Overview
Transurethral microwave thermotherapy (TUMT) is one of various procedures used for the treatment of lower urinary tract symptoms (LUTS) due to benign prostatic hypertrophy (BPH) in men. TUMT involves the insertion of a specially designed urinary catheter into the bladder, allowing a microwave antenna to be positioned within the prostate; there, it heats and destroys hyperplastic prostate tissue. An example of a TUMT device is shown below.
The goal of TUMT is to provide a 1-time efficacious treatment of LUTS due to BPH as an alternative to pharmacotherapy, transurethral resection of the prostate (TURP), transurethral needle ablation (TUNA), photoselective vaporization of the prostate (PVP), open prostatic enucleation, or other surgical therapies.
History and development
McCaskey used heat in the form of ultraviolet lamps to treat prostatism in 1921. In 1929, Corbus used diathermy probes for the same purpose.
Yerushalmi and associates reintroduced microwave therapy for prostatic enlargement in 1985 using a transrectal probe to treat patients with BPH who were otherwise poor operative candidates. [1]
The first TUMT clinical trials used a transurethral catheter in a series of ten 1-hour sessions, although the software and instrumentation allowed only a limited and often interrupted delivery of energy to the prostate. Intraprostatic temperatures reached 40-45°C; symptomatic improvement was suggested to be due to destruction of the alpha-adrenergic nerve fibers around the prostate, since an objective improvement of voiding parameters was not observed and histologic studies revealed that prostatic cells were not destroyed.
It has since been shown that prostate cells are not reliably destroyed until temperatures reach 45°C (113°F). The term thermotherapy was therefore coined to describe treatment temperatures above 45°C and hyperthermia for those below this level. However, the urethral-pain threshold was shown to be 45°C; therefore, higher energy and higher temperatures were achieved only with the introduction of urethral cooling during therapy.
Improvement in antennae design allowed better distribution of the energy to the transition zone of the prostate, the main source of adenomatous tissue. With thermotherapy, objective and subjective parameters reflected significant improvement. Histologic examination of specimens revealed cell destruction but no reliable cavitation. Patients invariably had severe prostatic edema and urinary retention, requiring the use of a urinary catheter.
High-energy thermotherapy has since been introduced; it can achieve temperatures greater than 70°C (158°F), causing thermoablation of prostatic tissue. Unlike thermotherapy, high-energy thermoablation causes prostatic cavities, resulting in greater improvement in symptoms and objective parameters. However, unlike with TURP, patients apparently do not notice an immediate improvement but instead experience a gradual change over a period of months.
In patients presenting with urinary retention, TUMT was originally considered to be insufficient therapy. Many of these patients, however, were older, had a larger prostate volume, and had more surgical comorbidities, making this subset more likely to benefit from a minimally invasive option. With the advent of high-energy TUMT, patients are now offered this less-invasive therapy, with a catheter-free rate of 82-91% in selected patients, although most also must continue medical therapy.
TUMT and non-BPH pathology
Microwave therapy may be of value to treat other types of prostate pathology, such as chronic prostatitis, with one study reporting a 25% complete and sustained improvement and a 50% rate of mild improvement in 45 patients. This article, however, will concentrate on the use of TUMT in the treatment of BPH.
For patient education information, see eMedicineHealth's Men's Health Center, as well as Enlarged Prostate.
Other Procedures
Urethral stent
The placement of a urethral stent is a simple alternative in patients with BPH. While placement usually requires only minimal anesthesia, the failure rate is high and more than one third of urethral stents are eventually removed, some with difficulty. Therefore, this should be used with caution.
Transurethral resection of the prostate
The criterion standard for treatment of BPH is TURP. Improvement in voiding symptoms is reportedly 80-90% at 1 year and 60-75% at 5 years. Over this same period, only 5% of patients reportedly needed a repeat resection. Unfortunately, TURP requires general, spinal, or epidural anesthesia, and potential risks include bleeding and absorption of hypoosmotic irrigating fluids, which can cause hyponatremia, hypertension, and mental status changes.
Long-term complications include incontinence (2-4%), urethral strictures (2-20%), and impotence (4.5-30%). The costs of the procedure are high because of operating-room time, surgeon time, and hospital stay.
