Practice Essentials
Radical cystectomy is a surgical procedure used to treat muscle-invasive bladder cancer without evidence of metastasis or with low-volume, resectable, locoregional metastases. Muscle-invasive bladder cancer is defined as tumors that invade the muscularis propria.
Signs and symptoms
Gross or microscopic hematuria is the initial presenting sign in 80-90% of patients with bladder cancer. Approximately 20% of patients have irritative symptoms such as urinary urgency, dysuria, or frequency.
See Presentation for more detail.
Diagnosis
Laboratory studies
The following studies are typically included in the workup:
-
Urine cytology
-
Bladder barbotage
-
Liver function tests and bone fraction of alkaline phosphatase
Imaging studies
An appropriate evaluation of hematuria includes radiographic (computed tomography [CT] scanning, ultrasonography, retrograde pyelography, intravenous pyelography [IVP]) or direct imaging (cystoscopy, ureteroscopy) of the entire urinary tract.
A bone scan is indicated if the patient has symptomatic bone pain, elevated calcium levels, or elevated alkaline phosphatase levels.
Magnetic resonance imaging (MRI) is used in some centers for evaluation of both local and metastatic disease; however, its staging accuracy is unknown.
See Workup for more detail.
Management
The criterion standard for the treatment of patients with stage T2-T4 bladder cancer is radical cystoprostatectomy for men and anterior pelvic exenteration for women. In addition, all patients should undergo bilateral pelvic lymphadenectomy.
Patients who undergo radical cystectomy may benefit from a cancer-specific survival advantage when neoadjuvant chemotherapy is given prior to surgery. The rationale of preoperative chemotherapy includes treatment of micrometastatic disease and pathologic downstaging.
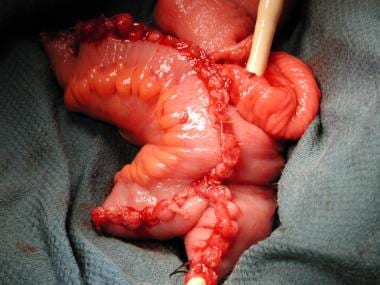
Many methods of urinary diversion following radical cystectomy are possible. These methods can be classified into 3 categories: incontinent urinary diversions, continent cutaneous urinary diversions, and orthotopic ileal neobladders. See the image below.
See Treatment for more detail.
Background
In the United States, bladder cancer is the fifth most common cancer (following lung, colon, prostate, and breast cancers), fourth in prevalence among men and eighth among women. More than 90% of bladder cancers are transitional cell in origin, while, in countries with high endemic schistosomiasis rates (eg, Egypt), squamous cell carcinoma (SCC) of the bladder is more common.
Bladder cancer can be axiomatically subdivided into non–muscle invasive and muscle-invasive disease. This article focuses primarily on the management of muscle-invasive transitional cell carcinoma (TCC) and the role of radical cystectomy. For a more in-depth review of the management of non–muscle invasive bladder cancer, see Bladder Cancer.
Lesions limited to the urothelium (pCIS), mucosa (pTa), or lamina propria (pT1) represent 70-80% of all newly diagnosed bladder cancer cases. Although prone to recurrences and, less commonly, progression to higher-stage disease, these lesions are typically managed with transurethral resection and selectively with intravesical chemotherapy, such as bacille Calmette-Guérin (BCG), mitomycin, or thiotepa. Patients with pT1 disease, particularly those with high-risk features (eg, multifocality, recurrence after intravesical therapy, extensive lamina propria invasion, concomitant carcinoma in situ [CIS]), are at considerable risk of disease progression and may benefit from early radical cystoprostatectomy.
Muscle-invasive bladder cancer, defined as tumors that invade the muscularis propria (pT2 or higher), requires more intensive therapy. To date, surgical resection via radical cystoprostatectomy (bladder and prostate) and pelvic lymph node dissection remain the criterion standard for determining accurate pathologic staging, optimizing curative potential, and minimizing the risk of tumor recurrence.
History of the Procedure
The first record of a radical cystectomy dates to the late 1800s. In 1949, Marshall and Whitmore described the basic surgical principles of radical cystoprostatectomy. In 1987, following the neuroanatomic mapping of the pelvic plexus by Schlegel and Walsh, nerve-sparing cystectomy became a surgical option that allowed for preservation of sexual function. [1]
For many years, radical cystectomy carried a significant perioperative mortality rate (5-10%). However, presumably because of improvements in surgical technique, the evolution of intensive care medicine, and the availability of new antibiotics, radical cystectomy is now a common procedure in major medical centers and carries a perioperative mortality rate of approximately 1-2%. [2] At high-volume centers with postoperative pathway care programs, an intensive care unit (ICU) stay is no longer routine and the median hospital stay is approximately 7 days.
