Background
Erectile dysfunction (ED), as defined by the International Consultation on Sexual Medicine (ICSM), is the consistent and recurrent inability to acquire or sustain an erection of sufficient rigidity and duration to engage in satisfactory sexual intercourse. [1] ED has a high prevalence, affecting up to 18.4% of men aged older than 20 years. [2]
Despite the relatively high prevalence of ED, our knowledge of this condition had remained limited until the 1970s. Since that time, advances in pharmacology and molecular biology techniques have improved our understanding of penile physiology and the pathophysiology underlying ED. [3, 4, 5] The process of achieving an erection requires coordination between psychological, vascular, endocrine, and neurological pathways, which combine to enable a physiologic response in the penile vasculature. In response to parasympathetic signaling received from the pudendal and pelvic splanchnic nerve plexuses, the penile cavernosal tissue releases nitric oxide (NO). NO induces relaxation of cavernosal smooth muscle through a cyclic guanosine monophosphate (cGMP)-mediated reduction in intracellular calcium. Filling of the cavernosal sinusoids obstructs venous outflow from the cavernosa by compression of the veins against the tunica albuginea, allowing for maintenance of an erection. The transient increase in cGMP into the penis is consequently ended by phosphodiesterase type 5 (PDE5). Detumescence is achieved via adrenergic receptor activation, which leads to rhythmic contraction of the cavernosal smooth muscle, promoting reduction in arterial diameter and inducing venous outflow. [3, 4, 5]
With the understanding of penile pathophysiology, many treatment options for ED have been developed. These treatments include oral phosphodiesterase-5 inhibitors (PDE5i), vasoactive agent administration via intracavernosal injection or intraurethral suppository, vacuum erection devices (VEDs), and implantation of a penile prosthesis (PP). [6] Optimal management of patients with ED requires a thorough assessment and understanding of erectile physiology, vascular and neurological pathophysiology, endocrine effects, the patient and partner’s expectations, drug pharmacokinetics, and surgical techniques.
History
Modern surgical treatment for ED began in the early 1970s when Scott, Bradley, and Timm developed the inflatable penile prosthesis (IPP). [7] This was made possible with the development of silicone, which greatly decreased infection rates with PPs and offered a resilient, biocompatible, and flexible material that is still used in current devices. [8] PP implantation has become the gold standard treatment for patients with medically refractory ED. According to recommendations from the ICSM, the previously held notion that PP is the last resort for ED should be reconsidered, because PP could be the best option for patients, depending on the clinical scenario. [6]
Medical management of ED
In this section, we will briefly cover medical management of ED. A more extensive discussion can be found in the Medscape article Erectile Dysfunction.
Lifestyle modifications
ED is usually a manifestation of generalized vascular disease, so in theory and in practice, it has been shown that lifestyle changes that improve cardiovascular health also have a positive impact on erectile function. [9] These changes include improving diet, increasing exercise, and decreasing tobacco use. [9, 10, 11]
Oral phosphodiesterase-5 inhibitors
Oral PDE5is continue to be the first-line drug therapies for ED. [12] The four main PDE5is that are currently marketed include sildenafil (Viagra), tadalafil (Cialis), vardenafil (Levitra), and avanafil (Stendra). It is important to recognize the side effects and safety profiles of PDE5is to ensure compliance and safety. PDE5is have additive effects on the NO pathway, resulting in their contraindication among patients who take any form of nitrates, as they can cause life-threatening hypotension. The American Heart Association consensus panel advises that nitrates should not be administered within 24 hours of sildenafil use. [13] Common side effects of PED5is include variable effects on olfaction, color changes in vision, vascular tone, insulin action, and platelet aggregation owing to the cross-reactivity of PDE5is with other phosphodiesterase receptors. Headache and gastroesophageal reflux disease (GERD) are also common manifestations of these effects. [14]
Intracavernosal injection therapy and intraurethral suppository
Alprostadil (Caverject Impulse), a synthetic form of prostaglandin E1 (PGE1), is the only US Food and Drug Administration (FDA)-approved intracavernosal injection (ICI) medication for ED. The Medicated Urethral System for Erections (MUSE) is an intraurethral suppository of PGE1 that is administered via the urethral meatus. These are effective alternative methods to deliver erectogenic drugs locally to the penis. Alprostadil works by binding to specific receptors on smooth muscle cells, activating intracellular adenylate cyclase to produce adenosine 3′,5′-cyclic monophosphate (cAMP) and inducing cavernosal smooth muscle relaxation, resulting in tumescence of the penis. [15] Alprostadil can be used alone or in combination with papaverine and phentolamine (better known as bi-mix or tri-mix).
Vacuum erection device
The vacuum erection device (VED) is a mechanical device that can induce an erection by creating a negative pressure environment around the penis. This causes dilation of the cavernous spaces by negative pressure and drawing venous blood into the penis. The constriction ring associated with VED at the base of the penis prevents venous outflow and in turn produces an erection.
