevents repeated cauterization of the same vessel. Waiting until the surgical capsule is reached before cauterizing the bleeding vessels is better.
Do not try to cauterize bleeding venous sinuses, because this is a waste of valuable time and may make the bleeding worse. This is one of the harder rules to follow.
Do not do any resecting or cauterizing if visibility is not adequate. The danger of accidentally perforating the prostatic capsule, undermining the trigone, injuring the bladder, resecting the ureteral orifices, or injuring the external sphincter muscle is significant if visibility is not adequate.
If bleeding is obscuring visibility, try increasing the irrigation fluid inflow rate or evacuating the clots and resected prostatic chips with an Ellik or other evacuator, then reexamine the last area of resection from a slightly different angle. If this fails, position the scope at the bladder neck and slowly pull the resectoscope out distally toward the verumontanum, while keeping the tip of the telescope close to the wall of the prostatic fossa and maintaining the same rotational angle. This can be repeated at a slightly altered angle until the bleeding source is found and cauterized.
Make sure the irrigation fluid is flowing adequately. A bleeding vessel at the bladder neck may often be visualized best by filling the bladder. This rotates the inner lip of the bladder neck distally and makes it visible for cauterization (see image below).
 Filling the bladder usually helps locate the bleeding vessels on the interior bladder neck. Limited resection of a covering lip of tissue is sometimes necessary, as illustrated here.
Filling the bladder usually helps locate the bleeding vessels on the interior bladder neck. Limited resection of a covering lip of tissue is sometimes necessary, as illustrated here.
Keep a constant watch on the outflow irrigation fluid color to help monitor blood loss. Also, keep track of the elapsed resection time because the complication rate increases in direct proportion to the actual duration of surgery. Optimal resection time is less than 60 minutes. Complication rates increase dramatically when the resection time is prolonged beyond 90 minutes.
Watch out for the tendency to extend the resection well beyond the bladder neck and into the trigone, which has been called “trigone creep.” This is easily done, especially if one has not already performed a bladder neck resection and created the margins or border for the proximal resection. Once the anatomical landmarks have been identified and the borders have been established, do not resect beyond them.
The raised edge of the resected posterior bladder neck often appears more obstructing than in actuality. If necessary, a midline incision through the bladder neck with a Collings knife can be made at the end of the case, which separates the bladder neck fibers and opens the passage, while avoiding injury to the ureteral orifices. It may also help prevent bladder neck contractures, especially in smaller prostates.
Unless very skilled, keep the scope relatively still while resecting, so that orientation with regard to the verumontanum is never in doubt. Expert resectionists can move the cutting loop and the resectoscope at the same time to carve the prostatic fossa, which is basically a curved concave surface when the hypertrophied adenoma is removed. This lateral-to-medial carving motion is very useful in dealing with larger prostate glands; however, losing orientation and landmarks is easy, which can result in an unintentional extension of the resection distal to the verumontanum.
Save the risky areas for the end of the case, especially the area around the verumontanum. It is often tempting, especially in larger prostate glands, to continue the resection distal to the verumontanum when tissue is present that appears to be obstructing, but this should be discouraged except for very skilled and experienced resectionists. Leaving a small amount of nonobstructing prostate tissue near the verumontanum is much better than risking permanent incontinence through an injury to the external sphincter muscle.
To help in resecting the tissue immediately around the verumontanum, inserting a finger in the rectum to raise the apical tissue is very useful. Another helpful technique is to stand and angle the scope downward toward the verumontanum, which exposes more apical tissue (see the image below).
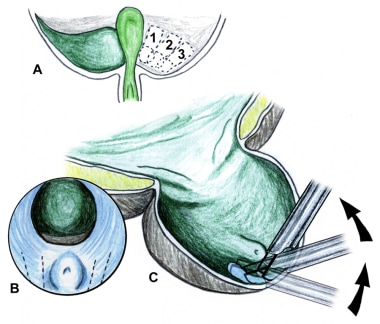 Apical resection near the verumontanum. Elevation of the resectoscope, which may require that the surgeon stand briefly, facilitates this portion of the procedure.
Apical resection near the verumontanum. Elevation of the resectoscope, which may require that the surgeon stand briefly, facilitates this portion of the procedure.
Right lateral lobe resection, left lateral lobe resection, and apical resection are shown in the videos below.
Nesbit resection technique
The Nesbit technique is probably the best-known and most commonly performed TURP method. It was first described by Reed M. Nesbit of Michigan in his landmark 1943 book on transurethral prostatectomy and is currently considered the standard approach to TURP surgery. [19] As originally described by Nesbit, the procedure is divided into 3 stages: (1) intravesical or proximal, (2) extravesical, and (3) apical.
To begin the intravesical portion, the resectoscope is positioned with the tip between the bladder neck and the midpoint of the prostatic urethra proximal to the verumontanum. This point is determined by the relative size of the intravesical prostate. Resection begins by removing the intravesical portion of the prostate and bladder neck tissue. This is removed, along with the immediately adjacent prostatic adenoma, starting at the 12-o’clock position and working clockwise (see the image below).
The initial cut is on one side or the other of the actual midline. The length of the cut is proportionate to the size of the gland. After this stage, care must be taken to avoid resecting proximal to the established resected margin. During this initial intravesical stage of the resection, only the internal sphincter, ureteral orifices, bladder neck fibers, and the margin of resection are visible for use as landmarks.
To begin the extravesical phase of the procedure, the resectoscope is repositioned just in front of the verumontanum, and the resection is continued from the previous distal resected margin to just proximal to the verumontanum, starting again at the 12-o’clock position (see the first image below). This channel is continued from the 12-o’clock to the 4-o’clock position on the left side and to the 8-o’clock position on the right side. The intent is to create a channel between the surgical capsule and the bulk lateral lobe tissue (see the second image below).
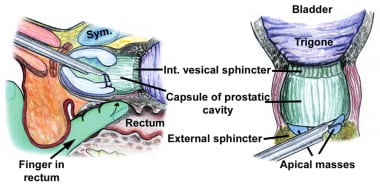 Demonstration of the Nesbit technique. Extravesical (second) and apical (third) portions. Inserting a finger in the rectum and tilting the resectoscope help expose tissue for removal.
Demonstration of the Nesbit technique. Extravesical (second) and apical (third) portions. Inserting a finger in the rectum and tilting the resectoscope help expose tissue for removal.