Six studies with a sufficient number of patients to allow a comparison of TUMT with TURP have been published. Symptomatic improvement and durability was greater after TURP than after TUMT, with a complementary better objective response as measured by maximal flow rate. TUMT yielded a lower incidence of retrograde ejaculation, newly onset erectile dysfunction, TURP syndrome, clot retention, and transfusion requirement.
In four trials (n=499) that compared TUMT with TURP, the response to treatment was similar. The rate of reoperation was significantly higher with TUMT (9.9%) than with TURP (2.3%). During long-term follow-up, incontinence was reported less frequently after TUMT (0.7%) than after TURP (3.9%), as was erectile dysfunction (6.3% vs 11.5%, respectively). [2]
Alpha-blocker therapy
When compared with alpha-blocker therapy, TUMT is associated with a slower symptomatic improvement (6 mo vs 6 wk) of symptoms but better long-term results. Alpha-blockade alone appears to be associated with a higher number of adverse effects.
Relevant Anatomy
The urinary bladder is derived embryologically from the urogenital sinus. Parasympathetic nerves stimulate the detrusor musculature of the bladder to contract, while alpha-adrenergic nerves from the pelvic plexus cause contraction and increased bladder outlet resistance in the prostatic stroma, capsule, bladder neck, and periurethral area.
The prostate, which originates from the mesenchyme surrounding the urogenital sinus, is a compound tubuloalveolar gland whose base abuts the bladder neck and whose apex merges with the membranous urethra at the urogenital diaphragm. The normal adult gland is cone-shaped and has a mean size of 4.4 cm transverse, 2.6 cm anteroposterior, and 3.4 cm in length. The prostate can be divided into zones, with one of the more common classifications based on studies by McNeal, who described anterior, peripheral, transitional, and central zones.
Overview of BPH
Etiology of BPH
The normal prostate is composed of a combination of glandular, stromal, and smooth muscle cells. BPH results from a proliferation of glandular elements and/or fibromuscular (stromal) elements. Unlike prostate cancer, which invariably originates in the peripheral zone of the prostate, BPH occurs in the transitional zone and the periurethral area.
The hyperplastic growth of prostate tissue is believed to be due, at least in part, to stimulation by dihydroxytestosterone (DHT), which is converted from testosterone by the action of 5-alpha reductase in the prostate. The only known risk factors for BPH are aging and intact testes.
Features of BPH lesions
BPH is a nodular regional growth with a variegated gross appearance. Nodules of varying sizes may appear anywhere in the prostate, although they are found more commonly in the transitional zone and periurethral areas. The prostatic capsule acts somewhat as a barrier to outward growth, so as nodules grow, they may compress the urethral lumen. No correlation exists between the size of the prostate or prostatic nodules and urethral occlusion.
Epidemiology of BPH
Adenomatous prostatic growth is believed to begin at approximately age 30 years. An estimated 50% of men have histologic evidence of BPH by age 50 years and 75% are thought to display such evidence by age 80 years. In 40-50% of these patients, BPH becomes clinically significant.
Pathophysiology of BPH
As urethral resistance to urinary flow increases, the bladder is initially able to maintain urinary flow parameters via detrusor muscle hypertrophy. Uncorrected, this initial adaptation leads to the replacement of the smooth muscle cells with collagen, resulting in decreased bladder compliance and, eventually, detrusor failure.
Irritative voiding symptoms, such as frequency, urgency, urge incontinence, and nocturia, severely affect patients' quality of life and perception of health. If left untreated, bladder outlet obstruction could lead to urinary stasis, predisposing patients to urinary tract infections and urosepsis, bladder dysfunction, bladder calculi, and renal failure.
Symptoms of LUTS
The clinical presentation of patients with LUTS is varied. Patients may present with storage symptoms, such as nocturia, urinary frequency, urgency, or dysuria. Obstructive symptoms, such as a weak urinary stream, double voiding, hesitancy, and a feeling of incomplete emptying, are more common when the bladder compensation is compromised. Others may present with urinary tract infections, bladder stones, abdominal pain, or even renal failure. In these situations, the kidneys should be evaluated for hydronephrosis. Patients with microscopic or gross hematuria must be evaluated for urothelial, prostatic, or renal neoplasms.
Symptom score
Symptom scores, such as the International Prostate Symptom Score (IPSS), are commonly used to quantify symptoms and to monitor the response to treatment.
