Cyanosis and the Clinical Assessment of Hypoxemia
Cyanosis is a bluish or purplish tinge to the skin and mucous membranes. (See the images below.)
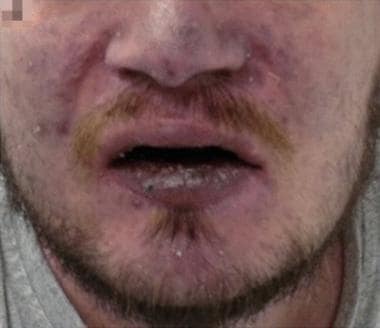 Cyanosis. Facial cyanosis in a patient with chronic hypoxemia. Courtesy of BioMed Central Ltd, Springer Nature [Patzig M, Laub C, Janssen H, Ertl L, Fesl G. Pseudo-subarachnoid haemorrhage due to chronic hypoxaemia: case report and review of the literature. BMC Neurol. 2014 Nov 18;14:219.].
Cyanosis. Facial cyanosis in a patient with chronic hypoxemia. Courtesy of BioMed Central Ltd, Springer Nature [Patzig M, Laub C, Janssen H, Ertl L, Fesl G. Pseudo-subarachnoid haemorrhage due to chronic hypoxaemia: case report and review of the literature. BMC Neurol. 2014 Nov 18;14:219.].
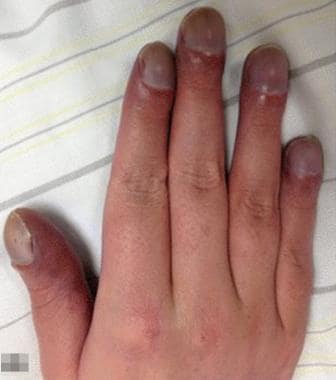 Cyanosis. Clubbed and cyanotic fingers in a patient with chronic hypoxemia. Courtesy of BioMed Central Ltd, Springer Nature [Patzig M, Laub C, Janssen H, Ertl L, Fesl G. Pseudo-subarachnoid haemorrhage due to chronic hypoxaemia: case report and review of the literature. BMC Neurol. 2014 Nov 18;14:219.].
Cyanosis. Clubbed and cyanotic fingers in a patient with chronic hypoxemia. Courtesy of BioMed Central Ltd, Springer Nature [Patzig M, Laub C, Janssen H, Ertl L, Fesl G. Pseudo-subarachnoid haemorrhage due to chronic hypoxaemia: case report and review of the literature. BMC Neurol. 2014 Nov 18;14:219.].
Before the era of rapid blood gas analysis, clinicians often assessed hypoxemia on clinical grounds alone, primarily by looking for cyanosis in the perioral area and fingers. [1, 2] Clinical assessment of hypoxemia is now known to be notoriously unreliable.
A host of factors, from natural skin pigment to room lighting, can affect detection of cyanosis. As with many other physical examination findings, significant interobserver variation occurs in detecting cyanosis. [3] Clinicians may diagnose cyanosis as an indicator of hypoxemia when the patient has normal oxygen saturation; alternatively, physicians may miss cyanosis when it should be present (the patient has very low oxygen saturation with normal hemoglobin). [4]
Approximately 5 g/dL of unoxygenated hemoglobin in the capillaries generates the dark blue color appreciated clinically as cyanosis. For this reason, patients who are anemic may be hypoxemic without showing any cyanosis.
Ancillary signs and symptoms of hypoxemia (eg, tachycardia, tachypnea, mental status changes) are nonspecific and of no value in reliably detecting hypoxemia. For example, patients may be dyspneic at rest for reasons other than hypoxemia (ie, they have normal partial pressure of oxygen [PaO2] and arterial oxygen saturation [SaO2]). Conversely, many patients who are chronically hypoxemic (low PaO2 and/or low SaO2) are perfectly lucid and without any obvious physical signs of their low oxygen state (at least while at rest).
Generation of Cyanosis
The requirement of 5 g/dL of reduced (ie, deoxygenated) hemoglobin in the capillaries translates into a reduced hemoglobin content of 3.4 g/dL in arterial blood. [5] For this reason, patients with normal hemoglobin manifest cyanosis at higher arterial oxygen saturation (SaO2) values than patients with anemia. Refer to the image below.