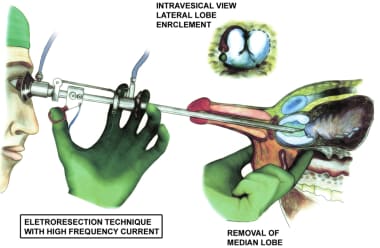 The Iglesias resectoscope used for the median lobe resection. Note resection along the inside margin of the capsule, which seals the perforating blood vessels and makes the rest of the resection relatively bloodless.
The Iglesias resectoscope used for the median lobe resection. Note resection along the inside margin of the capsule, which seals the perforating blood vessels and makes the rest of the resection relatively bloodless.
This encircling maneuver, combined with the intravesical and bladder neck resection performed earlier, results in the bulk of the 2 lateral lobes falling onto the floor of the prostatic fossa. In addition, they are essentially devascularized and can be resected easily with minimal bleeding. The bulk of the prostate tissue is resected during this part of the procedure.
The remaining tissue in the posterior lobe is then resected. Nesbit recommended the use of a finger in the rectum to aid in judging the relative thickness of the remaining tissue; this remains a good idea (see the image below).
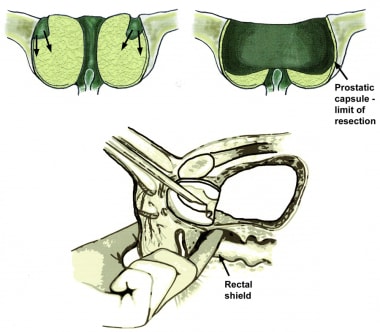 The prostatic capsule is the lateral limit of resection. Surgical removal usually starts at the proximal end. Tissue around the verumontanum is saved until the end. Inserting a finger in the rectum to lift the bladder neck and prostate can be helpful.
The prostatic capsule is the lateral limit of resection. Surgical removal usually starts at the proximal end. Tissue around the verumontanum is saved until the end. Inserting a finger in the rectum to lift the bladder neck and prostate can be helpful.
The final stage of the procedure is the apical stage, in which the remaining apical tissue around the verumontanum is carefully removed (see the image below). Again, Nesbit started the apical portion of the procedure with an anterior resection (12-o’clock position), using the verumontanum as the main landmark.
 Apical resection near the verumontanum. Elevation of the resectoscope, which may require that the surgeon stand briefly, facilitates this portion of the procedure.
Apical resection near the verumontanum. Elevation of the resectoscope, which may require that the surgeon stand briefly, facilitates this portion of the procedure.
The Nesbit technique has a number of significant advantages. By beginning at the roof of the prostate (12-o’clock position) and proceeding laterally along the capsule, the lateral lobes are released to fall posteriorly, making for an easier resection as one moves in that direction.
The main benefit of this technique is early control of the major prostatic blood vessels, starting with the perforating urethral vessels that enter posterolaterally at the bladder neck. This is followed by an encircling resection between the surgical capsule and the lateral lobes. This is intended to isolate and devascularize the bulk lateral lobe tissue, which allows even very large glands to be resected with relatively little blood loss.
Early resection of the bladder neck not only controls many of the blood vessels to the median and lateral lobes, it also helps clearly demarcate the proximal limits of resection and helps avoid subtrigonal tunneling and extravasation. Most of the resection is performed with the scope and the surgeon in a comfortable upright position, resecting from the top downwards. Finally, leaving the apical tissue for last allows the surgeon’s full attention to be directed to this critical surgical area.
The Nesbit technique has a few disadvantages. The procedure involves 3 distinct surgeries, each performed separately. The initial groove at the anterior prostate is made without any real landmarks, at the point where the prostate is thinnest and the risk of perforation is highest. An early perforation at the start of the resection greatly increases fluid absorption, hyponatremia, bleeding, and extravasation, leading to early termination of the surgery and increased morbidity.
For cases in which the lateral lobes are particularly crowded in the prostatic urethra, sufficient room may not be present to accommodate the falling lobes, causing them to bunch together and making further resection more difficult.
Resected grooves do not initially extend the full length of the prostate from the bladder neck to the verumontanum, which causes delays, mandates repositioning, interrupts the rhythm of the surgery, and leaves an incomplete resection of all lobes if the procedure needs to be terminated quickly. Finally, the lateral encircling grooves may uncover bleeders or venous sinuses, which can be awkward to control with interference from the large, bulky lateral lobes that are in the way medially.
Milner resection technique
William A. Milner was a highly skilled and respected urologist who established the Department of Urology at Albany Medical Center in Albany, New York. He received a working model of the Stern-McCarthy resectoscope from Joseph F. McCarthy in 1931 and then returned to Albany, where he developed his technique of TURP. This occurred more than 10 years before Nesbit published his book on transurethral prostatectomy.
In 1941, Milner reported on his first 700 cases, which had a combined mortality rate of only 2.6%. This rate was far superior to any alternate technique of prostate surgery then available. Milner became world-renowned for his exceptional surgical skill and TURP technique, particularly in transurethral resection of larger prostate glands. [20]
Almost 50 years and thousands of TURPs later, Milner personally instructed this author in his method, which differs slightly from the Nesbit technique described earlier. To the author’s knowledge, this is the first time his particular technique for transurethral prostatectomy has been published in the modern era, and it is offered here as a tribute to this highly skilled surgeon and teacher.
The initial resection groove is made at the 9-o’clock position and carried down to capsular fibers. This groove extends from the bladder neck to a point parallel to the verumontanum at the level of the ejaculatory ducts. Coagulation is not used, except for major bleeding sites when the resection has reached the surgical capsule. The groove is first extended upwards toward the 11-o’clock position and then downward toward the 7-o’clock position.
No attempt is made to encircle the bulk tissue. Rather, the intent is to resect the lateral lobe tissue from the inside out quickly and to reach the surgical capsule expeditiously, at which point the perforating and bleeding vessels can be cauterized if necessary. The next section in line is resected until an entire lobe is finished. The process is repeated on the opposite side (see the image below).
 The Milner technique is started with a deep incision directly into the lateral lobe at the 3 or 9-o'clock position and proceeds until the surgical capsule is reached. Further resection is then performed from this starting point.
The Milner technique is started with a deep incision directly into the lateral lobe at the 3 or 9-o'clock position and proceeds until the surgical capsule is reached. Further resection is then performed from this starting point.
