Practice Essentials
By definition, a microadenoma (seen in the image below) is a tumor less than 10 mm in diameter. Pituitary adenomas may secrete hormones, but most are clinically inactive. Many pituitary lesions are discovered while investigating other neurologic problems; these lesions are called incidentalomas. With the use of magnetic resonance imaging (MRI) increasing, the discovery of incidental microadenomas has become relatively common. Nonsecreting pituitary microadenomas do not need to be surgically resected. [1, 2] Secretory microadenomas, however, require further medical or surgical care.
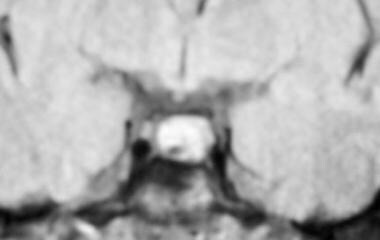 MRI showing a nonenhancing area in the pituitary consistent with a microadenoma, in a patient with hyperprolactinemia.
MRI showing a nonenhancing area in the pituitary consistent with a microadenoma, in a patient with hyperprolactinemia.
The microadenoma may be discovered during an investigation for the cause of a clinically diagnosed hypersecretory syndrome such as hyperprolactinemia, acromegaly, or Cushing syndrome. In more than 90% of cases, a nonsecreting microadenoma is a nonfunctioning pituitary adenoma, although a variety of other cystic, vascular, neoplastic, hyperplastic, or inflammatory processes may present in a similar manner.
Signs and symptoms of pituitary microadenomas
Patients with prolactin-secreting adenomas may present with galactorrhea/menstrual disorders.
Growth hormone–secreting adenomas cause acromegaly, with coarsening of facial features and increased width of the hands and feet. Progressive bony proliferation of the mandible both lengthens and thickens it, resulting in separation of the lower teeth and an underbite. Skin thickness is increased compared with age- and sex-matched controls (>2 mm in reproductive-aged women, >3 mm in men). The clinical changes may be subtle or absent at the time of presentation.
Corticotropin-secreting adenomas cause Cushing disease, characterized by weight gain, primarily in the facial, nuchal, truncal, and girdle areas (ie, centripetal, or "buffalo," obesity). Protein breakdown leads to thin, friable skin that bruises easily; this breakdown may form wide striae that are often purple. In addition, this protein breakdown often causes muscle weakness (in proximal muscles more than distal ones), wasting, and osteopenia with fragility fractures. Women frequently develop hirsutism and menstrual changes. In children, growth is arrested. Depression/anxiety is common.
Workup in pituitary microadenomas
Individualizing the evaluation of microadenomas with an evidence-based approach while considering signs, symptoms, the size of the lesion, imaging characteristics, and patient factors is key. Endocrine Society clinical practice guidelines recommend a complete biochemical assessment, even in asymptomatic patients. [1]
Because the excess secretion of growth hormone does not always produce the above-described clinical phenotype, especially early in the course of the disorder, a serum insulinlike growth factor-1 (IGF-1) level is recommended in all cases. [1] Hormonal hypersecretion in the setting of a sellar mass is typically caused by a pituitary adenoma, and prolactin-secreting microadenomas are the most frequent secretory microadenomas.. Screening for Cushing syndrome is advised, using an overnight dexamethasone suppression test
Magnetic resonance imaging (MRI) studies have shown a sensitivity and specificity of about 90% for secretory tumors. Enhancement with gadolinium and enhanced dynamic images improve the detection rate. However, the sensitivity for detection of corticotropin-secreting adenomas is much lower (60-75%); in contrast, the incidental finding of nonsecreting microadenomas is sufficiently common that the diagnosis of a corticotropin-secreting adenoma after positive clinical and biochemical testing may then require specialized tests such as petrosal sinus sampling to confirm the anatomic diagnosis. Cystic lesions, such as Rathke cleft cysts, typically have a low-intensity signal on T1-weighted images, whereas on T2-weighted images, cystic lesions may have a high-intensity signal. [3] Pituitary hemorrhage results in a high-intensity signal on T1- and T2-weighted images.
Management of pituitary microadenomas
For prolactinomas, therapy with a dopaminergic drug is the treatment of choice (see Hyperprolactinemia). The most common are bromocriptine and cabergoline.
For prolactin-secreting microadenomas, surgical removal is followed by recurrence in 10-50% of patients. Therefore, medical therapy is preferred. Secretory tumors are best removed by the transsphenoidal approach.
