Overview
Background
Radiofrequency catheter ablation (RFCA) has revolutionized treatment for tachyarrhythmias and has become first-line therapy for some tachycardias. Although developed in the 1980s and widely applied in the 1990s, formalized guidelines for its use in clinical practice were not developed until some years later. [1, 2, 3, 4, 5, 6, 7]
Catheters were first used for intracardiac recording and stimulation in the late 1960s, but surgical treatment for refractory tachyarrhythmias was the mainstay of nonpharmacologic therapy until it was superseded by catheter ablation. The initial energy source used was direct current (DC) from a standard external defibrillator. A shock was delivered between the distal catheter electrode and a cutaneous surface electrode; however, this high-voltage discharge was difficult to control and could cause extensive tissue damage.
RF energy, a low-voltage, high-frequency form of electrical energy familiar to physicians from its use in surgery (eg, electrocautery), quickly supplanted DC ablation. The relative safety of RF energy has contributed to the widespread adoption of catheter ablation as a therapeutic modality.
RF energy produces small, homogeneous, necrotic lesions by heating tissue. Lesion size is influenced, in part, by the length of the distal ablation electrode and the type of catheter (standard vs saline-cooled). With typical power settings and good catheter contact pressure with cardiac tissue, lesions are minimally about 5-7 mm in diameter and 3-5 mm in depth.
Catheter-based cryoablation was developed after RFCA, and it utilizes tissue cooling to cause tissue necrosis. Low-intensity cooling (cryomapping, -10°C) allows assessment of lesion efficacy and safety, prior to delivering the deeper cooling (-70°C) that causes irreversible tissue necrosis. Though not as versatile or widely used compared to RFCA, cryoablation is safer for ablation near the compact atrioventricular (AV) node.
A cryoballoon has been developed for pulmonary vein isolation, the minimal objective in catheter ablation of atrial fibrillation, and it appears roughly comparable to point to point RFCA. [8, 9, 10] The Fire and Ice Trial demonstrated noninferiority between cryoballoon and RFCA for the treatment of drug-refractory symptomatic paroxysmal atrial fibrillation, as well as comparable safety and efficacy, but cryoablation had a shorter procedure time, left atrial dwell time, and total fluoroscopy time that RFCA. [8, 9, 10]
Indications and Contraindications
Indications
There are three class I indications for catheter ablation. The first is symptomatic, recurrent supraventricular tachycardia (SVT) due to atrioventricular (AV) nodal reentrant tachycardia (AVNRT), Wolff-Parkinson-White (WPW) syndrome, unifocal atrial tachycardia, or atrial flutter (cavotricuspid isthmus form). For these conditions, catheter ablation is first-line therapy if that is the patient’s preference. [5]
The second indication is atrial fibrillation with lifestyle-impairing symptoms and inefficacy or intolerance of at least one antiarrhythmic agent. [3, 11] Both left atrial ablation for restoration of sinus rhythm and AV junction ablation for rate control are class I indications, depending on the circumstance.
The third indication is symptomatic, recurrent VT. [12] Catheter ablation is first-line therapy in idiopathic VT if that is the patient’s preference. In structural heart disease, catheter ablation is generally performed for drug inefficacy or intolerance, or as adjunctive therapy in patients with an implantable cardioverter-defibrillator (ICD) who are experiencing frequent ICD shocks.
Uncommon indications for catheter ablation include the following:
-
Symptomatic drug-refractory (inefficacy or intolerance) idiopathic sinus tachycardia
-
Lifestyle-impairing ectopic beats
-
Symptomatic junctional ectopic tachycardia
Radiofrequency catheter ablation (RFCA) has been applied to most clinical tachycardias, even to polymorphic VT and VF in preliminary studies. Success rates are highest in patients with common forms of SVT, namely AVNRT and orthodromic reciprocating tachycardia (ORT).
Contraindications
Few absolute contraindications to RFCA exist. Left atrial ablation and ablation for persistent atrial flutter should not be performed in the presence of known atrial thrombus. Similarly, mobile left ventricular thrombus would be a contraindication to left ventricular ablation.
Mechanical prosthetic heart valves are generally not crossed with ablation catheters. Women of reproductive age should not be exposed to fluoroscopy if any possibility exists that they are pregnant.
Periprocedural Care
Preprocedural planning
The preprocedural evaluation always includes a thorough history and physical examination, as well as a review of electrocardiograms (ECGs) (12-lead, if available) obtained during the tachycardia and in sinus rhythm. At a minimum, preprocedural blood work typically includes a complete blood cell count and an assessment of renal function and electrolyte levels.