When both lateral lobes have been resected, the posterior and median lobes are removed in a manner similar to the Nesbit technique, with one exception. Because Dr. Milner always used a Stern-McCarthy resectoscope working element (a 2-handed instrument), he did not have a free hand available to place a finger in the rectum. Instead, he would resect the posterior lobe to a slightly concave surface, depending on the size and shape of the rest of the prostatic fossa.
The apical tissue around the verumontanum is resected in a similar manner to that described previously. Prostatic tissue located distal to the verumontanum is carefully resected if it appears to be obstructing and if sufficient space is available to avoid injury to the external sphincter.
The final area of resection is the anterior tissue between the 11-o’clock and 1-o’clock positions. The anterior resected surface is left relatively straight and flat to avoid perforation. The risk of perforation in resection of the anterior tissue is relatively high because the prostate is thinnest here and because the absence of a visible distal landmark on the anterior surface risks inadvertent injury to the external sphincter. In addition, the anterior tissue is rarely obstructing; therefore, this area is resected last in case the procedure needs to be terminated early for any reason.
At first glance, the Milner technique seems to violate the main principles of the Nesbit school. By directly attacking the bulk tissue of the lateral lobes without devascularization from the encirclement technique used by Nesbit, additional bleeding might be reasonably expected.
However, when this technique is performed properly, very little blood loss actually occurs. The median lobe tissue has already been removed, if enlarged, which blocks the periurethral vessels. Perforating capsular vessels are cauterized directly. The key is the speed with which the tissue is resected directly perpendicular to the surgical capsule. This allows rapid access to the main bleeding vessels, which can then be easily cauterized and controlled.
The Milner technique has several key advantages. Because the initial groove is made at the point at which the prostatic adenoma is thickest, the risk of perforation or ureteral injury early in the resection is minimal. The initial resected grooves are the full length of the prostate, which speeds the surgery, avoids incomplete resections, and provides a new lateral/distal landmark from the very beginning of the procedure.
Identifying the full length of the surgical capsule early in the case makes visualizing how the rest of the surgery will proceed easier. It also helps avoid perforations of the surgical capsule by providing the surgeon with the full-length lateral border of the resection at the start of the case.
With the Milner technique, completely finishing the resection of one prostatic lobe before starting on the opposite side is much easier. This allows early termination of the surgery if necessary. Resecting the anterior portions of each lobe allows the remaining tissue to then fall into the posterior section, much as in the Nesbit technique. This method avoids the problem of the bulk lateral lobe tissue impeding either the resection or the cauterization of surgical capsular bleeders.
Leaving the anterior prostatic tissue for last minimizes the risk of anterior perforation. It also allows the distal margins of the resection to be established when working on the distal anterior tissue when the primary distal landmark, the verumontanum, is not visible. This margin is established by the distal anterior edge of the already resected adjacent lateral lobes. Finally, it converts the resection into reasonable parts (right side, left side, floor, and roof) compared to Nesbit’s somewhat arbitrary stages (proximal or intravesical, extravesical, and apical).
Other approaches to resection
As mentioned previously, the critical issue is having a plan for the resection and carrying it out in an orderly fashion. This is far more important than where the resection starts or the particulars of the specific technique involved. Still, some of the other methods that have been advocated for TURP surgery and the reasons for their development are notable.
Nathaniel Alcock described his method as starting with one lateral lobe and completely resecting the lower portion from the bladder neck to a point just proximal to the verumontanum. This is supposed to allow the lateral lobe tissue to fall toward the floor of the prostatic urethra. New grooves are then made in the cleft anteriorly, and the upper half of the lateral lobe is then removed.
In practice, Alcock’s technique works reasonably well only for larger prostates in which the lateral lobes are sufficiently heavy to allow them to fall after being undermined. In small prostates, this would cause the lateral lobes to rise superiorly and might make completing the resection more difficult.
Robert Barnes preferred to start his resection at the posterior lobe, which helps guarantee a good flow of irrigation into the bladder. It also allows for a good resection of the posterior lobe, which can be difficult later when the floor of the prostatic fossa is full of clots and resected debris.
Holtgrewe and others suggest a variation of the Nesbit technique, in which the resection begins at the 1-o’clock position with the resectoscope tip positioned in the middle of the prostatic fossa. This is extended clockwise to the 6-o’clock position. A similar maneuver is performed on the opposite side. Once this stage is complete, the resectoscope is repositioned just proximal to the verumontanum and the bulk tissue is resected in quadrants, beginning with the area between the 12-o’clock and the 3-o’clock positions. Apical tissue is removed last. [21]
Other variations include starting the resection at the 4- to 5-o’clock positions and the 7- to 8-o’clock positions, with the intention of blocking the main blood vessels coming into the prostate from the bladder neck area at these points. These and other techniques have been used at some point, and each has their proponents. Most importantly, the surgeon should know how to perform at least one type of TURP well and stick to it.
Posterior lobe resection
Posterior lobe resection is considered important by some and unnecessary by others. A few have recommended starting with the posterior resection so that the surgeon has a clear view without debris, resected prostatic chips, or blood clots in the way. Advocates of this position point out that resection of the posterior lobe removes tissue with the potential for developing cancer and makes the TURP more complete. Others suggest that the posterior lobe is usually not sufficiently enlarged to cause any obstruction; therefore, extensive resection is not particularly important.
Adequate resection of the posterior lobe requires good visualization. This means that if not performed at the start of the case, any resection performed previously needs to be very hemostatic. All prostatic chips should be irrigated into the bladder and evacuated.
An Iglesias working element is usually preferred to allow for digital monitoring of the posterior resection from a finger in the rectum inside an appropriate sheath, which provides tactile sensory feedback to avoid perforation. Performing this resection with the Stern-McCarthy working element is also possible, but this requires greater skill and experience.
The posterior lobe resection is performed in the standard fashion while sweeping the distal end of the resectoscope up and carving a shallow groove with the cutting loop, using a scooping motion to create a concave surface. Relatively thin cuts are made to avoid inadvertent perforation. Care must be taken to avoid any resection or cauterization of the verumontanum. Unroofing of the seminal vesicles may occur. This has no significant sequelae and may be considered proof of an adequate posterior resection.
The depth of the resection is up to the operating surgeon. Certainly, in a difficult or prolonged case, spending too much time on a lengthy posterior resection is not prudent. A more extensive posterior resection is recommended only if visualization is good, the patient is tolerating the procedure without problems, and reasonable time is available. Otherwise, only bulky posterior tissue should be removed.
Tips for resecting larger prostates
The following tips may be useful in the resection of larger prostates.