However, studies have failed to document a strong correlation between symptom scores and physiologic changes due to BPH.
Patients may have minimal voiding symptoms that may interfere significantly with the quality of life; conversely, they may have significant voiding symptoms that may not interfere significantly with quality of life.
Indications for TUMT
Candidates for transurethral microwave thermotherapy (TUMT) include persons with moderate to severe voiding symptoms due to benign prostatic hypertrophy (BPH), those with side effects to medical therapy, those in whom medical therapy has failed, and those who choose to not be treated medically.
Contraindications to TUMT
Active urinary infection is a contraindication to urethral instrumentation. Patients should be evaluated for prostate or urothelial cancer when necessary and treated appropriately. Those with neurogenic voiding dysfunction must also be treated appropriately.
Patients with a history of TURP or severe pelvic trauma should not undergo transurethral microwave thermotherapy (TUMT) because of potential alterations in pelvic anatomy.
Patients with glands smaller than 30 g or a prostatic urethral length of less than 3 cm respond poorly to TUMT, as do patients with glands larger than 100 g and patients with a prominent median bar.
Patients with metallic implants, penile prostheses, artificial urinary sphincters, severe urethral stricture disease that prohibits proper probe placement, severe peripheral vascular disease with claudication, or Leriche syndrome should not undergo TUMT. Patients with a significantly decreased pain response should be approached with caution.
Patients who desire future fertility should be cautioned about the potential risk for postoperative retrograde ejaculation and erectile dysfunction.
Patients with pacemakers need clearance from their cardiologists; pacemakers may need to be turned off during therapy. Regardless, performing TUMT in this group should be approached with apprehension.
Hip replacement is no longer a contraindication to TUMT. Acute urinary retention was once thought to be a contraindication to TUMT; however, high-energy TUMT has shown promising initial results in select patients. [3]
Patient History and Physical Examination
All patients considered for TUMT require a thorough history and physical examination. The history of present illness should include the presence, onset, progression, and severity of LUTS. The past medical history should include the patient's urologic history (including sexually transmitted diseases, stones, trauma, and bladder function) along with other concomitant medical problems (eg, diabetes).
Medicines containing alpha sympathomimetics, such over-the-counter cold remedies, may cause symptoms of bladder outlet obstruction and should be withheld when possible. A family history should focus on a history of urologic cancer, and a social history should focus on risks for cancer such as a smoking history and occupational exposure.
The physical examination should be systematic and meticulous. The patient should be evaluated specifically for distended bladder, urethral meatal stenosis, and nodularity. Variables such as rectal tone, prostatic size, consistency, and landmarks should also be assessed.
TUMT-Associated Tests
Complete blood count
A complete blood count (CBC) is not required before TUMT, but it stratifies patient risks.
A platelet count and platelet function studies should be performed if patient history and/or physical examination suggest a qualitative or quantitative platelet deficiency.
Serum chemistries
All patients should be evaluated for renal insufficiency and electrolyte abnormalities.
Patients with a baseline creatinine level greater than 1.7 mg/dL due to obstruction should be considered for methods other than TUMT, such as TURP or open enucleation.
Prostate-specific antigen
Serum prostate-specific antigen (PSA) levels may be measured to help determine if a patient is at risk for prostate cancer.
If clinically indicated or suspected, a prostate biopsy should be performed.
For unknown reasons, patients with a higher PSA level at baseline, but without cancer, may actually respond more favorably to TUMT than will persons with lower PSA levels.
Urinalysis and/or urine culture and sensitivities
All patients must be free of urinary tract infection prior to TUMT.
Transrectal ultrasonography
Transrectal ultrasonography is suggested before performing TUMT to assess prostate size and to evaluate for prostatic pathology.
Patients with prostate volumes estimated to be less than 30 mL or greater than 100 mL respond less favorably to TUMT than do patients with moderately sized glands; consider other therapies for these patients.
Renal ultrasonography
Renal ultrasonography is noninvasive, provides information about renal anatomy, and may demonstrate pathology.
Patients should undergo renal ultrasonography to evaluate for hydronephrosis due to bladder outlet obstruction if they have a history of urinary retention or have an elevated creatinine level.