Experimental therapies
Regenerative therapies have been used for the treatment of ED, albeit without robust clinical data. These include low-intensity shock wave therapy (LiSWT), stem cell therapy (SCT), and platelet-enriched plasma (PRP). There is currently no FDA approval for these alternative therapies. The Sexual Medicine Society of North America (SMSNA) position statement states that the current studies are limited in value and these therapies should be reserved for clinical trials and not offered in routine clinical practice. [16] The American Urological Association (AUA) guidelines on ED concur that these therapies should be considered experimental. [17]
Indications
A penile prosthesis (PP) is a device that is surgically implanted into the penis. The surgery involves placing PP cylinders into the corpora cavernosa. [18] Patient selection is key for PP success. The patient is considered a good candidate for PP if he has failed nonsurgical management of ED, if he is not a candidate for nonsurgical management, or if he cannot tolerate nonsurgical management. [18] PP remains a highly effective treatment option for select patients with ED. [6] Careful patient selection and meticulous surgical techniques are vital for superior patient outcomes. [19]
Peyronie disease
Patients with concomitant moderate to severe ED and Peyronie disease (PD) benefit from implantation of a PP as treatment for both conditions. [20] It is more challenging to implant in patients with PD owing to more difficult corporal dilation secondary to plaque distortion and fibrosis, as well as owing to residual curvature after implant placement and inflation. [21] In cases of residual curvature after PP implantation, adjunctive maneuvers (in order of increasing complexity), such as manual modeling, tunical plication, plaque incision, or plaque excision with grafting, can be performed to straighten the penis. [22, 23] The main goal of these maneuvers is to reduce curvature to allow for satisfactory intercourse.
Female to male transgender
Gender-reaffirming surgery for gender dysphoria has become more common with increased societal acceptance and encouragement of transgender individuals. [24] Erection is not achieved by the creation of a neophallus alone, as the flaps used to create the phallus lack erectile tissues. Instead, PPs are implanted within the neophallus for erections. [25] Modern conventions suggest that patients should wait at least one year after phalloplasty with stable urethral issues, have a neophallus with protective sensitivity, and not require secondary modification procedures. [26]
Challenges for PP implantation in this setting include the lack of corpora cavernosa, which results in difficulty anchoring the prosthesis, leading to potential malposition. The lack of tunica albuginea also increases the risk of distal erosion in these patients. [27] The neurovascular supply of the neophallus and the significant scarring associated with phalloplasty not only make implantation more challenging but also increase the incidence of infection. Despite these challenges, PP implantation in transgender males with a neophallus has been successful. Up to 60% of original implants were still present at follow-up of PP implantations. [28] New techniques and transgender-specific devices potentially offer improved outcomes in the future. [28]
Types of Penile Prosthesis
Malleable penile prosthesis
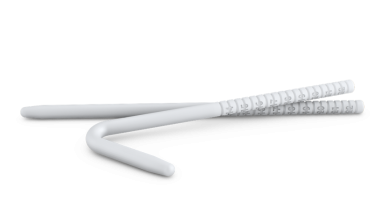
A malleable penile prosthesis (MPP) involves two flexible rods implanted into each corporal body, which can be manipulated to be rigid in an erect state, allowing for penetration, but can be bent to remain functionally and socially acceptable when not in use. [29] The ideal candidates for these devices are patients with limited manual dexterity (eg, those with Parkinson disease) or patients with other significant health issues for whom concealment is not a concern. [30] The devices currently available in the United States include Coloplast Genesis, Boston Scientific Spectra and Tactra, and Rigicon Rigi10 (see the images below). MPPs are used more widely throughout the world than in the United States, mainly because of lower cost. Patients’ satisfaction with the MPP devices is approximately 77%, which is slightly lower than with the inflatable PP. [31] MPPs come in a fixed length, which can be trimmed or lengthened with rear-tip extenders (RTEs), and in various diameters. Because they have comparably lower rates of infections, they are often utilized during revision surgery for infection or for treatment of priapism. [32, 33, 34]
Two-piece inflatable penile prosthesis
The only two-piece PP currently available in the US market is the Ambicor by Boston Scientific (see the image below). This device consists of two inflatable cylinders and a pump with no additional reservoir. It enables an erection in patients with ED and a history of multiple abdominal surgeries, in whom traditional reservoir placement is considered a high risk, or in patients who are averse to reservoir placement but who have sufficient manual dexterity to use the pump. [29] Patients’ satisfaction is higher with Ambicor compared with malleable PPs (90.1% versus 74.2% in one study of 142 patients). [35] In these devices, fluid is located at the posterior part of the cylinders and pump. Inflation is performed by squeezing the pump, which transfers fluid to the anterior part of the cylinders, creating a fully rigid erection. [36] Ambicor comes in three different girths (12.5 mm, 14 mm, and 15 mm) and in standardized lengths (14-22 cm), which can be augmented with the use of RTEs.
Three-piece inflatable penile prosthesis
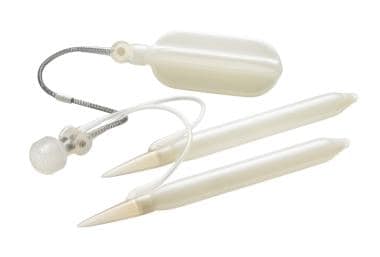
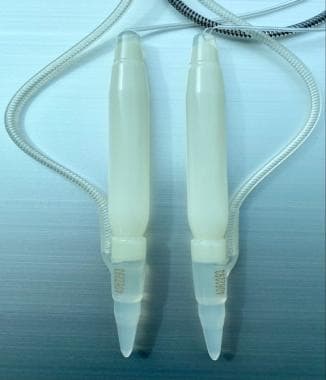
Three-piece inflatable implants offer the most functional similarity to the natural flaccid and erect states of the penis. [7] The three-piece inflatable PPs consist of a scrotal pump that transfers fluid from the reservoir to the cylinders, resulting in an erect state. When the penis is flaccid, the fluid is stored in the reservoir. The reservoir is traditionally placed in the space of Retzius through the medial floor of the inguinal canal at the level of the external inguinal ring. Alternative placement of the reservoir includes submuscular, subfascial, or subcutaneous. [37, 38] These “ectopic” positions avoid the potential complications of placement into the space of Retzius, including bowel, vascular, and bladder injury, while minimizing the increased risk of palpability and reservoir herniation. [39] The available three-piece devices in the United States include Boston Scientific 700-CX, 700-CXR, and 700-LGX, and Coloplast Titan and Coloplast Titan Narrow Base (see the images below). These devices come in standard sizes, which can be adjusted with RTEs.