Pathophysiology
As with most neoplasms, bladder carcinogenesis is a complex multistep process that is not fully understood. Activation of proto-oncogenes, loss or inactivation of tumor suppressor genes, and abnormal growth factor or receptor expression have been implicated.
Multiple mutations of chromosome 9 have been identified in superficial bladder cancer cells. Increased expression of the epidermal growth factor receptor and increased mutations of tumor suppressor genes (eg, TP53 and Rb) are common in patients with advanced bladder cancer. Mutations and nuclear accumulation of TP53 have been correlated with an increased grade, stage, and recurrence risk.
The risk of progression to muscle-invasive disease is associated with tumor grade, stage (Ta vs T1), size, number of lesions (solitary vs multiple lesions), previous tumor recurrence, and presence of CIS.
Etiology
Environmental risk factors
Tobacco use accounts for up to 50% of all bladder cancer cases; people who smoke heavily quintuple their risk. Former smokers are at less of a risk for the disease than active smokers. The risk associated with second-hand smoke is unclear.
Exposure to aromatic amines found in some dyes, paints, solvents, leather dust, inks, combustion products, rubbers, and textiles is a risk factor.
Prior radiation therapy is a risk factor. Women who have undergone pelvic radiation (eg, for cervical cancer) have a 2- to 4-fold increased incidence rate. Survival rates are poorer in men who have undergone radiation for prostate cancer than in men of similar age and stage who have not undergone radiation. [3]
Treatment with cyclophosphamide (Cytoxan, Neosar) and ifosfamide (Ifex) may lead to the development of bladder cancer through their metabolite acrolein. Following high-dose cyclophosphamide treatment, the 12-year prevalence of bladder cancer is as high as 11%.
Low daily fluid intake may be a contributing factor; the relative risk in persons who drink 6 cups of water per day is 0.49 compared with that in persons who drink one cup of water per day.
Schistosomiasis caused by the parasite Schistosoma haematobium can cause SCC; this is common in Egypt and the Nile River Valley.
Long-term phenacetin use is a risk factor; this agent is no longer approved for use in the United States.
Long-term placement of indwelling catheters is a risk factor. Patients who have indwelling catheters for longer than 10 years should undergo bladder surveillance via cytology and cystoscopy.
Artificial sweeteners (saccharin, cyclamate), when administered in high doses to laboratory animals, are risk factors for bladder cancer; no similar evidence has been shown in humans.
The use of Aristolochia fangchi, a Chinese herb, has been implicated as a risk factor for both upper and lower tract TCC.
Coffee and tea are not risk factors for bladder cancer.
Epidemiology
United States data
In the United States, up to 600,000 people have bladder cancer. In 2020, an estimated 81,400 new cases of bladder cancer were diagnosed, and 17,980 persons died of the disease. [4]
In 2008, the male-to-female incidence ratio was 2.9:1, and the male-to-female mortality ratio was 2.4:1. [5]
Bladder cancer is more common in whites than in African Americans. The average age at diagnosis is 65 years.
Bladder cancer diagnoses increased by 36% from 1984-1993. More than 90% of bladder cancers are TCC.
Screening of asymptomatic individuals is not currently recommended.
International data
In 1996, an estimated 310,000 new cases of bladder cancer were diagnosed worldwide. The incidence rate in Western Europe and North America is higher than in East Asian countries.
In developing countries, many bladder cancers are SCCs caused by the parasite S haematobium. In high-prevalence regions, SCC of the bladder has enormous health implications (eg, SCC is the most common solid tumor in Egyptian men).
Presentation
Gross or microscopic hematuria is the initial presenting sign in 80-90% of patients. Approximately 20% of patients have irritative symptoms such as urinary urgency, dysuria, or frequency. This presentation is typical in patients with diffuse CIS, which can be confused with a urinary tract infection and can result in a delayed diagnosis.
With the more routine use of cross-sectional imaging, many bladder lesions are incidentally diagnosed. Patients with muscle-invasive disease can present with incidental or symptomatic obstructive hydroureteronephrosis or, less commonly, with metastatic deposits. These factors make bladder cancer a very uncommon incidental finding on autopsy.
Indications
Indications for radical cystectomy include the following:
-
Infiltrating muscle-invasive bladder cancer without evidence of metastasis or with low-volume, resectable locoregional metastases (stage T2-T3b)
-
Superficial bladder tumors characterized by any of the following:
Refractory to cystoscopic resection and intravesical chemotherapy or immunotherapy (up to 71% of such patients may progress to stage T2 within 5 years of initial recurrence [6] )
Extensive disease not amenable to cystoscopic resection
Invasive prostatic urethral involvement
-
Stage-pT1, grade-3 tumors unresponsive to intravesical BCG vaccine therapy
-
CIS refractory to intravesical immunotherapy or chemotherapy
-
Palliation for pain, bleeding, or urinary frequency
-
Primary adenocarcinoma, SCC, or sarcoma
Indications for urethrectomy include the following:
-
Tumor in the anterior urethra
-
Prostatic stromal invasion that is noncontiguous with the primary
-
Positive urethral margin during radical cystectomy
-
Diffuse CIS of bladder, prostatic ducts, or prostatic urethra (a relative indication)
Rarely, radical cystoprostatectomy is indicated for salvage treatment for recurrent prostate cancer or intractable hematuria following primary therapy with radiation.