Patients with hematuria may also benefit from renal ultrasonography to evaluate for renal parenchymal pathology, especially if intravenous pyelography or computed tomography (CT) scanning is contraindicated.
Voiding velocity
The voiding velocity is a noninvasive, but also nonspecific, electronic recording of urinary flow rate.
Patients void into a specially designed funnel that contains an electronic sensor that records urine volume and velocity and plots it against time. Results include a peak flow rate, average flow rate, and total volume voided. This information is not used to evaluate detrusor contractility. Voiding velocity can be used to monitor response to treatment.
For accuracy, the patient should void at least 125-150 mL. Because of variations among voids, a minimum of 2 voids should be collected.
Other causes of a slow stream may include inadequate detrusor contraction or other causes of bladder outlet obstruction.
A man without evidence of obstruction should have an average velocity of 12 mL/s and an average peak velocity of 20 mL/s.
Patients with initially lower flow rates may respond better to TUMT than will patients with higher rates, if their detrusor function is normal.
Postvoid residual
The postvoid residual (PVR), the volume of urine remaining immediately after micturition, is measured with urinary catheterization or ultrasonography.
Patients usually void to completion; however, those with neurogenic bladder or bladder decompensation due to chronic outlet obstruction may retain significant quantities of urine.
The PVR does not necessarily correlate with signs and symptoms of prostatism and does not predict surgical outcome.
Patients with a higher PVR have slightly higher rates of failure of watchful waiting, and they are at an increased risk of complications, such as urinary tract infections and renal failure.
Cystourethroscopy
All patients considered for TUMT should undergo cystoscopy to rule out urethral stricture, to evaluate for the presence of bladder or urethral pathology, to measure prostatic urethral length, and to evaluate prostatic lobe (especially median lobe) size.
Patients with lateral lobe hypertrophy respond much better to TUMT than do those with median lobe hypertrophy or a median bar.
Pressure-flow study
A pressure-flow study simultaneously measures the bladder pressure and flow rate during voiding. It can be performed through a urethral or suprapubic urinary catheter.
Candidates include patients whose voiding velocity and postvoid residual measurements are not sufficient to determine whether poor flow is due to bladder outlet obstruction or to poor detrusor contraction, such as patients with neurologic disease or detrusor failure.
Cystometrography
Cystometrography (CMG) involves the measurement of bladder pressures during filling. Patients are awake and respond to the sensation of filling with a verbal response to the examiner.
Generally, liquids (eg, water, normal saline, contrast) are used for bladder filling, although some use gases (eg, carbon dioxide).
Cystometrography evaluates bladder capacity, the presence or absence of uninhibited detrusor contractions, and estimated bladder compliance. While this may add little information in routine cases, it may be of value in patients with known or suspected neurological impairment.
Patients who have adequate bladder contractions have better outcomes after TUMT than do those with poor detrusor contractions.
Urethral pressure profile
A urethral pressure profile test measures pressures along the length of the urethra. In patients with known or suspected urethral obstruction, this test helps to determine the location of the lesion. This is not necessary in most cases.
Video urodynamics
Video urodynamics involves performing cystometrography or a penile flow study under fluoroscopic guidance, using contrast as the filling medium; anatomic information, in addition to pressure information, is derived.
Video urodynamics should be reserved for complex cases (eg, patients with neurogenic bladder or incontinence) or for cases in which specific sites of obstruction need to be identified.
Patient Preparation
In preparation for TUMT, patients need to be counseled about the therapy’s risks, benefits, alternatives, and expected results. Specific protocols and manufacturer guidelines differ per TUMT machine and must be meticulously followed.
All patients receive appropriate preprocedure antimicrobial therapy, and an appropriate oral analgesic (eg, ibuprofen, ketorolac, morphine) and an anxiolytic (eg, benzodiazepine) may be preadministered. The penis is prepared with an antiseptic solution, and the urethra is anesthetized with 10-20 mL of 1-2% lidocaine gel. The treatment catheter is then positioned properly per guidelines. A rectal probe to monitor temperature (if used) is inserted, and the treatment program is started.