Practice makes perfect. A relatively inexperienced resectionist may not be able to handle a 100-g prostate by TURP, even after carefully reading this review. Only practice, good technique, and increasing confidence allow the eventual accomplishment of this skill.
Be confident. Initially, performing transurethral resections on prostates substantially larger than 100 g and completing the surgeries in less than 90 minutes of operating time may not seem possible; however, using the tips and suggestions listed here, it can be accomplished safely.
Get comfortable. Use table movements to present the patient and prostate at the most comfortable angle and position. Tilting the table too much initially is not a good idea, but changing the table height and judicious use of the Trendelenburg position can help by bringing the resecting area close to your comfort level. Elbow rests also help stabilize the instrument during the resection. Make sure the patient is at the very end of the cystoscopy table so that deflection of the resectoscope is not restricted by the edge of the table.
Use every possible advantage. Finasteride has been shown to reduce bleeding, particularly in patients with larger prostates. This appears to take approximately 2 months, but even 2 weeks of finasteride therapy may help. If possible, a longer course of therapy is probably better.
Use continuous flow. Continuous-flow techniques have not been conclusively proven to be substantially better than standard, intermittent-flow TURP surgery, but most urologists feel that the clearer vision with fewer interruptions allows more efficient resection. It also helps maintain a lower intravesical pressure, which should lessen fluid absorption and reduce the risk of TUR syndrome and hyponatremia. Continuous-flow resection is particularly useful in patients with very large prostates and those with small or contracted bladders.
Cut with confidence. Failure to perform a perineal urethrostomy when appropriate is one of the most common errors in clinical TURP practice. A perineal urethrostomy allows a larger resectoscope instrument (28F) to be used safely without risking stricture formation. It takes only a few minutes to perform and allows better movement of the resectoscope while reducing the risk of permanent urethral injury and strictures. Meatotomies should also be performed if any question exists about meatal adequacy.
Use only the best equipment. Use equipment with which you are comfortable and experienced. A case involving a very large prostate is not the time to try out a new type of instrument.
Have adequate spares and supplies. Make sure enough irrigating fluid is available to handle a longer than average case. Have spares of critical equipment (eg, a light source and cables, an electrosurgical unit, an electrical cord, cutting loops, telescopes, and a complete spare resectoscope set) immediately available in case a problem arises in the middle of a case. Do not forget to check the instruments yourself just before you start the surgery to make sure all the parts are secure and there are no leaks.
Bigger is better. If possible, use a larger (eg, 28F) resectoscope sheath for bigger prostates. Although performing the surgery with a small sheath is possible and may help prevent strictures, it is easier to resect a markedly enlarged prostate gland with the larger instrument. If the urethra does not allow the sheath to pass with ease, perform a perineal urethrostomy. Make sure the entire resectoscope sheath is adequately lubricated, not just the tip.
Smaller is easier. Avoid cutting off a large chunk of tissue by separating it from the capsule. These freely floating prostatic sections cannot be evacuated through the sheath and must be cut into smaller pieces for removal. This is much easier to do when they are fixed in place in the prostate rather than floating freely in the bladder.
Always have a plan. Always have a plan for the procedure that involves completion of one area of resection at a time. This is even more important for larger glands than for average-sized or small-sized prostates.
Stay focused on the goal. The goal is to remove the important bulk obstructing tissue in the least possible time. Thus, try to spend most of the available resecting time on the bulk tissue of the median and lateral lobes. Do not make the novice’s mistake of wasting time on small, nonobstructing tissue tags or small bleeders at the expense of getting the main bulk tissue resected. This is not a beauty contest, and no prize exists for the most beautiful prostatic fossa.
Relatively little adenomatous tissue is present in the anterior and posterior prostatic urethra; therefore, spending much time on the resection in these areas is not efficient unless it is necessary to the overall surgical plan. If things are going well and time remains at the end, these areas can be resected, but they should be considered optional.
Be generous with hemostasis. While the emphasis on safely performing TURP on a large prostate is speed and efficiency, hemostasis is an area in which extra time and diligence are required and well-spent. Spend extra time checking for bleeding sites that can be cauterized, but try not to waste time on bleeders until the surgical capsule is reached. Avoid cauterizing venous sinuses because they usually cannot be stopped by cautery alone; this also wastes valuable time.
Keep track of the time. Your assistant should inform you when 30 minutes of operating time has elapsed and then should notify you every 15 minutes after that point. This allows you to judge your progress and adjust the surgery accordingly. If the entire prostate cannot be completely resected in the remaining time, concentrate on finishing at least the median lobe (if obstructive) and one lateral lobe. This often works quite well in relieving symptoms, even for patients with very large prostates.
Prostate resection is an art. The secret to safely performing a TURP on a prostate larger than 100 g is the ability to carve and sculpt the prostatic fossa to produce the typical concave cavity, which can only be accomplished by withdrawing the resectoscope as the cutting loop is activated and retracted. The external portion of the instrument must be moved simultaneously to the contralateral side. The movable length of the average cutting loop is only 2.3 cm, while some prostates extend 6-10 cm.
For example, if the right lobe is being resected, activate the cutting loop while withdrawing the resectoscope and angling the instrument’s external portion to the patient’s left side. This creates the internal, curved surface by moving the cutting loop from medial to lateral and back again as it is being withdrawn, thus obviating the need to resect the same area 2-3 times to make a single groove. To do this safely, you must know the landmarks and borders of the resection and the scope’s exact location and orientation to those landmarks.
Know your landmarks. Never resect tissue if the exact location or surgical orientation with regard to the relevant landmarks is uncertain. Taking an extra minute for reorientation is much better than risking permanent incontinence from iatrogenic injury to the external sphincter.
Get into a rhythm. Maintaining a regular rhythm is the most efficient way to perform a TURP. Working steadily with a clear plan and long, smooth strokes over the entire length of the prostate allows for better results and increased surgical capability.
Take your time. This is the single most important rule. If attempts are made to rush or speed up, trouble is inevitable. If bleeding is encountered that is hard to control, remember hemorrhage control techniques.
Know when to quit. Be prepared to quit the resection with very little notice if the patient gets into trouble. If possible, try to finish at least one obstructing lateral lobe.
Check and double-check. Intraoperatively, regularly check laboratory values for patients with very large prostates. Use IV furosemide early if extensive bleeding is present and presumed fluid absorption occurs, as noted by a rise in blood pressure or a decrease in hemoglobin and/or serum sodium. If serum sodium reaches 125 mg/dL or the patient becomes symptomatic from hyponatremia, consider early termination even if the procedure is not complete. Pay extra attention to hemostasis because a return visit to the OR should be avoided if possible.