Patient Selection and Preoperative Counseling
Inflatable PPs have a high satisfaction rate for both patients and their partners, with a 95-99% satisfaction rate reported in a large series. [40] However, they are not the best choice for all patients; careful patient selection is crucial for optimal surgical outcomes and patients’ satisfaction. In addition to ED, factors that must be considered prior to PP implantation include the patient’s past medical and surgical history, overall health status, reasons for the decision to pursue PPs, and postsurgical expectations. [41] The patient’s and their partner’s expectations should be evaluated to ensure that they are attainable and realistic.
Certain patients have a higher risk of suboptimal outcomes and dissatisfactions. Trost et al identified these traits and came up with the mnemonic “CURSED patient,” which stands for Compulsive, Unrealistic, Revision, Surgeon shopping, Entitled, Denial, and Psychiatric. [42] It is vital to identify these patients to establish a healthy relationship and have realistic expectations with them to increase patient satisfaction, thereby reducing physical and emotional complications postoperatively as well as legal ramifications. [42] In addition, polysubstance abusers have been found to have a higher risk of implant infection postoperatively. [43]
An in-depth discussion about indications for PP implantation, preoperative preparation, operative procedure, postoperative care, and expectations of PP function and use needs to be undertaken with patients and their partners prior to surgery. Discussion regarding complications that can occur intraoperatively and postoperatively is also necessary. This should include sensation, appearance, sexual function, decreased postoperative penile length, mechanical life, postoperative pain, infection, injury during surgery, and erosion. [44]
Prior to PP implantation, patients should be free of systemic, cutaneous, and symptomatic urinary tract infections (UTIs). As many men undergoing PP implantation have multiple comorbidities, patients need to be medically optimized. Most anticoagulation therapies are stopped or bridged 3-7 days before surgery, depending on the decision made by the patient’s primary care physician and/or cardiologist. [45] Aspirin 81 mg (also known as "baby aspirin") may be safely continued throughout the perioperative period. [41] Diabetic patients are at increased risk for infection after implantation. [46] As such, optimization of glycemic control is recommended before PP implantation. Antibiotic-coated devices are now the standard of care and should be used when available. [47] Preoperative antibiotics should be administered to all patients with consultation to local antibiograms, as organisms not covered by standard antibiotic regimens have been detected in up to one-third of PP infection cases. [48]
Surgical Steps
The steps that are implemented in PP surgery are reviewed in detail. These include the following:
-
Preparation of operative field
-
Surgical incision
-
Corporal incision and dilation
-
Insertion of prosthesis reservoir
-
Insertion of prosthesis cylinders and pump
-
Connection of device
-
Surgical wound closure
These steps have been carefully analyzed, and checklists have been developed to improve patient safety and surgical performance. [49]
Preparation of operative field
Various antibiotic combinations can be administered intravenously on the day of surgery, prior to PP insertion. The most common antibiotic combination found in a multi-institutional study (consistent with AUA guidelines) was vancomycin and gentamicin. [48, 50] In certain circumstances, adding an antifungal agent is warranted for perioperative coverage. [48] Non-narcotic multimodal analgesia (acetaminophen, gabapentin, and/or meloxicam) administered in the preoperative area prior to anesthesia induction can significantly reduce postoperative pain and reduce postoperative narcotic usage. [51]
Removal of hair follicles in the operating room is performed by shaving (ie, cutting the hair close to the skin with a razor) or by surgical clipping (ie, cutting the hair with hair clippers to leave approximately 1 mm of hair). [52] It is recommended that patients do not personally shave the surgical area prior to arriving for surgery owing to the increased risk of self-induced skin trauma, which can lead to increased bacterial colonization in these trauma-induced areas. [53, 54] Preoperative hair removal from the genitalia cannot be considered the same as removing hair follicles from other parts of the body, because the skin of the genitalia is delicate and elastic with irregular skin folds. Therefore, it is difficult to use surgical clippers on the genitalia without causing unavoidable skin trauma. Furthermore, using razors on the genitalia results in less skin trauma and decreases the likelihood of surgical site infections (SSIs) when compared with surgical clippers. [52, 53]
Preparation of the surgical field by using chlorhexidine-alcohol has been documented throughout the surgical literature, including PP surgery, to reduce the rate of SSIs more effectively than povidone iodine scrub. [54, 55] Based on these data, some surgeons provide patients with chlorhexidine wash in the outpatient setting and instruct patients to wash the surgical area up to several days prior to surgery. A Cochrane review of this specific practice did not demonstrate benefit of preoperative bathing with chlorohexidine wash when compared with preoperative bathing with soap. [56]
Surgical incision and approach
The following three surgical incisions and approaches are utilized for PP surgery:
-
Penoscrotal (PS)
-
Infrapubic (IP)
-
Subcoronal (SC)
Prior to incision, a Foley catheter is inserted to drain the bladder. It is also utilized after the surgical incision to help identify the urethra when exposing the corpora cavernosa.