TUMT Equipment
Several machines are currently in use for TUMT in the United States. Some of the more common machines are the Targis and Prostatron (Urologix; Minneapolis, Minn), the TherMatrx Dose-Optimized Thermotherapy system (AMS; Minnetonka, Minn), the Urowave (Dornier MedTech; Kennesaw, Ga), the Prolieve system (Boston Scientific; Natick, Mass), and the ProstaLund CoreTherm (ProstaLund Operations AB; Lund, Sweden; marketed by ACMI). Each machine has its own specifics and guidelines for treatment. (See the images below.)
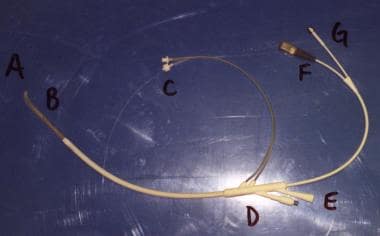 Transurethral microwave thermotherapy catheter. Catheter tip and balloon (A) are shown. Thermosensing unit and microwave antennae (B) are located just proximal to the balloon. These are to be positioned within the prostatic urethra. Coolant-circulating ports (C) are shown. Thermosensor port (D) is shown. Drainage port (E) is indicated. Microwave ports (F and G) are shown.
Transurethral microwave thermotherapy catheter. Catheter tip and balloon (A) are shown. Thermosensing unit and microwave antennae (B) are located just proximal to the balloon. These are to be positioned within the prostatic urethra. Coolant-circulating ports (C) are shown. Thermosensor port (D) is shown. Drainage port (E) is indicated. Microwave ports (F and G) are shown.
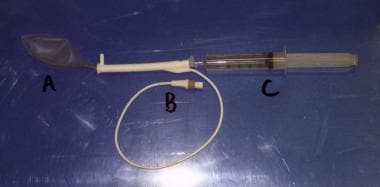 Transurethral microwave thermotherapy rectal thermosensing unit. Rectal balloon (A) is shown with thermosensors. Connection port (B) is shown. A syringe (C) is used to inflate the balloon.
Transurethral microwave thermotherapy rectal thermosensing unit. Rectal balloon (A) is shown with thermosensors. Connection port (B) is shown. A syringe (C) is used to inflate the balloon.
For example, the Targis system is a small, portable machine that delivers power from 0-60 W at a frequency of 902-928 MHz. The 21F catheter contains either a 2.8-cm or a 3.5-cm helical bipolar antenna that provides impedance matching with the prostatic tissue so that thermal energy is delivered with minimal antennae self-heating; the shape of the antenna allows preferential heating at the anterolateral prostate. The rectal thermosensing unit is composed of a balloon with 5 thermosensors that continuously monitors the rectal temperature and provides an automatic shut-down mechanism if rectal temperatures reach 42.5°C (108.5°F).
High prostate-tissue temperatures of 60-80°C (140-176°F) persist throughout therapy while a urethral coolant circulates at 8°C (46.4°F) to maintain the urethral temperature at 39-41°C (102.2-105.8°F) (which is estimated by maintaining the rectal temperature below 42°C [107.6°F]). The device decreases the power in 1-W increments when the rectal temperature reaches 42°C (107.6°F) and in 3-W increments if no response occurs. While the procedure originally took 60 minutes to complete, software and catheters (eg, Cooled ThermoCath, Urologix) are now available that provide equal outcomes in only 28.5 minutes.
In contrast, the Prostatron is a larger machine that uses a monopolar, rather than bipolar, antenna. The initial software program, Prostasoft 2.0, was a low-energy protocol with maximum energy of 60 W; treatment took 60 minutes, and noticeable symptomatic, but not objective, improvement occurred. The higher-energy Prostasoft 2.5 allowed a stepwise increase in energy without interruptions to achieve intraprostatic temperatures of 75°C (167°F) and used urethral cooling with 20°C (68°F) water. The treatment similarly took 60 minutes, and results were better than with the initial software.
With the introduction of even more powerful software, Prostasoft 3.5, only 30 minutes of treatment is required. Compared with Prostasoft 2.5, patients have reported a slightly higher level of pain early in the treatment, due to the initial higher power, but eventually, the same level of comfort is achieved. This protocol is associated with a slightly higher rate of postprocedure urinary retention.
Many of the other systems work similarly overall, with minor alterations. For example, the ProstaLund contains intraprostatic thermosensors to monitor temperatures, the Urowave applicator enlarges during treatment to ensure maximal urethral contact, and the Prolieve system includes a 46F dilating balloon as part of the treatment protocol.