Take extra care at the end. Be careful when placing the Foley catheter at the end of the case to avoid perforating the prostatic capsule. Make sure the catheter drains easily, with clear fluid return, before concluding the surgery.
Foley catheter insertion is depicted in the video below.
Practice still makes perfect. If first attempts at larger prostates are not totally successful, continue applying these principles to all prostates until sufficient skill is attained. All expert resectionists went through a similar period during which their skills needed to be improved to allow them to handle more demanding cases.
Final inspection and removal of rectoscope
After the completion of the resection, the resectoscope is withdrawn into the bulbous urethra so that the sphincter, verumontanum, and prostatic fossa can be observed. Significant hanging or retracted tissue tags that were previously hidden by the sheath may now fall into view (see the image below). These are seen most easily with the scope inverted to look at the roof of the prostatic fossa.
 Slow withdrawal of the resectoscope at the end of the case sometimes helps demonstrate a flap of tissue hanging down from the roof. These should be carefully resected to avoid a ball-valve effect.
Slow withdrawal of the resectoscope at the end of the case sometimes helps demonstrate a flap of tissue hanging down from the roof. These should be carefully resected to avoid a ball-valve effect.
Prostatic tissue evacuation and resected prostatic tissue are shown in the video below.
These remnants and tags should be resected only if they contain significant prostate tissue. The tags can be physically lifted from the inner bladder surface by the cutting loop before applying current and resecting them. This helps prevent bladder injuries. Mucosal flaps can be left alone because they will slough by themselves. Some tissue tags retract into the bladder from the vesical neck; look for these specifically. They can be seen most easily when the bladder is full, which will rotate the inner lip of the bladder neck outwards and make these tags more visible.
If some additional resection of apical tissue proves necessary, placing a finger in the rectum to elevate the apex is very useful for examination or resection. In addition, standing up straightens the angle of attack on the apical tissue and is sometimes helpful (see image below).
 Apical resection near the verumontanum. Elevation of the resectoscope, which may require that the surgeon stand briefly, facilitates this portion of the procedure.
Apical resection near the verumontanum. Elevation of the resectoscope, which may require that the surgeon stand briefly, facilitates this portion of the procedure.
In very large prostates, the prostatic apical tissue bulges distal to the verumontanum before the inferior margin of the external sphincter curves proximally. Some consider the resection incomplete unless this tissue, located somewhat distal to the verumontanum, is removed. Any surgery in this area must be performed with great care to avoid injury to the external sphincter.
The most common area of injury to the external sphincter is at the 12-o’clock position, where direct visualization of the verumontanum is not possible. Therefore, be extremely careful at the distal surgical margins, particularly when resecting at the 12-o’clock position opposite the verumontanum, to avoid inadvertent damage to the external sphincter.
The resectoscope is then reintroduced into the bladder and prostatic fossa to be absolutely certain that all bleeding sites have been controlled. All arterial and venous bleeders of significance are electrocoagulated, except for the venous sinuses, which cannot be controlled by cautery.
One can safely assume that adequate hemostasis has been obtained when the irrigant flowing out of the bladder is completely clear. Any persistent hematuria suggests a bleeding vessel that needs cauterization. A few extra minutes spent on hemostatic control at the end of the procedure is time well spent and may avoid an unplanned return to the operating room.
Some patients maintain a lower blood pressure during the case. When their blood pressure returns to normal, additional bleeding occurs. Sites that were not actively bleeding earlier now start to lose blood. Having the anesthesiologist allow the patient’s blood pressure to rise to his normal level and reinspecting the prostatic fossa for additional bleeding sites is useful before terminating the procedure.
An Ellik evacuator (see image below) or similar device is then used to remove all the prostatic chips and large blood clots from the bladder. This final irrigation and evacuation should be performed gently to avoid disrupting blood clots forming on bleeding sites.
Before the resectoscope is removed at the end of the case, the bladder is given a final inspection to make absolutely sure no residual prostatic chips are present, and the prostatic fossa is given one final look to be certain that no treatable bleeders remain. A useful tip for finding small bleeding vessels at the end of the case is to slow down the flow of irrigation. This allows even a small bleeding vessel to become visible for cauterization. Irrigation from the catheter should be absolutely clear before taking the patient off the operating room (OR) table.
A laceration or perforation may be located in the posterior bladder wall that is not easy to find unless specifically sought. If a suprapubic trocar or tube has been placed, this should also be routinely examined cystoscopically to make sure any bleeding is controlled.
Any bladder diverticula should be carefully inspected to make sure no resected prostatic chips are hiding inside that could clog and block the postoperative Foley catheter. The bladder is partially filled with fluid to facilitate introduction of the catheter. The resectoscope is then removed.
Special Considerations
TURP for intractable prostatitis
A radical TURP is the complete removal by transurethral resection of all the prostatic tissue possible. While similar to a standard transurethral resection (see Technique), a radical TURP requires superior transurethral surgical skill; takes more time; is more difficult to perform; and carries slightly greater risks of perforation, extravasation, dilutional hyponatremia, and bleeding.
The radical TURP is performed by extending the limits of resection laterally and circumferentially to the prostatic capsule until all visible prostatic tissue has been removed. The resection is continued posteriorly until no more prostatic tissue can be felt by the surgeon’s guiding finger in the rectum. The use of continuous-flow irrigation and drainage, allowing an uninterrupted resection under low hydrostatic fluid pressure, is recommended for this surgery.
Radical TURP has been used with some success for treating chronic prostatitis, especially in otherwise intractable cases. An overall improvement has been reported in approximately 33% of patients treated, and the remainder are generally no worse. A success rate of up to 67% has been reported in patients with documented chronic bacterial prostatitis who underwent the procedure. However, the incidence of incontinence and impotence is significantly higher after radical TURP compared to standard TURP.