The choice of approach depends on surgeon’s preference and the patient’s prior surgical history and/or medical comorbidities. Multiple studies have compared the PS versus the IP approach and specifically evaluated operative time, penile length, patient satisfaction, waiting period for initial device activation, infection rate, and urethral injury. [57] There are currently no studies that compare the SC approach with the other PP surgical approaches in the literature. Approximately 60-80% of PP surgeons use the PS approach. [58, 59] When comparing the PS approach versus the IP approach, there are no differences observed in postoperative penile length, patient satisfaction, or infection rate. [57, 58, 59, 60]
The PS approach provides excellent exposure to the penile corpora and spongiosum, facilitating placement of the prosthesis cylinders and pump, especially in patients with complicated penile anatomy and a history of penile surgery. A ventral phalloplasty (also known as a scrotoplasty) can be performed at the time of wound closure to eliminate a redundant amount of scrotal skin, which can create the appearance of a larger phallus without increasing penile length. [57, 58, 59, 60, 61] The disadvantage of the PS approach is the blind placement of the PP reservoir and possible postoperative scrotal complications that can delay the PP activation. There is also a risk of increased scrotal wound dehiscence when performing simultaneous scrotoplasty on diabetic patients. [57, 58, 59, 60, 62]
The IP approach is associated with shorter operative time, quicker activation of the PP device, and less scrotal pain. The disadvantage of the IP approach is increased difficulty with placing the distal penile prosthesis cylinders, increased likelihood of unintentional scrotal PP pump migration, increased difficulty with dilation of the corpora in the obese population with a large pannus, and more potential complications when performing penile prosthesis revision surgeries via the IP approach. Injury of the penile dorsal nerve, which can lead to decreased penile sensation, is also a recognized risk associated with the IP approach. [57, 58, 59, 60, 63]
The SC approach can be performed to obtain more penile exposure when performing concurrent penile procedures (eg, Peyronie curvature correction with plication and/or Peyronie plaque incision and grafting). It is also possible for the SC approach to be performed under local anesthesia, but this is not routinely done in the United States. Investigators report a mean operative time of 53 minutes at a high-volume surgery center (in Korea) utilizing the SC approach. The group does not recommend using the SC approach for PP revisions. [64] This approach usually requires the longest operative time of the three approaches. Recognized and reported complications for the SC approach include sensorineural alterations, skin loss, glans necrosis, and lymphedema. [57, 64, 65, 66]
Corporal incision and dilation
Once the surgical incision is carried down to the tunica albuginea with adequate exposure, stay sutures are placed on the lateral and medial aspects of both the left and right corporal tunica albuginea. Longitudinal corporotomies are made onto the tunica albuginea between the stay sutures at the shortest length necessary for cylinder insertion, which is approximately 1.5 cm. [63, 67] To accommodate the device, the majority of implant surgeons perform corporal serial dilation by using blunt instruments and sequentially increasing in caliber until the proximal and distal corporal space is approximately 12 French in diameter. While dilating each corporal body, it is important to direct the dilators laterally and away from the urethra to avoid urethral injury and/or inadvertent crossover into the adjacent corpora. [63, 67] The measurements of each proximal and distal corpora are then recorded and communicated to the surgical team. The appropriately sized device is prepared on the surgical back table. The left and right corpora should have a similar corporal length, with a difference of less than 1 cm in total length. [41]
Insertion of prosthesis reservoir
The majority of prosthesis reservoir placement is in the extraperitoneal space anterior to the bladder and deep to the pubic bone, referred to as the perivesical space or space of Retzius (SOR). Prior to placement of the reservoir in the SOR, the bladder must be emptied to minimize the risk of bladder injury. The perivesical space is accessed by piercing the transversalis fascia near the medial aspect of external ring and above the pubic bone. With an awareness of the lateral external iliac vessels, blunt dilation into the retroperitoneal space is performed with caution by dissecting medially after entering the perivesical space. [68]
A study measured the distance between the external inguinal ring (EIR) and bladder as well as the distance between the EIR and the external iliac vein (EIV) of cadavers. When the bladder was filled to 200 cc, the average distance from the EIR to the bladder was 2.61 cm. In cadavers, when the bladder was decompressed, the average distance between the EIR and bladder was 6.45 cm. The EIV average distance from the EIR was 3.23 cm. [69]
In another study, researchers measured the distance between the EIR and bladder, as well as the distance between the EIR and EIV, in patients who underwent an MRI scan of the pelvis for prostate cancer workup. These patients did not have any prior surgical intervention or radiation treatment to the pelvis or inguinal region. The average EIR to EIV distance was 3.0 cm, and the average EIR to bladder distance was 1.8 cm, with a weak correlation between bladder volume and distance between the EIR and the bladder. [70] Even though this study did not measure the EIR to bladder distance of each patient when the bladder was filled versus decompressed, it does demonstrate that the distance to the bladder and the reservoir in the SOR is relatively close even when the bladder volume is low.
Ectopic placement of the reservoir is defined as placement anywhere but the SOR, a procedure which has recently gained popularity especially among younger implanting urologists. It is performed for select patients with a history of pelvic surgery or radiation. It is also performed at times based on the surgeon’s preference to avoid injuring the bladder or iliac vessels. The most popular location utilized outside the SOR is the high submuscular (HSM) space (between the transversalis fascia and rectus abdominis muscle), which is 6-8 cm above the external inguinal ring posterior to the belly of the rectus muscle. [68] Investigators have compared reservoir placement with the SOR and HSM approach with cross-sectional abdominopelvic imaging after IPP placement. Compared with SOR reservoirs, HSM reservoirs were significantly less likely to induce a mass effect on the bladder or iliac vessels. HSM reservoirs were also located 5 times farther from the bladder and iliac vessels. [71]
Insertion of prosthesis cylinders and pump
The PP device that is prepared on the surgical back table includes the prosthesis cylinders and the penile pump. For modern devices, the cylinders and the pump are already connected to the device prior to its placement into the corpora cavernosa. Once the appropriate device has been selected and prepared on the back table, the device is soaked in an antibiotic solution if it is a Coloplast Titan three-piece PP device. AMS three-piece PP devices are pre-prepared with a rifampin and minocycline coating called “Inhibizone,” which slowly elutes these two agents into the surrounding microenvironment. [72] The Coloplast Titan has a hydrophilic coating that allows various antibiotic agents to be absorbed in order to also slowly elute the preferred antibiotic into the surrounding tissue and to discourage bacterial attachment and biofilm formation. [72]
Historically, rifampin and gentamicin were originally used as an antibiotic dipping solution for the Coloplast device prior to corporal insertion. With the rise of antibiotic resistance and increased focus on local antibiograms, vancomycin and gentamicin antibiotic dipping solution has recently been reported to have the lowest infection rate compared with other antibiotic dipping solutions. [72] Studies have demonstrated that adding antifungal solution and bupivacaine to the PP antibiotic dipping solution did not diminish the efficacy of the antibiotic solution. Bupivacaine may also improve analgesia during the postoperative period. [73]
Placement of the PP cylinders should occupy the corpora cavernosa anatomy, which extends distally into the proximal portion of the glans penis and proximally near the ischiopubic rami. [74] Inflatable implants require a Furlow tool, a device that can place a Keith needle, which is threaded to the distal portion of the PP implant. The Furlow device guides the threaded Keith needle through the distal corporal body and out of the glans. This allows the distal end of the device to reach the distal aspect of the penis. The proximal end of the implant can usually be seated at the ischiopubic ramus by using the end of a Debakey forceps or hand guidance. [63, 67] It is routine after placement of the cylinders to ensure the device is in the appropriate position by inflating and deflating the device. If placement is appropriate, the corporotomies can be closed.