During any TUMT procedure, patients may experience mild perineal warmth, mild pain, and a sense of urinary urgency. Rarely is pain significant enough to require stopping the therapy. Most patients do require some oral analgesics during treatment.
Outcome and Prognosis
Urologists and patients can be overwhelmed with information and results from various instruments. Unfortunately, it is difficult, if not impossible, to truly evaluate the efficacy of one treatment over another. Most TUMT studies involve a limited number of patients, a short follow-up time, or an inherent selection or reporting bias. Therefore, all data must be interpreted with caution.
TUMT has been compared with sham (placing the catheter but not running the program), showing a decrease in symptom scores of an average of 11 points (compared with 5 for sham) at 6 months in 220 patients. Selected groups have a reported significant symptomatic improvement of up to 24 months using the Targis machine, with a similar improvement in quality of life. In these studies, the mean maximum flow rate increased from 7.3 mL/s to 14.5 mL/s at 6 months, remaining stable at 1 year. The mean postvoid residual decreased from 199 mL to 34.8 mL at 6 months, which also remained stable at 12 months. Prostatic volume decreased from 57 mL to 42 mL, and cavitation was observed in 77% of patients. A substantial decrease in voiding pressures occurred. Only 13% of patients required retreatment within 1 year.
The low-energy Prostasoft 2.0 yielded early symptomatic improvement that was not durable and that did not have a complementary objective improvement, with two-thirds of patients requiring supplemental treatment. Higher-energy protocols have resulted in objective flow rate improvement in addition to symptomatic improvement. In 2000, de la Rosette et al reported that, 6 months after treatment with Prostasoft 3.5, the IPSS decreased by 11 points, with an increase in maximum flow rate of 5 mL/s. [4] Indwelling catheter time postprocedure was 18 days. No serious complications occurred.
The ProstaLund Feedback Treatment has been compared with TURP and has not shown inferior outcomes, but selection and reporting bias and a limited number of patients inhibit one from truly being able to use this data in a meaningful manner.
Complications
Primary risks
The main risks associated with TUMT include urinary retention, infection, and postoperative pain. One report cited a 13% risk of infection, an 11% risk of retention, and a 3% rate of acute incontinence. Patients undergoing TUMT are at an increased risk for urinary tract infections compared with TURP, possibly due to necrotic-tissue sloughing or to a catheter left indwelling for a longer duration.
Sexual dysfunction
While changes in sexual function due to the role of the prostate, bladder neck, and local neural tissue are observed in association with all forms of BPH, the overall reported rate of changes in sexual function is 17% with TUMT, compared with 36% with TURP. Retrograde ejaculation is observed in 48-90% of patients after TURP and in 0-28% of patients after TUMT. However, this appears to be a trade-off for better urinary flow patterns after TURP than after TUMT. A study by Marra et al concluded that transurethral microwave thermotherapy along with transurethral incision of the prostate and transurethral needle ablation should be considered for men aiming to maintain normal ejaculation although these treatments provide less symptomatic benefit compared with transurethral resection of the prostate. [5]
Erectile dysfunction is observed after any treatment for BPH. Newly onset or worsening erectile dysfunction after TUMT is uncommon if a patient previously had normal erections. Although causes have not been fully elucidated, psychogenic factors, bladder neck trauma, and neurogenic voiding dysfunction probably play roles. Lower-energy TUMT protocols appear to yield a lower incidence of erectile dysfunction compared with higher-energy protocols, but this occurs at the expense of better urinary results.
In 1997, Francisca et al reported no change in sexual performance after low-energy TUMT, compared with a sham procedure in 147 patients. [6] However, Arai et al reported an 18.2% rate of erectile dysfunction after TUMT using a high-energy protocol. [7]
Overall, patients' satisfaction with their sex lives seems to be higher among those who have undergone TUMT rather than TURP, with 55% of patients who have undergone microwave thermotherapy reportedly being very satisfied, compared with a 21% of patients who have undergone TURP. However, only 27% of this population is satisfied with their urinary flow after TUMT, compared with 74% of patients who are satisfied after TURP.