Decaestecker et al conducted a retrospective analysis to assess the outcomes and side effects of TURP in the treatment of recurrent acute bacterial prostatitis. [22] They identified 23 cases from 2004 to 2013 in which TURP was performed for this indication in 21 patients; 2 of the patients in their cohort underwent the procedure twice. During a follow-up period of from 3 to 108 months (median, 44 months), 12 patients became free of symptoms, 2 became disease-free after expereriencing postoperative attacks (of those 2 patients, 1 experienced such attacks twice); and 8 were not cured and experienced rapid recurrences. For 3 patients, the follow-up period was of only several months' duration. One of the patients for whom the procedure failed developed orchiepididymitis shortly after the procedure, and another developed orchiepididymitis 1 year later. No incontinence or bladder neck contracture was noted. The investigators concluded that TURP is an acceptable procedure for the treatment of refractoryrecurrent bacterial prostatitis. [22]
TURP for palliation in prostate cancer
Theoretically, entirely removing all prostatic cancer tissue should be possible using a radical TURP if the procedure is skillfully and completely performed in selected cases in which the prostate cancer is well localized some distance from the prostatic capsule. While practiced by a few urologists, no published data are available regarding the efficacy of radical TURP as a potentially curative therapy for prostate cancer. Therefore, TURP is not currently considered a definitive therapy for prostate cancer, but only a palliative measure for relief of obstructive symptoms.
In patients with known prostate cancer, the risk of urinary incontinence and bleeding after TURP is generally higher because of their underlying disease. During a TURP, arterial vessels in prostate cancers are not able to contract the way they normally do in patients with benign prostatic tissue. More fibrinolysins are released from cancerous tissue during surgery, which also increases bleeding.
However, a review of more than 3000 cases by Crow et al failed to show any significant difference in overall complication rate or hospital stay after TURP in patients with prostate cancer compared to patients with benign disease. [23] These findings may be due to patient selection bias, relatively minimal or clinically insignificant cancers, or alterations in the standard TURP technique in patients known to have prostatic malignancies.
Performing only a limited TURP in patients known to have extensive localized prostate cancer is judicious and prudent. A channel is made through the prostate but is not carried routinely all the way down to the surgical capsular fibers. Resection around or even near the verumontanum is not recommended because of the increased risk of postoperative urinary incontinence in these patients. In patients who have undergone previous radiation therapy to the prostate, the risks of postoperative incontinence and hematuria are substantially increased compared to the average patient undergoing TURP.
Because of the release of fibrinolysins and other chemicals by prostate cancer cells, a bleeding diathesis or coagulopathy is more likely in patients with prostate cancer who undergo transurethral surgery compared to the general population. Epsilon aminocaproic acid (Amicar) is often helpful in these patients, particularly in cases in which diffuse bleeding is present and no specific vessels can be identified. Pretreatment with hormonal agents may be helpful in reducing bleeding in this patient population.
TURP should be avoided immediately after definitive radiation therapy for prostate cancer because of the high incidence of intractable postoperative incontinence. At least 3 months should separate radiation completion and TURP, but 6 months or longer is preferred, especially after brachytherapy (radioactive seed implantation). Allowing time for prostate shrinkage following definitive radiation therapy often obviates the need for surgical intervention.
TURP should also be avoided prior to planned radiation therapy. Hormone therapy can usually shrink the prostate adequately for voiding and improves the efficacy of the radiation. In cases in which the patient has undergone recent prior TURP and is planned for prostate/pelvic radiation, the radiation therapy should be delayed until at least 4 weeks after the TURP has been completed. Postponing the radiation therapy for 6-8 weeks may help to minimize bladder neck contractures and incontinence.
TURP should not be performed in patients in whom brachytherapy (radioactive seed implantation) or cryotherapy is being considered. Hormone ablation therapy and alpha-adrenergic blockers may be sufficient in these patients to avoid the need for a TURP.
Clinically significant tumor dissemination does not appear to occur from TURP, although the literature suggests that patients with prostate cancer and obstructive symptoms are more likely to have higher-stage disease. Disease progression and mortality rates in patients treated with TURP are similar to those in patients not treated with TURP who are matched for pathological stage and histological grade.
Repeat TURP for staging appears to be unnecessary except in the rare circumstance in which a small, localized, high-grade tumor is found and substantial tissue is left behind after an incomplete initial TURP.
Surgical Alternatives to TURP
Surgical alternatives to TURP are designed to decrease blood loss, inpatient hospitalization, and fluid absorption, while still removing or destroying the obstructing prostatic tissue. These include transurethral vaporization of the prostate (TUVP), bipolar TURP, photoselective vaporization of the prostate (PVP), and holmium laser enucleation.
Reports comparing these various prostatic ablative techniques by Van Melick et al, [24, 25] Eaton and Francis, [26] and Gilling et al [27, 28] show that they all demonstrate improvement that is roughly equivalent to TURP in terms of urodynamics, symptom scores, and uroflowmetry parameters for at least 7 years.
Noble and colleagues compared these techniques in a randomized, controlled trial from a strictly economic point of view. They concluded that noncontact laser therapies tended to be the most costly surgical treatment option, while TURP was the most cost-effective. [29]
Electrovaporization of prostate
Electrovaporization of the prostate (ie, TUVP, electrical vaporization, or VaporTrode method) uses a ridged or pitted cylindrical metal roller electrode instead of the standard wire loop. This roller electrode conducts the electrical cutting current at very high energy levels, resulting in complete vaporization of the prostatic tissue it contacts. This method results in relatively good hemostasis with less bleeding and fluid absorption than the standard TURP.
Overall results are very similar to TURP, although flow rates are not quite as good and postoperative irritative symptoms are reported more frequently. Electrovaporization must be performed relatively slowly, which tends to limit the size of the gland that can be treated, and little or no tissue remains at the end of the procedure for pathological analysis. Newly developed modified vaporization/resection electrodes offer the opportunity to treat larger glands with electrovaporization and still retain sufficient tissue for pathological examination.
Bipolar TURP
Bipolar TURP uses electrosurgical resection, but cutting energy is delivered in a bipolar fashion rather than the straight monopolar current used in the traditional TURP. When the cutting current is applied, a plasma corona or field of ionization is formed between them, disrupting the molecular tissue bonds and effectively cutting the tissue. The original bipolar wire loops used two parallel wires spaced about 2 mm apart, but current models now use a single wire loop with the electrical ground return built into the shaft of the loop.
The instrumentation and surgical TURP technique are virtually identical, except that a special bipolar electrical generator is required, along with specially modified loops (slightly more expensive than standard monopolar TURP loop electrodes) and resectoscopes. A fluid warmer is recommended, particularly with bipolar instrumention, not only because it increases patient safety by preventing hypothermia, which can occur with longer procedures, but also because warmed saline for irrigation decreases the lag time and allows for faster cutting.
