Overview
Background
A radical orchiectomy is one aspect of the definitive treatment of testicular cancer.
Testicular cancer generally affects young men between puberty and age 35 years. The median age at diagnosis is approximately 33 years. [1] Successful treatment incorporates a number of modalities, including radical orchiectomy, retroperitoneal lymph node dissection, chemotherapy, and radiation. [2, 3]
Indications
A radical orchiectomy is indicated in the management of a suspected testicular tumor. A testicular tumor should be suspected in any patient with the physical findings of a painless, firm, and irregular mass arising from the testicle. Confirm this with Doppler ultrasonography of the scrotum.
Most cases of testicular tumors demonstrate hypoechoic hypervascular intratesticular lesions. Elevated levels of alpha-fetoprotein and/or human chorionic gonadotropin should also suggest a testicular tumor (germ cell type).
Contraindications
In some patients with a testicular mass, causes other than a testicular tumor need to be excluded before radical orchiectomy is performed. For example, if a patient presents with testicular enlargement but a history consistent with an orchitis, then antibiotic therapy would be indicated prior to surgery.
Similarly, if a patient with a history of congenital adrenal hyperplasia presents with bilateral multifocal testicular masses, a frozen section biopsy would be indicated at the time of the operation to confirm that the lesions are hyperplastic nodules of adrenal rests rather than multiple germ cell tumors.
A study that assessed the clinical outcome of testicular sex cord stromal tumors (TSCST) according to management and stage reported that testis-sparing surgery may be feasible and effective in case of small tumors. [4] Another study concluded that testis-sparing surgery performed for small testicular masses may represent a safe procedure with optimal results in terms of functional and oncologic end points. [5] A testis-sparing approach may also be appropriate for a prepubertal patient with a testis mass and a normal serum alpha-fetoprotein level if examination of an intraoperative frozen section reveals benign histology. [6]
Outcomes
Seminoma
After treatment with radical orchiectomy and external-beam radiation therapy, the 5-year disease-free survival rate is 98% for stage I tumors and 92-94% for stage IIA tumors. For higher-stage disease that has been treated with radical orchiectomy followed by chemotherapy, the 5-year disease-free survival rate is 35-75%. [7]
Nonseminomatous Germ Cell Tumors
For stage I tumors, which are treated with radical orchiectomy and retroperitoneal lymph node dissection, the 5-year survival rate is 96-100%. [8] For low-volume stage II disease, which is treated with radical orchiectomy and chemotherapy, the 5-year disease-free survival rate is 90%. For bulky stage II disease, which is treated with radical orchiectomy followed by chemotherapy and retroperitoneal lymph node dissection, the 5-year disease-free survival rate is 55-80%.
Scrotal Violation
Inguinal orchiectomy is the standard treatment for suspected testicular carcinoma. Scrotal violations that occur during scrotal orchiectomy, open testicular biopsy, and fine-needle aspiration may compromise the patient’s prognosis. Thus, patients with a scrotal violation often are subjected to potentially morbid local therapies. In addition, surveillance protocols usually exclude patients with scrotal violations.
Much of the current bias in managing scrotal violation stems from a 1925 article by Dean, who reported a 24% local recurrence rate after simple orchiectomy. [9] However, the validity of this report has been questioned, and numerous articles have suggested that scrotal violation may not necessarily confer a worse prognosis.
In particular, 2 articles have addressed this issue. In 1995, Capelouto et al reviewed all published series of patients with testicular cancer in whom scrotal violation occurred. [10] They then performed a meta-analysis to choose a subset on the effect of scrotal violation on patient prognosis for critical analysis. Although the local recurrence rates among the scrotal violation studies contained statistically significant differences, the overall local recurrence rates were minimal (2.9% vs 0.4%, respectively). In all groups analyzed, the rates of distant recurrence and survival were statistically similar.
In 1995, Leibovitch et al published a retrospective review of 78 of 1,708 patients (4.6%) with nonseminomatous testis cancer who presented to Indiana University School of Medicine following scrotal violation. [11] The overall relapse rate among patients with pathological stage A disease associated with scrotal violation was increased. The finding was exclusively attributed to local recurrences in 7.7% of cases following scrotal violation, compared with no local recurrences in patients who underwent standard radical orchiectomy. However, the overall patient survival rate was comparable to that in patients without scrotal violation.
Inguinal orchiectomy and high spermatic cord ligation remain the standard of care for diagnosis and initial management of testicular cancer. Scrotal violation alone, without tumor contamination, does not impart a worse prognosis in patients with testicular cancer. In the absence of gross tumor spillage, close observation alone may be adequate management for scrotal violation. Therefore, consider local or systemic therapies only upon evidence of a local recurrence.