Owing to the inherent rigidity of the devices, malleable implants are placed into the corporal space via the corporal incisions without the assistance of the Furlow device. Corporotomies for MPPs and two-piece devices need to be larger in comparison with the inflatable three-piece devices. The tubing between the reservoir and the scrotal pump is adjusted, then connected per connector kits provided by the specific PP company. The PP pump is then placed dependently to either the posterior or anterior scrotum.
Surgical wound closure
Wound irrigation and hemostasis of the surgical bed are achieved, and the wound is then closed in multiple layers. The use of closed surgical drains for the scrotum after IPP placement has been debated. The surgical drain is placed to avoid scrotal hematomas in the postoperative period; however, there is concern for increased risk of infection to the prosthesis device with the surgical drain in place. [75] A multi-institutional study found no difference in PP infection rates when patients had a postoperative scrotal surgical drain versus no surgical drain. The penile cylinders are left partially inflated to place pressure on the corpora cavernosa to achieve additional hemostasis. To prevent scrotal hematomas, compression of the scrotum is performed by using a Kerlix scrotal wrap, scrotal support underwear, or other maneuvers. Additional prophylaxis used in the perioperative setting, to help prevent venous thromboembolism (VTE), does not increase the likelihood of scrotal hematomas, if a scrotal surgical drain is also placed. There is also no benefit of pharmacologic over mechanical VTE prophylaxis in the PP perioperative setting. [76]
Postoperative Care
Recent years have seen an increase in the number of PP procedures performed in ambulatory surgery centers (ASC) and a downward trend in PP surgeries requiring inpatient admissions. [77] When patients are scheduled for PP surgery with the intention of it being performed in the ASC, the patient’s medical insurance and/or significant medical comorbidities (eg, significant heart disease, difficulty with anesthesia) will ultimately dictate whether the patient will require inpatient admission. [78] Researchers showed that PP surgery performed in ASC demonstrated no increased infection rate compared with inpatient surgeries (1.7% versus 4.4%). [79] Since PP surgeries performed in the inpatient setting involved patients with significantly more medical comorbidities, the ASC infection rate was not reported as a “lower incidence of infection rate,” when compared with the inpatient group infection rate. The average procedural time was shorter in the ASC in comparison with inpatient PP surgeries. The risks of PP revision surgery and device erosion were both similar in the ASC and the inpatient group. [79]
Antibiotics have historically been prescribed to patients in the postoperative setting to prevent future prosthetic infections. Although the AUA acknowledges that there is no adequate evidence to guide the duration of postoperative antibiotic therapy, the AUA does refer to the orthopedic literature on prosthetic joints, which states that antibiotic prophylaxis should be discontinued within 24 hours of surgery. [50] Regardless of this best practice statement, approximately 67% of patients are prescribed postoperative antibiotics, the most common being fluoroquinolones or cephalosporins. It has been shown in a nationwide analysis that postoperative antibiotic therapy does not reduce the odds of removal of a PP device. [80] Investigators performed a prospective study on a population of patients who were deemed to be at increased risk for PP infection prior to surgery. The study demonstrated postoperative antibiotic use in the high-risk population was not associated with a lower risk of explant for infection (4% PP infection rate without postoperative antibiotics versus 5% PP infection rate with postoperative antibiotics). [81]
Patients who undergo PP surgery will require postoperative multimodal pain control. Postoperative multimodal pain regimens, including intraoperative local anesthesia administration to the surgical site, when compared with a narcotic-only postoperative pain regimen, were associated with a lower amount of narcotics administered during admission, lower amount prescribed on discharge, and a lower percentage of patients refilling narcotics after discharge. [51, 82]
The time to initial device activation can range from 1-6 weeks, depending on surgical approach, PP device, and surgeon’s preference. Sexual intercourse is usually withheld for approximately 4-6 weeks. [57, 63, 67] Once patients are taught how to activate and deactivate their device in the outpatient setting, they are also encouraged to inflate and deflate their device on a daily basis to diminish penile capsule formation. [67] When the three-piece device is inflated, the reservoir fluid is transferred to the cylinders. Deflation of the three-piece device allows saline to flow from the cylinders into the reservoir. Complete deflation of the PP cylinders allows full expansion of the reservoir. In order to allow full expansion of the reservoir, patients are also instructed to completely deflate the device for the first 3 months postoperatively to avoid diminished capsule formation over the reservoir, which would limit the PP reservoir’s expansion during future use. [18] This principle does not apply to two-piece or MPP devices, because they do not have reservoirs.