The main advantage of bipolar TURP is increased patient safety because saline irrigation is used, which virtually eliminates TUR syndrome and dilutional hyponatremia. It also allows larger prostates to be resected without the usual time limitations. Indeed, in a prospective, nonrandomized study comparing bipolar TURP with monopolar TURP in patients with BPH, Madduri et al found a lower incidence of bleeding and dilutional hyponatremia with the bipolar procedure, with the mean reduction in postoperative serum sodium concentration being 0.99 mEq/L for patients treated with bipolar TURP, and 3.60 mEq/L for those treated with monopolar TURP. The investigators also found that bipolar TURP was used to treat prostates with a significantly greater mean size than those that were operated on with the monopolar technique. [30]
When used for bladder tumors, the bipolar instrumentation greatly reduces the risk of an obturator reflex because the electrical effect is directed superficially and away from any deep tissue or nerves.
Collateral and penetrative tissue damage is reduced compared to standard monopolar TURP surgery. This is because the electrical energy is directed between the wire loop and its shaft within the resectoscope (bipolar method) rather than into the peripheral tissue from the active wire loop electrode to the grounding pad (monopolar TURP). This explains why less granulation tissue is formed with bipolar instrumentation.
Additionally, less tissue charring occurs, which helps in identifying the surgical capsule and other landmarks. The resected prostate chips are cleaner and have less coagulation or desiccation defects, which simplifies pathological examination.
The movement of the wire loop in the bipolar instrument is somewhat slower compared with standard TURP, but hemostasis is perhaps slightly better; consequently, the total surgical time is approximately the same. Direct pressure on a bleeding site is the recommended technique while a coagulating current is used with bipolar technology. Overall intraoperative and postoperative bleeding, hospital stay, and late complications are essentially identical between the 2 technologies.
Photoselective vaporization of prostate
PVP uses a high-power potassium-titanyl-phosphate (KTP) laser, also called the “greenlight” laser. KTP laser energy at 532 nm is highly absorbed by oxyhemoglobin and only penetrates 1-2 mm deep into the prostatic tissue, making it theoretically superior to other types of prostatic laser vaporization procedures. Modern KTP lasers produce 80 watts or more of average power and 240 watts of peak power, which vaporizes the prostatic tissue fairly rapidly. The limited tissue penetration, compared to Nd:YAG lasers, minimizes the adverse effects.
The PVP procedure has excellent hemostasis because the blood vessels are rapidly sealed by the KTP laser energy. No tissue is available for analysis, but the procedure can be performed relatively quickly. It can safely be performed even in patients who cannot be taken off their anticoagulant medications, which is a major advantage over TURP. In the hands of skilled operators, typical operating times are less than 1 hour for glands up to 100 g.
Holmium enucleation of prostate
Holmium enucleation of the prostate uses holmium laser energy to carve out the two lateral lobes of the prostate in an endoscopic version of an open enucleation. The tissue removed is generally too large to be removed through the resectoscope; therefore, a tissue morcellator must be introduced and the tissue, floating free in the bladder, must be captured and fragmented, while avoiding contact between the morcellator and the bladder wall.
This method offers good hemostasis and allows tissue to be saved for histological evaluation. However, it is very technically challenging and can be quite time-consuming.
Minimally Invasive Procedures for BPH
Minimally invasive surgical therapies for BPH, such as free-beam laser therapy, radiofrequency ablation, transurethral needle ablation, prostatic urethral stents (UroLume), and alcohol injection, are relatively simple procedures that can usually be performed in an outpatient setting, often with decreased postoperative catheterization time.
For patients at very high medical risk who cannot safely undergo significant anesthesia or surgery, these minimally invasive treatments may offer some benefit. Prostatic urethral stents, for example, have been suggested as a reasonable BPH treatment alternative when medical therapy has failed and the medical risks of surgery are unacceptably high. Stent migration, dysuria, and pain are relatively common complications but are easily reversible with stent removal.
These minimally invasive methods do not allow tissue to be saved for pathological analysis and do not remove the entire adenomatous prostate; thus, retreatment and even TURP is sometimes required later. Up to 25% of patients who receive these minimally invasive treatment alternatives ultimately undergo a TURP within 2 years.
Post-Procedure
Postoperative Physical Therapy
Urinary incontinence is a common complication following prostate surgery. Pelvic-floor exercises are usually recommended, but there is no evidence to support their effectiveness. A review of 2 randomized trials of incontinent men postsurgery compared one-to-one sessions with a physical therapist to standard care and lifestyle advice only. High rates of incontinence persisted in both groups after 12 months. Pelvic floor muscle training after prostate surgery is unlikely to be effective. [31]
Guidelines
Guidelines Summary
The American Urological Association (AUA) updated its guideline on surgical management of lower urinary tract symptoms (LUTS) attributed to benign prostatic hyperplasia (BPH) in 2020. Recommendations are listed below; unless otherwise specified, recommendations are based on clinical principles. [32]
Evaluation and Preoperative Testing
The initial evaluation of patients presenting with bothersome LUTS possibly attributed to BPH should include the following:
-
Medical history, utilizing the AUA Symptom Index (AUA-SI)
-
Physical examination
-
Urinalysis
Prior to surgical intervention for LUTS attributed to BPH, clinicians should do the following:
-
Consider assessment of prostate size and shape via abdominal or transrectal ultrasound, cystoscopy, or by preexisting cross-sectional imaging (ie, magnetic resonance imaging [MRI]/ computed tomography [CT])
-
Assess post-void residual (PVR)
-
Consider uroflowmetry
-
Consider pressure flow studies when diagnostic uncertainty exists
Surgical Therapy
Surgery is recommended for patients with any of the following resulting from BPH:
-
Renal insufficiency
-
Refractory urinary retention
-
Recurrent urinary tract infections (UTIs)
-
Recurrent bladder stones or gross hematuria
-
LUTS, in patients unresponsive to, or unwilling to use, other therapies
Clinicians should not perform surgery solely for an asymptomatic bladder diverticulum. However, consider evaluation for bladder outlet obstruction.
Surgical approaches for men with LUTS attributed to BPH are listed below.
Transurethral resection of the prostate (TURP):
-
Offer TURP as a treatment option.
-
Use a monopolar or bipolar approach to TURP, depending on clinician expertise with these techniques.
Simple prostatectomy:
-
In patients with large prostates, consider open, laparoscopic or robotic assisted prostatectomy, depending on clinician expertise with these techniques.
Transurethral incision of the prostate (TUIP):
-
Offer TUIP as an option for patients with prostates ≤30 g.