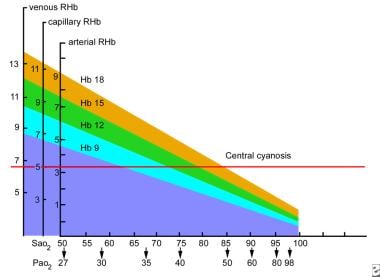 Cyanosis. Oxygen and hemoglobin values at which central cyanosis occurs. The threshold for central cyanosis is a capillary reduced hemoglobin content of 5 g/dL, which can occur at varying values of the two parameters that are measured most commonly, arterial oxygen saturation (SaO2) and arterial hemoglobin content. The vertical axis shows values for venous, capillary, and arterial reduced hemoglobin (RHB, g/dL blood), and the horizontal axis shows percentage saturation of hemoglobin in arterial blood (SaO2) along with the corresponding partial pressure of oxygen (PaO2, mmHg). Each diagonal line represents a different hemoglobin content (g/dL). For example, central cyanosis can manifest when SaO2 is 79% in a patient with a hemoglobin of 15 g/dL.
Cyanosis. Oxygen and hemoglobin values at which central cyanosis occurs. The threshold for central cyanosis is a capillary reduced hemoglobin content of 5 g/dL, which can occur at varying values of the two parameters that are measured most commonly, arterial oxygen saturation (SaO2) and arterial hemoglobin content. The vertical axis shows values for venous, capillary, and arterial reduced hemoglobin (RHB, g/dL blood), and the horizontal axis shows percentage saturation of hemoglobin in arterial blood (SaO2) along with the corresponding partial pressure of oxygen (PaO2, mmHg). Each diagonal line represents a different hemoglobin content (g/dL). For example, central cyanosis can manifest when SaO2 is 79% in a patient with a hemoglobin of 15 g/dL.
Consider the following examples:
-
A patient whose hemoglobin content is 15 g/dL (hematocrit approximately 45%) would not generate 5 g/dL of reduced (ie, deoxygenated) hemoglobin in the capillaries until their SaO2 level reached about 79% (partial pressure of oxygen [PaO2] 47 mmHg).
-
When hemoglobin content is 9 g/dL (hematocrit approximately 27%), the threshold SaO2 level for manifesting cyanosis is lowered to about 65% (PaO2 35 mmHg). At this level of hypoxemia, the patient would also have other manifestations of hypoxemia (eg, respiratory symptoms, mental status changes) apart from cyanosis.
-
With a hemoglobin content of less than 9 g/dL, the patient would likely succumb from hypoxemia before cyanosis became evident.
If hypoxemia is suspected for any reason, some measurement of the oxygen level is necessary (eg, arterial blood gas determination, pulse oximetry). No reliable alternative is available to measure of PaO2 or SaO2 when diagnosing hypoxemia or assessing the need for supplemental oxygen therapy. [6] At the same time, one should not rely on the absence of cyanosis as reassurance that hypoxemia is not present.
Most often, cyanosis is detected in the lips and fingers. (See the image below.)
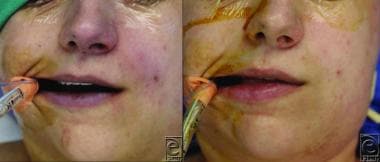 Cyanosis. Left: Facial cyanosis in a patient with benzocaine-induced cyanosis. Right: Resolution of cyanosis in the same patient following administration of methylene blue. Courtesy of HMP Global Learning Network, HMP Omnimedia [Jain A, Patel A, Hoppe IC. Benzocaine-induced cyanosis. Eplasty. 2016 May 16;16:ic18.].
Cyanosis. Left: Facial cyanosis in a patient with benzocaine-induced cyanosis. Right: Resolution of cyanosis in the same patient following administration of methylene blue. Courtesy of HMP Global Learning Network, HMP Omnimedia [Jain A, Patel A, Hoppe IC. Benzocaine-induced cyanosis. Eplasty. 2016 May 16;16:ic18.].
Causes of Cyanosis
Cyanosis is classified as being either peripheral or central. Peripheral cyanosis spares the oral mucosa but causes a dusky or bluish discoloration of the hands and feet; when unaccompanied by hypoxemia (as determined by blood gas analysis), peripheral cyanosis is caused by peripheral vasoconstriction. In addition to hands and feet, central cyanosis is apparent at the lips, tongue, and sublingual tissues. The majority of cyanosis is caused by cardiac or respiratory etiologies.