Setting expectations and detailed informed consent in the preoperative setting are imperative to improve postoperative patient satisfaction. It should be noted that even if these discussions are performed appropriately, preoperative counseling is crucial, because the partner’s satisfaction directly correlates with the patient’s satisfaction. [83] Patient dissatisfaction is often associated with perceived loss in penile length, even if the patient is counseled about this in the preoperative setting. It may be important to measure the patient’s penile length prior to surgery, because it has been shown that there can be a 0.5-0.8 cm decrease in penile length after PP implantation. [84, 85] Reasons for decreased penile length after PP placement include postoperative fibrotic changes, loss of tunica albuginea elasticity, lack of glans penis engorgement, an increase in prepubic and peripenile fat, apoptosis of erectile tissues, denervation atrophy with hypoxia, improper sizing at the time of surgery, improper preoperative and postoperative measurements, and patient recall bias. [84, 85]
To better understand patient satisfaction, a validated questionnaire, the Satisfaction Survey for Inflatable Penile Implant (SSIPI), has been developed to help providers identify postoperative concerns or issues that need to be addressed and/or improved upon. [86] Health literacy has also been shown to be directly correlated with patient satisfaction following PP surgery. Being able to identify patients with lower health literacy can lead to increased preoperative counseling in this population, which can improve patient expectations and quality of life following prosthetic surgery. [87]
Lack of penile glans engorgement after surgery can be addressed with oral sildenafil or intraurethral alprostadil (MUSE), which have both been demonstrated to improve glans engorgement after PP placement. [88, 89] One study demonstrated that daily PP inflation, 1 hour per day for 1 year postoperatively, expands the capsule surrounding the cylinders, resulting in an increase in patient’s penile length of approximately 0.5 cm to 1.0 cm. This increase in the patient’s perceived penile length in turn increases the patient’s satisfaction. [90]
Complications
Infection
Signs and symptoms that a PP implant is infected include the following:
-
Prolonged pain over device components
-
Fixation of the pump to the scrotal wall
-
Erosion of the device visible through the skin, with associated purulent drainage
In the setting of overt device infection, the entire device needs to be surgically removed, as the entire device is considered in continuity. [91, 92] When the device is removed, the prosthetic surgeon employs an antibiotic irrigation solution to wash out the infected surgical field, and the surgeon may replace the corporal space with another PP device. Immediate replacement after washout of the infected surgical field is encouraged to prevent corporal body fibrosis and penile shortening, which can occur if the device is not immediately replaced at the time of surgery, thus making future implantation of a PP device more difficult for the surgeon. [91, 92] The Mulcahy salvage protocol (washout with PP device replacement) has been modified since its introduction in 1996, owing to rising bacterial resistance and increased prevalence of fungal infections observed in the obese and diabetic population. [93, 94] In order to minimize the number of foreign objects placed in an infected field, the MPP (no scrotal pump or reservoir) has been the PP device chosen to replace the infected PP device. The malleable implant salvage technique (MIST) has a post infection-free rate of 93%. Six months after the MPP is placed, the three-piece PP, if requested by the patient, can then replace the MPP. [95]
Differentiating between expected postoperative pain and PP pain related to infection can be challenging. Postoperative pain gradually subsides over 3-6 weeks after surgery. In the circumstance where pain is continuous, a trial of antibiotics can help differentiate between infectious and noninfectious pain. If the pain improves while the patient is on antibiotics and if the pain recurs when the antibiotics are discontinued, an infectious cause is more likely. An increase in white blood cell (WBC) count and erythrocyte sedimentation rate (ESR) can also help in the diagnosis of substantial PP infection that causes persistent penile pain. [91]
Even with optimization of medical comorbidities prior to surgical implantation, PP infection has been associated with poorly controlled diabetes. Hemoglobin A1c (HbA1c) has been utilized as a tool by prosthetic surgeons to help identify patients who have long-term, poorly controlled diabetes, even if their glucose is controlled perioperatively, and patients who have an increased risk of infections after PP implantation. Researchers reported in a multi-institutional study that patients with a HbA1c ≥ 8.5% were significantly more likely to have an infected PP. [96] Other studies also demonstrated that an increase in HbA1c was associated with an increased rate of PP revisions caused by infections. [97]
In contrast, multiple studies have demonstrated that the comorbidities associated with diabetes might be the cause for an increased likelihood of PP infection, rather than solely the HbA1c or glucose level. A multi-institutional study demonstrated that HbA1c and preoperative glucose levels were not associated with higher postoperative infection rates or explantations. This study also revealed that a history of diabetes-related complications was a significant predictor of higher PP revision rates. [98] Dick et al performed a systematic review in 2021 regarding HbA1c as a predictor of PP infection, reporting that no conclusion can be made about HbA1c and its association with PP infection rate. [99] Investigators also performed a systematic review in 2021, reporting that neither HbA1c nor blood glucose was a predictor of PP infection in men with diabetes.