Contraindications
Contraindications to radical cystectomy include the following:
-
Bleeding diathesis
-
Evidence of gross, unresectable metastatic disease (unless performed for palliation)
-
Medical comorbidities that preclude operative intervention (eg, advanced heart disease, poor pulmonary mechanics, advanced age)
Relevant Anatomy
The bladder is an extraperitoneal muscular urine reservoir that lies behind the pubis symphysis in the pelvis. At the dome of the bladder lies the median umbilical ligament, a fibrous cord that is anchored to the umbilicus and that represents the obliterated urachus. This ligament contains vessels that must be ligated when divided. The ureters, which transport urine from kidney to bladder, approach the bladder obliquely and posterosuperiorly, entering at the trigone. The intravesical ureteral orifices are roughly 2-3 cm apart and form the superolateral borders of the trigone. The trigone consists of the area between the interureteric ridge and the bladder neck. The bladder neck serves as an internal sphincter, which is sacrificed during a radical cystectomy.
In males, the seminal vesicles, vas deferens, ureters, and rectum border the inferoposterior aspect of the bladder. Anterior to the bladder is the space of Retzius, which is composed of fibroadipose tissue and the prevesical fascia. The dome and posterior surface of the bladder are covered by parietal peritoneum, which reflects superiorly to the seminal vesicles and is continuous with the anterior rectal peritoneum. In females, the posterior peritoneal reflection is continuous with the uterus and vagina.
The vascular supply to the bladder arrives primarily via the internal iliac (hypogastric) arteries, branching into the superior, middle, and inferior vesical arteries, which are often recognizable as lateral and posterior pedicles. The arterial supply also arrives via the obturator and inferior gluteal artery and, in females, via the uterine and vaginal arteries. Bladder venous drainage is a rich network that often parallels the named arterial vessels, most of which ultimately drain into the internal iliac vein.
Recent extensive anatomic pathology studies have determined that initial lymphatic drainage from the bladder is primarily into the external iliac, obturator, internal iliac (hypogastric), and common iliac nodes. Following the drainage to these sentinel pelvic regions, spread may continue to the presacral, paracaval, interaortocaval, and paraaortic lymph node chains. For a more detailed explanation of lymphatic drainage, see Treatment.
For more information about the relevant anatomy, see Bladder Anatomy.
-
Mobilizing the lateral peritoneum after transection of the vas deferens with preservation of the spermatic cord.
-
Pelvic lymph node dissection with removal of lymphatic tissue from the genitofemoral nerve to the obturator nerve and from the pelvic sidewall to above the bifurcation of the iliac vessels.
-
Mobilization of the distal ureters into the bladder, taking care to preserve vascular supply. The bladder is lifted to the left; the pouch of Douglas is seen between the bladder and rectum.
-
Exposure and ligation of the lateral pedicle, which extends from the internal iliac vessels over the distal ureter on its way to the bladder.
-
Opening the pouch of Douglas between the rectum and posterior bladder wall.
-
Pelvic ileal neobladder ready for anastomosis to the urethra.
-
The bowel segment of the Indiana pouch includes the entire ascending colon, a small portion of the transverse colon, and about 7-8 cm of the terminal ileum.
-
The colon has been opened on its antimesenteric border, and the terminal ileum has been plicated by application of the gastrointestinal anastomosis (GIA) stapler to reduce the lumen to approximately 16F. A 14F catheter is shown traversing the efferent limb. The ileocecal valve also has been bolstered with 2-0 absorbable sutures.
-
Dissection of the lateral pedicle extending from the internal iliac vessels over the distal ureters. These vessels can be ligated, allowing full mobilization of the ureter down to the bladder.
-
The posterior peritoneum has been entered, and the rectum is retracted to the right. The posterior plane between the bladder and prostate anteriorly and the rectum posteriorly can then be entered using a combination of sharp and blunt dissection; this defines the posterior pedicles.
-
The rectum is retracted down and to the left, and the articulating stapler is applied to the posterior pedicle. Three such applications of the stapler typically advance the dissection all the way to the lateral endopelvic fascia. The apex can then be addressed in a manner similar to a radical prostatectomy.
-
The ureters are anastomosed to the posterior wall of the pouch and are shown from behind the pouch. They are brought through the wall and sutured to the pouch with interrupted absorbable 4-0 sutures. An antirefluxing anastomosis has traditionally been recommended, using the LeDuc technique. Both anastomoses are stented.