Acute myocardial infarction
The risk of acute myocardial infarction is not negligible using TUMT. A 3.9-year follow-up study compared 888 TURP patients with 478 TUMT patients; both treatments were associated with a higher incidence of acute myocardial infarction, especially more than 2 years after therapy. The death rate from cardiovascular disease in patients after these therapies was higher than it was in the general population.
Urinary retention
Prostatic edema is expected after microwave therapy, leading to a risk of urinary retention, especially with higher-energy protocols. Early studies on several low-energy protocols reported a 6-36% need for indwelling catheterization for up to 1 month, while 10% of patients undergoing high-energy protocols require catheterization for more than 3 months. Patients with larger prostates are more prone to long-term catheterization because of increased edema. Therefore, many protocols suggest leaving a catheter in for a few days to 2 weeks in all patients.
A slow process of improvement in urinary flow is characteristic of high-energy TUMT. Coagulated tissue must be absorbed, and the treated area must be reorganized before sufficient voiding is achieved. Patients may notice an improvement over a period of many months.
Patients maintained on alpha blockers after TUMT may experience fewer urinary symptoms and have a decreased incidence of retention. Other protocols suggest placing a temporary prostatic bridge catheter to prevent prostatic obstruction immediately after TUMT. Proponents of the bridge catheter describe a potentially decreased incidence of urinary tract infection, compared with an indwelling catheter or clean intermittent catheterization, and a better immediate peak flow rate, symptom score, and quality of life compared with not using a bridge catheter.
Patients should return to the clinic for follow-up. If a catheter is placed, it can be removed at home or in the clinic. All patients should be instructed to watch for an inability to void, painful voiding, high fevers, abdominal pain, or other problems. Posttreatment convalescence is relatively rapid, with most patients able to void and a mean recovery time of less than 5 days at home. This suggests that some patients return to full activity relatively early.
Histologic findings
Unlike with TURP or open prostatectomy, no histologic specimen is obtained with TUMT. Patients with a PSA level within the reference range and negative prior prostatic biopsy findings may still be at risk for clinically silent prostate cancer.
Few studies have evaluated the histologic effect of TUMT on prostatic tissue in vivo. Khair et al performed radical prostatectomies after TUMT in 9 patients with prostate cancer—7 within 1 week, and 2 more than 1 year later. [8] At 1 week, hemorrhagic necrosis and devitalized tissues without inflammation were observed, with necrosis seen in benign, stromal, and cancerous areas without skips. The mean volume of necrosis was 8.8 mL, and the average amount of necrosis was 22%.
One year later, only nonspecific chronic inflammation and desquamated metaplasia with evidence of periurethral fibrosis occurred. The mean volume of necrosis remaining was 0.2 mL, which was less than 1%, implying that cells were sloughed away. No differences were observed between BPH and cancerous elements.
Additional complications
Various other, rare complications have been reported following TUMT. These include, but are not limited to, urethrorectal fistula, bladder perforation, bowel irradiation, chronic pain, urethral injury, prostatitis, a pressure sensation, urinary urgency, urethral tear, anal irritation, urethral stricture, infertility, and retrograde ejaculation. Proper intratreatment physician and nursing observation are vital to decrease these risks.
Efficacy of TUMT
In conclusion, TUMT is a safe and effective, minimally invasive, alternative treatment for symptomatic BPH. TUMT can be performed in a 1- to 2-hour office visit, without intravenous sedation. The procedure therefore is an alternative for patients who are at high surgical and anesthetic risk. It is not effective for patients with a large median lobe or a very large prostate, and it results in less-significant improvement in urinary flow patterns than does TURP.
TUMT appears to balance efficacy against patient tolerability, although this balance might be tenuous for patients long-term.
-
The Targis machine is a small, portable transurethral microwave thermotherapy device.
-
Transurethral microwave thermotherapy catheter. Catheter tip and balloon (A) are shown. Thermosensing unit and microwave antennae (B) are located just proximal to the balloon. These are to be positioned within the prostatic urethra. Coolant-circulating ports (C) are shown. Thermosensor port (D) is shown. Drainage port (E) is indicated. Microwave ports (F and G) are shown.
-
Transurethral microwave thermotherapy rectal thermosensing unit. Rectal balloon (A) is shown with thermosensors. Connection port (B) is shown. A syringe (C) is used to inflate the balloon.