Asymptomatic bacteriuria is historically treated prior to prosthetic implantation, but this practice has not been proved to prevent PP infections. Researchers demonstrated that bacteria on skin, rather than bacteria found in the untreated urine specimens, were present on cultures taken from the infected and explanted PP. From these results, these investigators suggested that treating asymptomatic bacteriuria and even obtaining preoperative urine cultures may be of limited value. [100] Next-generation DNA sequencing of the infected devices can also be used to help identify bacteria that are not identified by conventional culture from the infected prosthesis. [101]
Smoking is well known throughout the surgical literature to increase the likelihood of surgical site infections (SSI) and anesthesia-related complications. [102, 103] Smokers were approximately 4 times more likely to experience overall postoperative PP complications related to infections when compared with nonsmokers. [104]
Immunocompromised patients (as a result of chronic corticosteroid use, history of solid organ transplant, or congenital or acquired immune deficiency) have been previously hypothesized to be at increased risk for PP infection. It has been demonstrated that reoperation rates are not significantly different between immunosuppressed and non-immunosuppressed patients. [105]
Infected PP implants that were explanted in the summer and fall were 2 to 3 times more likely to grow gram-positive bacteria in comparison to implants explanted in the spring. PP fungal infections were seen to be more common when relative humidity was greater than 55%. [106]
Patients treated by prosthetic surgeons who had a PP implant case volume of less than 31 cases per year were 2 times more likely to require reoperation for PP infection compared with those whose surgeons had a PP implant case volume of more than 31 cases per year. [107] Performing concurrent penile surgeries at the time of PP, such as circumcision and hydrocelectomy, was also associated with an increased likelihood of PP infection. [104]
Malfunctioning of device
A literature survey of PP durability demonstrates a PP survival rate of approximately 95.9% at 8 years. Based on a comprehensive review of the literature focusing on the long-term outcomes of PP implantation, the 5- and 10-year survival rates of the PP are 90.4% and 86.6%, respectively. [108] Common defects of the PP include fracture of the tubing derived from the pump and connecting to the reservoir or cylinders. It is unusual to have a defect isolated in the reservoir. Although rare, intraoperative needle puncture of the device must be suspected if the device never inflated after the surgery. [109]
Patients with a malfunctioning implant typically report that the pump no longer inflates the cylinders and that the sound of air is heard upon pressing the pump. When fluid leaks out of the system, it will not draw fluid from the reservoir and will remain compressed. If the device is 1-2 years old, it is recommended to repair or replace the malfunctioning component rather than replacing the entire device. It is recommended to use its antibiotic, washout protocol when replacing device parts. [109, 110] Of interest, 14% of patients who experience device malfunction do not pursue revision of their prosthesis. [111]
Device corpora cavernosa crossover
When two cylinders wind up in the same corporal body, either proximally or distally, one cylinder has crossed over to the adjacent corporal body. This is due to the laxity of the fenestrated penile septum located between the corpora. [112] This is best avoided by pointing the corporal dilators laterally at the 3 or 9 o’clock position when dilating right and left corporal body, respectively. Crossover can be addressed when recognized during surgery by removing the cylinders, placing one dilator on the correctly dilated corpora, and re-dilating a new cavity in the corporal body that experienced crossover. [109]
Device extrusion/erosion
Erosion of the cylinder tips may be seen under the foreskin. This may be associated with pain for the patient or his partner. The cylinder pressing on the glans may result in extrusion into urethral mucosa. Cylinders tips protruding from the meatus in a delayed fashion may be due to infection or pressure necrosis on the urethra. Erosion of the scrotal pump through the skin is most likely due to device infection. Because the device is exposed to the outside of the body, it is technically treated as an infection. If the device is extruded but not eroded, the device may be relocated into an appropriate location via a corporal or glanular approach. [109, 113]
Urethral perforation
About 1-3% of PP implants are complicated by urethral perforation. [114] Urethral perforation usually occurs during corporal dilatation, passage of the cylinders with the Furlow tool, or during Peyronie curvature correction via penile modeling. [115] If urethral injury is suspected, irrigation through the corporotomy will identify the area of injury. The prosthesis procedure should be aborted if there is a urethral injury. A single cylinder can be left as a place holder on the other corpora for 6-12 weeks. A urethral catheter is left in place to allow the injury to heal for 3-6 weeks. [115]
Proximal crural perforation
The proximal crural bodies course laterally, and during proximal dilation, the scissors and dilators should be passed in a down and lateral direction. In scarred corporal bodies, the tendency is to aggressively dilate downward, and with this maneuver, a proximal perforation of the crus may occur. [109] The surgeon can still proceed with the operation. A nonabsorbable suture can be placed through the proximal end of the cylinder or the rear-tip extender (RTE) to keep this proximal cylinder secured at the point of the corporotomy (also known as a purse string suture) to prevent the cylinder from inadvertently sliding proximally. [109] Fibrosis of the area of perforation occurs within 6-8 weeks, with no long-term consequences.
Vascular/visceral injury or erosion from reservoir placement
Injury to the adjacent vasculature, urinary bladder, and bowel are potential complications of reservoir placement. Erosions of the reservoir into the bladder (the patient may present with increased urinary urgency without UTI) or bowel are also rare. [109] Aborting the procedure and allowing several weeks of catheter drainage is typically sufficient to manage bladder injuries/erosions, because the placement of the reservoir is considered an extraperitoneal bladder injury. The bladder injury/erosion might be complex and still require repair. [116] If bowel or vascular injury occurs, the procedure must be aborted, general or vascular surgery are consulted, and all injuries must be repaired prior to terminating the PP procedure. Intraperitoneal placement or migration of the reservoir from the SOR is neither a complication nor a danger to the patient, and the reservoir can be placed near the pelvic side wall. [109, 117]
Hypermobile glans
Hypermobility of the glans penis after implant cylinder insertion, more commonly referred to as floppy glans or supersonic transporter (SST) deformity, can pose difficulty with penetration during intercourse. SST deformity is named after the Concorde supersonic transport aircraft (see the image below). The floppiness can be in any direction and is seen more commonly in uncircumcised patients. If pulling the foreskin in the proximal direction does not allow for the cylinders to situate more distally into the glans, then a glanspexy can be performed by fixating the tunica albuginea to the glans via a hemi-circumscribing incision with nonabsorbable sutures. Glans floppiness should not be confused with undersizing of the PP cylinders. [109]
Outcomes of PP implantation
Patients are generally very satisfied with a PP implantation. Despite the controversial methods used to obtain patients’ satisfaction, such as a validated questionnaire specifically assessing post-PP implantations, several studies reported a high satisfaction rate for patients and their partners after a PP implantation. MPPs have a satisfaction rate of approximately 77%. There was no difference between the two brands used (Coloplast versus Boston Scientific). [31] IPPs have a 90.9% satisfaction rate in patients with three-piece IPPs versus MPPs. [40] Reasons for this difference in satisfaction may include the more natural cosmetic penile rigidity and flaccidity of IPPs. [19] Despite this, investigators observed that most patients with a MPP felt satisfied and would have the same device implanted again, if given the opportunity. [118] Researchers found that the two-piece IPP also provides an excellent satisfaction rate, with more than 85% of patients providing an overall rating of at least satisfied. [119] This satisfaction rate is slightly lower compared with the three-piece IPP, perhaps due to the reduced axial penile rigidity. [120] Investigators reported a very high satisfaction rate with the three-piece IPP, reaching up to 95-99%. [40, 121] This high satisfaction rate, which is consistent across different countries, confirms that the three-piece IPP received the highest satisfaction rating among patients. [40, 122] A summary of satisfaction rates is displayed in the Table below.