Periprocedural Care
Pre-Procedure Planning
Measure the serum tumor markers alpha-fetoprotein, beta-human chorionic gonadotropin, and lactate dehydrogenase. The serum half-lives of alpha-fetoprotein and beta–human chorionic gonadotropin are 5-7 days and 24-36 hours, respectively; these values are clinically useful in determining when the tumor marker levels can be expected to normalize after treatment.
Perform chest radiography.
Abdominal and pelvic computed tomography scanning is indicated once the diagnosis is confirmed.
Administer cefazolin intravenously at a dose of 1 g 30-60 minutes prior to incising the skin if placement of a testicular prosthesis is being considered.
Patient Preparation
Anesthesia
Administer general anesthesia.
Positioning
Place the patient in the supine position.
Shave the lower abdomen and scrotum. Prepare and drape the penis, scrotum, and lower abdomen.
Monitoring & Follow-up
Postoperatively, the authors recommend bed rest for 24 hours, scrotal fluffs or a jock strap for 2-3 days, and resumption of routine activities after 2-3 weeks.
Follow-up depends on the type of tumor and the treatment used. [12, 13]
Patients With Seminomas
Low-stage seminomas (I, IIA, IIB) are treated with radiation. Higher-stage seminomas (IIB, IIC, III) are treated with chemotherapy.
Every 2 months for the first year and every 3 months for the second year, perform a physical examination, chest radiograph, and evaluation of tumor markers.
Perform a computed tomography scan every 4 months for the first 2 years and every 6 months for a minimum of 5 years.
Patients With Nonseminomatous Germ Cell Tumors Who Elect Surveillance
Perform a physical examination, chest radiograph, and evaluation of tumor markers every month for the first year and every 2 months for the second year.
Perform a computed tomography scan every 3 months for the first 2 years and every 6 months for a minimum of 5 years.
Complications
Complications associated with radical orchiectomy include the following:
-
Bleeding, resulting in scrotal hematoma or retroperitoneal hematoma
-
Infection of the wound
-
Ilioinguinal nerve injury, resulting in hypoesthesia of the ipsilateral groin and lateral hemiscrotum
Technique
Radical Orchiectomy
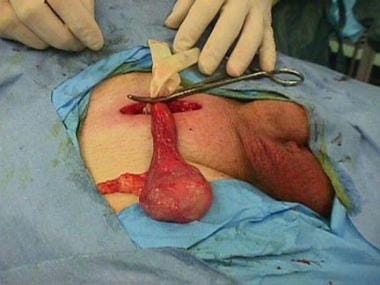
Make an incision in the skin 2 cm superior and parallel to the inguinal ligament, following the line connecting the internal and external rings (see image below).
If the testicular mass is too large to be delivered through this incision, then the incision can be extended toward the scrotum.
Continue the incision through the subcutaneous fat and the Camper and Scarpa fasciae.
Identify the external oblique fibers and external inguinal ring.
Incise along the direction of the fibers from the external inguinal ring.
Identify and mobilize the ilioinguinal nerve and separate it so as not to resect it during the radical orchiectomy.
Take the 2 leaves of the external oblique fascia with snaps and lift them anteriorly in order to dissect free the spermatic cord along its length.
Isolate the spermatic cord at the pubic tubercle. Wrap a Penrose drain tightly around it and secure it with a snap to form a tourniquet.
Stretch the point of entry of the cord into the scrotum with a finger and deliver the testicle into the operative site.
Identify the gubernaculum and then clamp, divide, and ligate.
Confirm the diagnosis with inspection. A frozen section is rarely necessary.
Occasionally, the diagnosis is unclear, even when the testicle has been delivered. In cases of uncertainty, remove the testicle because the risks of replacing a testis tumor outweigh the impact of losing one testicle.
Take the spermatic cord in 2 portions (a vascular portion and the vas deferens) by doubly clamping, dividing, and ligating above the tourniquet. The cord must be ligated as close as possible to the internal ring to facilitate complete removal of cord tissue in case a later retroperitoneal lymph node dissection is required. Leave long silk sutures on the cord to allow identification of the cord stump.
Remove the testicle from the operative field and send it for pathology.
Inspect the wound and especially the scrotum for adequate hemostasis.
At this point, a testicular prosthesis [14] may be placed into the hemiscrotum.
Close the external oblique fascia and external ring in a running fashion, appose Scarpa fascia in an interrupted fashion, and close the skin.
-
Right radical orchiectomy. Note the inguinal approach and the use of the tourniquet.