Differential cyanosis
Differential cyanosis is defined as a difference in oxygen saturation (SaO2) of at least 5% or a difference in partial pressure of oxygen (PaO2) of at least 20 mmHg between the arms and legs. The typical clinical finding of differential cyanosis is usually cyanotic toes with normal fingers. This is seen in neonates with patent ductus arteriosus (PDA) and pulmonary arterial hypertension. The deoxygenated blood in the pulmonary artery goes through the PDA, empties into the aorta, and flows to the lower half of the body. The upper half of the body continues to received oxygenated blood from the left ventricle.
Reverse differential cyanosis (cyanotic fingers and normal toes) is seen in infants with transposition of the great arteries (TGA) with coarctation of the aorta or interrupted aortic arch [7] and TGA with suprasystemic pulmonary vascular resistance. In this setting, the descending aorta is filled with oxygenated blood from the pulmonary circulation, and the lower extremities have higher oxygen saturation than the upper extremities.
Under guidelines from the American Academy of Pediatrics, American Heart Association, and the American College of Cardiology, asymptomatic neonates should be screened for congenital heart defects with pulse oximetry at two sites, including the right hand and foot, after 24 hours of life or before discharge. Overall, the percentage of severe congenital heart diseases diagnosed postnatally has increased from 58% with clinical examinations alone to 72% with pulse oximetry, making differential cyanosis rare in neonates. [8]
In adults, differential cyanosis is pathognomonic for a large untreated patent ductus arteriosus (PDA) associated with Eisenmenger syndrome (shunt reversal into a right-to-left shunt due to progressive pulmonary vascular disease). [9, 10, 11] In the setting of Eisenmenger syndrome (interrupted aortic arch with ventricular septal defect) in pregnancy, differential cyanosis is a rare condition in which cyanosis is evident in both the fingers and the toes, but the SaO2 level (measured by pulse oximetry) is much lower in the toes. [12]
Methemoglobinemia
Methemoglobinemia is a condition in which the iron within the hemoglobin (Hb) molecule is oxidized from the ferrous (Fe+2) to the ferric (Fe+3) state and, for this reason, cannot bind to oxygen. In addition, methemoglobin (MetHb), the oxidized form of hemoglobin, shifts the oxygen-Hb dissociation curve to the left, hindering the release of oxygen in tissues. These two conditions compromise oxygen exchange and supply to tissues, causing a cyanosis that does not resolve with oxygen therapy, which is a clue to the diagnosis. [13]
Methemoglobinemia usually occurs as a drug reaction, especially to nitrite or nitrate-containing compounds (eg, nitroglycerin) [14] and to some topical anesthetics (lidocaine-prilocaine cream). [15, 16] (See the image below.) Dahshan and Donovan reported a case of severe methemoglobinemia from topical benzocaine in a toddler. [17] Dapsone, a drug used in human immunodeficiency virus (HIV) and non-HIV conditions, can also cause methemoglobinemia. [16, 18]
 Cyanosis. Left: Facial cyanosis in a patient with benzocaine-induced cyanosis. Right: Resolution of cyanosis in the same patient following administration of methylene blue. Courtesy of HMP Global Learning Network, HMP Omnimedia [Jain A, Patel A, Hoppe IC. Benzocaine-induced cyanosis. Eplasty. 2016 May 16;16:ic18.].
Cyanosis. Left: Facial cyanosis in a patient with benzocaine-induced cyanosis. Right: Resolution of cyanosis in the same patient following administration of methylene blue. Courtesy of HMP Global Learning Network, HMP Omnimedia [Jain A, Patel A, Hoppe IC. Benzocaine-induced cyanosis. Eplasty. 2016 May 16;16:ic18.].
Methemoglobinemia can also be congenital; recessive congenital methemoglobinemia is an autosomal recessive disorder due to deficiency of nicotinamide adenine dinucleotide (NADH)-cytochrome b5 reductase (cytb5r). [19] A rare hereditary methemoglobinemia has been described in a family with Hb M-Hyde Park (Hb M-Akita) (HBB:c.277C > T; p.His93Tyr). Interestingly, Garcia Sanchez et al reported the case of a cyanotic infant with methemoglobinemia from a cow's milk protein allergy. [20]
Methemoglobinemia resulting from toxic ingestion or inhalation in suicide attempts/completions is also found in the literature, [21, 22, 23] including an unusual case in which a patient had concurrent methemoglobinemia and carboxyhemoglobinemia following pesticide ingestion. [21]
MetHb imparts an intense bluish tinge to the skin; therefore, the cyanosis of methemoglobinemia is not related to reduced hemoglobin but to oxidized hemoglobin. [24, 25] Although excess metHb reduces the measured SaO2, PaO2 is not affected, because metHb does not affect transfer of oxygen from the atmosphere to the lungs. A low PaO2 in a patient with excess metHb suggests a concomitant pulmonary problem.