Table. Satisfaction rates among different types of PP (Open Table in a new window)
Author |
Year |
Type of implants |
Number of patients |
Satisfaction rate (%) |
Lux et al [120] |
2007 |
Two-piece IPP |
146 |
85 |
Natali et al [121] |
2008 |
MPP |
16 |
75 |
Two-piece IPP |
66 |
81 |
||
Three-piece IPP |
33 |
97 |
||
Falcone et al [119] |
2013 |
Two-piece IPP |
23 |
86.4 |
Song et al [122] |
2013 |
Two-piece IPP |
40 |
81.1 |
Three-piece IPP |
161 |
93 |
||
Casabe et al [31] |
2016 |
MPP |
60 |
77 |
Cayan et al [40] |
2019 |
MPP |
349 |
70.8 |
Two-piece IPP |
26 |
84.6 |
||
Three-piece IPP |
508 |
90.9 |
||
Bayrak et al [35] |
2020 |
MPP |
81 |
74.2 |
Two-piece IPP |
61 |
90.1 |
IPP, inflatable penile prosthesis; MPP, malleable penile prosthesis; PP, penile prosthesis.
Future of PP
PP has become an important treatment modality for ED refractory to medical therapy. Although patient satisfaction is high, obtaining the proper insurance coverage for the procedure is still a challenge for the patient. Insurance policies that exist today may not align with guidelines, recommendations, or sexual medicine practices. Investigators reviewed 100 insurance plans from 306 regional healthcare markets. Approximately 70% of plans required strict criteria in order for the patient to be approved for PP surgery, and 20% of plans did not cover PP surgery. [123] Insurance coverage criteria were highly variable by state and plan. Step-wise mandates that generally need to be taken in order to be covered include the following [123] :
-
Trial, contraindication, or intolerance of pharmacologic therapy (61% of insurance plans)
-
Consideration of prior pharmacologic therapy (30% of insurance plans)
-
Trial of ICI, MUSE, and/or VED
In the United States, 28 out of the 50 states have Medicaid coverage for PP surgery, but there are no obvious criteria for prior authorization for the patient to obtain Medicaid coverage for PP surgery. [124] Insurance policies that dictate coverage for PP surgeries will hopefully improve access for patients who are deemed appropriate for PP surgery by AUA and sexual medicine guidelines.
A “remote controlled” PP has been reported in preclinical studies that removes the need for manual pumping. The pump is connected to an electronic device, conformed by a microprocessor that activates the reservoir pump, and a rechargeable battery is installed with an antenna capable of connecting to a mobile device. [29, 125] The PP may also incorporate elements to enhance partner pleasure such as a “vibrating” penile implant. A touchless prosthesis designed to achieve a set shape from magnetic induction is also being investigated. [126, 127]
There are also technological advancements in transgender PP surgery, allowing the PP to be fixed to the pubic bone. The neophallus-specific PP, Zephyr Surgical Model 475 FtM, has been available in Europe since March 2016 but is not available in the United States. The 2021 model will have a detachable glans component to allow the individual to customize the neophallus. [29] Researchers reported that 80% of the patients did not require PP revision. About 86% of the patients were able to have penetrative sexual intercourse, and about 93% were either satisfied or very satisfied with the prosthesis. [128]
Conclusion
Having been the gold standard treatment for ED before medical therapy, PP implants have now become the gold standard for treating medical refractory ED. As PP implants have gained popularity throughout the past few decades, the surgical techniques and postoperative management have also improved. PP technology will continue to advance in the years to come. The focus on PP research and improvement on PP education will allow outside providers to have a better understanding of PP surgery and therefore facilitate the conversation between healthcare providers and patients to ensure better patient-guided healthcare decisions.
-
Coloplast Genesis. Courtesy of Coloplast Corp. .
-
Boston Scientific Spectra. Courtesy of Boston Scientific.
-
Boston Scientific Tactra. Courtesy of Boston Scientific.
-
Rigicon Rici10. Courtesy of Rigicon, Inc.
-
Boston Scientific Ambicor two-piece PP. Courtesy of Boston Scientific.
-
Boston Scientific 700. Courtesy of Boston Scientific.
-
Coloplast Titan. Courtesy of Coloplast Corp.
-
Coloplast Titan Narrow Base. Courtesy of Coloplast Corp.
-
British Airways Concorde supersonic transport aircraft. Courtesy of Wikimedia Commons (Eduard Marmet) (https://commons.wikimedia.org/wiki/File:British_Airways_Concorde_G-BOAC_03.jpg).