Transurethral vaporization of the prostate (TUVP):
-
Bipolar TUVP may be offered.
Photoselective vaporization of the prostate (PVP):
-
Consider PVP as an option using 120W or 180W platforms.
Prostatic urethral lift (PUL):
-
Consider PUL as an option in patients with prostate volume < 80 g and verified absence of an obstructive middle lobe.
-
PUL may be offered to eligible patients concerned with erectile and ejaculatory function.
Transurethral microwave therapy (TUMT):
-
TUMT may be offered, but patients should be informed that surgical retreatment rates are higher with TUMT than with TURP.
Water vapor thermal therapy:
-
Water vapor thermal therapy may be offered if prostate volume is < 80 g; however, patients should be informed that evidence of efficacy, including longer-term retreatment rates, remains limited.
-
Water vapor thermal therapy may be offered to eligible patients who desire preservation of erectile and ejaculatory function.
Transurethral needle ablation (TUNA):
-
TUNA is not recommended.
Laser enucleation:
-
Consider holmium laser enucleation of the prostate (HoLEP) or thulium laser enucleation of the prostate (ThuLEP), depending on clinician expertise with either technique, as prostate size–independent treatment options.
Aquablation:
-
Aquablation may be offered, provided prostate volume is between 30 and 80 g.
Prostate artery embolization (PAE):
-
PAE is not recommended outside the context of a clinical trial.
Medically Complicated Patients
HoLEP, PVP, and ThuLEP should be considered in patients who are at higher risk of bleeding, such as those on anticoagulant drugs.
-
Hugh Hampton Young. Image courtesy of the William P. Didusch Museum of the American Urological Association.
-
Cold-cut prostatic punch designed by Hugh Hampton Young in 1909. This instrument was used blindly, without any telescope or coagulation. The hollow inner sheath has a sharpened circular tip that cuts any tissue caught within the fenestration when the inner sheath is advanced. Image courtesy of the William P. Didusch Museum of the American Urological Association.
-
Davis-Bovie generator of the 1930s. Image courtesy of the William P. Didusch Museum of the American Urological Association.
-
Reinhold Wappler. Image courtesy of the William P. Didusch Museum of the American Urological Association and ACMI.
-
Original 1932 Stern-McCarthy resectoscope with rack-and-pinion working element.
-
Reed M. Nesbit. Image courtesy of the William P. Didusch Museum of the American Urological Association.
-
Demonstration of the Iglesias resectoscope, with the free hand allowing a finger in the rectum to elevate the floor of the prostate.
-
Benign prostatic hypertrophy of the lateral and median lobes. Various configurations.
-
Basic anatomy of the prostate, sagittal section.
-
Anatomy of the prostate and bladder, posterior view.
-
Anatomy of the prostate and urethra.
-
Blood supply to the prostate.
-
Blood supply to the prostate. Note the two main branches: urethral and capsular.
-
The male urethra.
-
Technique of perineal urethrostomy. Incision is made over sound (A-B). Optional stay sutures used to help open urethra (C-D). Wound closed in layers with absorbable material (E-F).
-
Median lobe resection. Loop without current is used to gently lift the posterior flap of the median lobe tissue now lying on the bladder surface. Resection can now be performed without risk of bladder injury.
-
Perforation at the inferior bladder neck can easily occur if the line of resection is too straight or is not carved to fit the shape of the prostate in this area.
-
Filling the bladder usually helps locate the bleeding vessels on the interior bladder neck. Limited resection of a covering lip of tissue is sometimes necessary, as illustrated here.
-
Apical resection near the verumontanum. Elevation of the resectoscope, which may require that the surgeon stand briefly, facilitates this portion of the procedure.
-
Slow withdrawal of the resectoscope at the end of the case sometimes helps demonstrate a flap of tissue hanging down from the roof. These should be carefully resected to avoid a ball-valve effect.
-
Beware of resecting folds of tissue that may build up in front of the edge of the beak of the resectoscope because this may cause a perforation. Additional urethral dilation or use of a smaller resectoscope sheath can help prevent this from occurring.
-
Resection of the prostate at the bladder neck. With a finger in the rectum for guidance, the loop without current can be used to lift a flap of prostatic tissue prior to cutting. This helps avoid perforation and subtrigonal tunneling.
-
Resection of an enlarged median lobe allows the lateral lobes to come closer together.
-
Demonstration of the Nesbit technique. First cut at the 12-o'clock position, intravesical portion.
-
Demonstration of the Nesbit technique. Extravesical (second) and apical (third) portions. Inserting a finger in the rectum and tilting the resectoscope help expose tissue for removal.
-
The Iglesias resectoscope used for the median lobe resection. Note resection along the inside margin of the capsule, which seals the perforating blood vessels and makes the rest of the resection relatively bloodless.
-
The prostatic capsule is the lateral limit of resection. Surgical removal usually starts at the proximal end. Tissue around the verumontanum is saved until the end. Inserting a finger in the rectum to lift the bladder neck and prostate can be helpful.
-
The Milner technique is started with a deep incision directly into the lateral lobe at the 3 or 9-o'clock position and proceeds until the surgical capsule is reached. Further resection is then performed from this starting point.
-
An Ellik evacuator is used to remove resected prostate chips from the bladder.
-
Inserting a finger in the rectum (A) and applying external suprapubic pressure (B) can assist in locating and coagulating bleeding vessels by altering the orientation of their flow and bringing the bleeding sites into view.
-
Appearance of a bleeding vessel at the bladder neck and the vascular supply of the prostate.
-
Transurethral resection of the prostate. Preparation, positioning, instruments, and cystoscopy. Video courtesy of Dennis G Lusaya, MD, and Edgar V Lerma, MD.
-
Transurethral resection of the prostate. Right lateral lobe resection. Video courtesy of Dennis G Lusaya, MD, and Edgar V Lerma, MD.
-
Transurethral resection of the prostate. Left lateral lobe resection. Video courtesy of Dennis G Lusaya, MD, and Edgar V Lerma, MD.
-
Transurethral resection of the prostate. Apical resection. Video courtesy of Dennis G Lusaya, MD, and Edgar V Lerma, MD.
-
Transurethral resection of the prostate. Prostatic tissue evacuation and resected prostatic tissue. Video courtesy of Dennis G Lusaya, MD, and Edgar V Lerma, MD.
-
Transurethral resection of the prostate. Foley catheter insertion. Video courtesy of Dennis G Lusaya, MD, and Edgar V Lerma, MD.