Nonsecreting pituitary microadenomas rarely grow to produce mass symptoms and rarely become clinically secretory, so they are best merely observed. Observational studies show that some enlargement of nonsecreting pituitary adenomas may occur in 10% of patients, whereas reduction in size occurs in 6%. [2]
Pathophysiology
Most pituitary tumors are sporadic. Some are part of genetic syndromes such as multiple endocrine neoplasia type 1 (MEN1), McCune-Albright syndrome, or Carney complex. Clonal analysis shows almost all are monoclonal in origin from a genetically mutated single cell. The cause of sporadic tumors is unknown. Familial pituitary tumors are described, and some have been found to result from germline mutations, such as aryl hydrocarbon receptor–interacting protein (AIP). [4] The role of genetic mutations was highlighted in a report suggesting that patients with pituitary tumors from four Irish families shared a common mutation with a patient from the 18th century who had pituitary tumor–mediated gigantism. [5]
Of the secretory tumors, the most common are prolactinomas. [1] Other secretory tumors may secrete (1) corticotropin, causing Cushing disease; (2) growth hormone, causing acromegaly; (3) gonadotropins with clinical presentations reflective of severity and sex (rare); (4) or thyroid-stimulating hormone (TSH), causing hyperthyroidism (rare). Most clinically nonsecreting adenomas are gonadotropin in origin and secrete fragments of beta or alpha subunits of gonadotropin peptide. Such clinically inactive microadenomas are of little clinical consequence. [1, 2]
Epidemiology
Frequency
United States
Autopsy studies find pituitary adenomas, almost all being microadenomas, in 10-14% of cases. They occur in persons of all ages, with no sex predisposition or geographic variation. In the largest meta-analysis of autopsy studies, in which 18,902 pituitaries were examined in 32 reports, pituitary microadenomas were found in 10.7% of autopsies, while macroadenomas were found in less than 1%. [6]
In a surgical series of 1120 sellar masses, 91% were noted to be pituitary adenomas. Of these, 42% were non-functioning pituitary adenomas. Nineteen percent of secretory lesions were found to be prolactinomas, while 15% were growth hormone secretory, and 14% were corticotropin secretory. [1]
The use of MRI now may identify previously unsuspected presumed pituitary microadenomas. MRI studies in unselected populations have reported presumed microadenomas in 10-38% of individuals. [7]
The frequency of microadenomas and the scarcity of macroadenomas at autopsy indicate that microadenomas only rarely progress to macroadenomas and that most macroadenomas clinically present during life.
Mortality/Morbidity
Microadenomas do not cause excess mortality. These tumors generally are too small to cause pain, diplopia, or pressure on the optic chiasm. Otherwise-normal anterior and posterior pituitary function remains intact. Any morbidity is caused by excessive hormone secretion or patient anxiety.
Secretory microadenomas are typically treated medically or surgically.
Nonsecreting microadenomas are best clinically followed without treatment. Their natural history is that tumor enlargement is uncommon and is rarely clinically significant. There is tumor enlargement in 10% of patients, decreased size in 6%, and no change in about 83%. [1, 2] Acute changes from hemorrhage may rarely occur, but this is less common than with macroadenomas.
A population-based, retrospective, open-cohort study by Toulis et al indicated that prolactinomas in males, but not females, increase their risk of incident cardiovascular disease (CVD). The investigators found that males in the study had an incident CVD rate of 14.8 per 1000 person-years, compared with 1.8 per 1000 person-years for females. [8] However, the majority of the men in this report likely had macroadenomas, and thus, many probably had some degree of hypopituitarism, which is associated with increased cardiovascular morbidity. This may not apply to patients with microadenomas and has not been noticed in clinical practice.
A retrospective study by Machado et al found that in patients with Cushing disease, those with microadenomas had a higher cortisol/adrenocorticotropic hormone (ACTH) ratio than did those with macroadenomas. The study also found that hirsutism, facial plethora, and muscular weakness and atrophy occurred more often in the patients with microadenomas, while nephrolithiasis, osteopenia, hyperprolactinemia, and galactorrhea occurred at a higher rate in those with macroadenomas. [9]
A study by Harbeck et al indicated that in patients with hypothalamic-pituitary disorders, those with microadenomas suffer from headache and depression more frequently than do those with macroadenomas. [10] This is not appreciated in clinical practice, however, and the report's results may have arisen from incidental findings of microadenomas occurring when patients with headache and depression have had MRI studies.
A study by Nakhleh et al indicated that in patients with pituitary apoplexy, those with pituitary microadenomas have a better prognosis than do patients with pituitary macroadenomas. The investigators reported that in patients with macroadenomas, 30%, 44.4%, and 22.2% had hyponatremia, a random cortisol level below 200 nmol/L, and secondary hypothyroidism, respectively, on admission compared with 20%, 40%, and 16.7%, respectively, of those with microadenomas. At median 3-year follow-up, the rate of corticotropic deficiency and secondary hypothyroidism was 14.3% in patients with microadenomas, and 65% in those with macroadenomas. [11]
Race
No race predilection exists.
Sex
Microadenomas may occur in either sex. Prolactinomas, the most common secretory microadenoma, are diagnosed more frequently in women, possibly because of the more striking presenting features such as amenorrhea and/or galactorrhea.
Age
Microadenomas may occur at any age, as shown by autopsy studies, but the clinical prevalence appears to increase with advancing age.
-
MRI showing a nonenhancing area in the pituitary consistent with a microadenoma, in a patient with hyperprolactinemia.