MetHb can be measured in a co-oximeter, which is incorporated into most hospital blood gas machines. The co-oximeter also measures carboxyhemoglobin, hemoglobin content, and SaO2. Note that standard pulse oximeters, which measure SaO2 using two wavelengths of light, do not measure metHb (or carboxyhemoglobin). However, pulse oximeters that use eight wavelengths of light do have the ability to measure carboxyhemoglobin and metHb. [26]
Sulfhemoglobinemia
Sulfhemoglobinemia is a rare condition caused by sulfur binding with hemoglobin so that oxygen cannot be bound. Unlike metHb, the iron moiety remains in the reduced state (HbFe+2). Sulfhemoglobin is similar to metHb in causing low SaO2 but not affecting PaO2, and in imparting an intense bluish color to the skin. Although it is most often associated with the use of drugs such as phenacetin, metoclopramide, dapsone, phenzopyridine, and trimethoprim-sulfamethoxazole, [27] hydrogen-sulfide-producing intestinal bacteria, such as Morganella morganii, have also been reported to be etiologic agents. [28]
Pseudocyanosis
Pseudocyanosis can mimic peripheral cyanosis, although there is no response to attempted “blanching” of the skin by applying pressure, and the presence of a bluish tinge to the skin and/or mucous membranes is not associated with either hypoxemia or peripheral vasoconstriction. For confirmation of the diagnosis, obtain pulse oximetry or arterial blood gas. Pseudocyanosis is generally due to drug exposure (eg, amiodarone) and, much more rarely, metal exposure (chrysiasis, argyria). [29]
Baernstein et al described blue-gray discoloration in a man who ingested colloidal silver for years for urinary tract infections due to a neurogenic bladder; his oxygen levels were normal. [30] Zillich et al described a girl with intensely blue skin from food coloring. [31]
-
Cyanosis. Facial cyanosis in a patient with chronic hypoxemia. Courtesy of BioMed Central Ltd, Springer Nature [Patzig M, Laub C, Janssen H, Ertl L, Fesl G. Pseudo-subarachnoid haemorrhage due to chronic hypoxaemia: case report and review of the literature. BMC Neurol. 2014 Nov 18;14:219.].
-
Cyanosis. Clubbed and cyanotic fingers in a patient with chronic hypoxemia. Courtesy of BioMed Central Ltd, Springer Nature [Patzig M, Laub C, Janssen H, Ertl L, Fesl G. Pseudo-subarachnoid haemorrhage due to chronic hypoxaemia: case report and review of the literature. BMC Neurol. 2014 Nov 18;14:219.].
-
Cyanosis. Left: Facial cyanosis in a patient with benzocaine-induced cyanosis. Right: Resolution of cyanosis in the same patient following administration of methylene blue. Courtesy of HMP Global Learning Network, HMP Omnimedia [Jain A, Patel A, Hoppe IC. Benzocaine-induced cyanosis. Eplasty. 2016 May 16;16:ic18.].
-
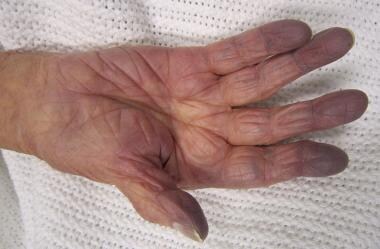
Cyanosis. Cyanosis of the hand in someone with low oxygen saturations. Courtesy of Wikimedia Commons [author James Heilman, MD, https://commons.wikimedia.org/wiki/File:Cynosis.JPG].
-
Cyanosis. Oxygen and hemoglobin values at which central cyanosis occurs. The threshold for central cyanosis is a capillary reduced hemoglobin content of 5 g/dL, which can occur at varying values of the two parameters that are measured most commonly, arterial oxygen saturation (SaO2) and arterial hemoglobin content. The vertical axis shows values for venous, capillary, and arterial reduced hemoglobin (RHB, g/dL blood), and the horizontal axis shows percentage saturation of hemoglobin in arterial blood (SaO2) along with the corresponding partial pressure of oxygen (PaO2, mmHg). Each diagonal line represents a different hemoglobin content (g/dL). For example, central cyanosis can manifest when SaO2 is 79% in a patient with a hemoglobin of 15 g/dL.