An echocardiogram is frequently obtained to exclude structural heart disease. Other tests that are indicated in specific situations include exercise testing with or without cardiac imaging (especially for exercise-induced tachyarrhythmias), cardiac computed tomography (CT) scanning, cardiac magnetic resonance imaging (MRI), or coronary angiography.
The patient should fast overnight before the procedure. Cardiac medications with electrophysiologic effects (eg, beta blockers, calcium channel blockers, digoxin, and class I and III antiarrhythmic drugs) are often tapered or discontinued before the procedure. Anticoagulants may or may not be held prior to the procedure. For example, performing left atrial ablation for atrial fibrillation on warfarin may reduce thromboembolic complications. [4, 13]
Patient preparation
Catheter ablation typically requires that the patient be under conscious sedation with intravenous tranquilizers and narcotics. General anesthesia is used in children and selected adults.
Monitoring and follow-up
After generic supraventricular tachycardia (SVT) ablation or idiopathic ventricular tachycardia (VT) ablation, some physicians empirically treat patients with 4 weeks of aspirin therapy with the aim of potentially reducing the risk of thromboembolic sequelae.
Anticoagulation with warfarin or one of the newer agents is typically employed for at least 1 month after ablation for patients presenting in persistent atrial flutter and for a minimum of 2 months after left atrial ablation for patients with atrial fibrillation. [11]
Echocardiography is not routinely performed unless a complication (eg, pericardial effusion) may have occurred. Postprocedural electrophysiologic testing is not routinely performed unless recurrent tachyarrhythmias are suspected.
Technique
Approach considerations
Typically, two to five electrode catheters are percutaneously inserted via the femoral or internal jugular veins; they are positioned within the left heart, the right heart, or both. Multiple catheters are needed to induce and map various tachyarrhythmias before radiofrequency catheter ablation (RFCA).
Cannulation of the coronary sinus is helpful to map left-sided accessory pathways or to evaluate other left-sided tachyarrhythmia substrates like atrial fibrillation.
For left-heart catheterization, one of the following two approaches may be taken:
-
Transseptal catheterization via the interatrial septum
-
Retrograde catheterization across the aortic valve
The former can be guided by intracardiac ultrasonography and is required for catheter ablation of atrial fibrillation and other left atrial tachyarrhythmia substrates. The latter is typically reserved for ventricular tachycardia (VT) ablations or accessory pathway ablations, though transseptal catheterization access can be utilized for either and is generally the technique of choice for left-sided accessory pathways.
Anticoagulation with intravenous heparin is used to reduce the risk of periprocedural thromboembolism.
Atrial fibrillation
RFCA of the atrioventricular (AV) junction is the simplest catheter ablation procedure performed in patients with atrial fibrillation. AV nodal modification is less effective and is not frequently performed, except in an attempt to avoid pacemaker implantation. Both of these approaches are used to achieve good rate control in atrial fibrillation, but unlike ablation techniques in atrial tissue, neither one restores normal sinus rhythm. In addition, AV junction ablation mandates permanent pacemaker implantation.
Catheter ablation of atrial tissue to cure atrial fibrillation continues to evolve. The procedure is technically demanding and is both more risky and less successful than AV junction ablation. Nevertheless, the observation of Haissaguerre et al [14] that pulmonary vein foci can trigger atrial fibrillation has stimulated much additional research, and there is considerable scientific excitement that this common tachyarrhythmia may be amenable to a curative catheter procedure.
For catheter ablation of atrial tissue for atrial fibrillation, the most commonly used technique is a wide circumferential ablation around the pulmonary veins (see the image below). The goal is to electrically isolate rapid electrical activity arising from inside the veins, or adjacent to the pulmonary vein ostia, from the rest of the left atrium.
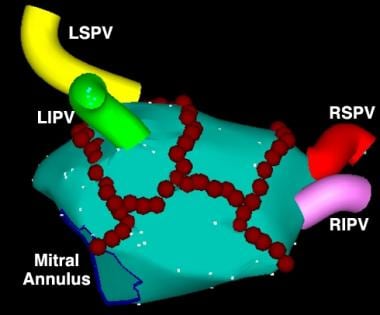 Catheter Ablation. Early version of an electroanatomic map of the posterior left atrium, illustrating the pulmonary veins: right superior pulmonary vein (RSPV), right inferior pulmonary vein (RIPV), left superior pulmonary vein (LSPV), and left inferior pulmonary vein (LIPV). Red circles represent discrete radiofrequency (RF) applications, predominantly delivered in a circumferential pattern around the pulmonary veins. This ablation strategy can isolate foci in or near the pulmonary vein opening that initiate atrial fibrillation (AF) or alter the substrate of the left atrium to inhibit fibrillatory activity due to reentry. Image courtesy of American College of Cardiology Foundation.
Catheter Ablation. Early version of an electroanatomic map of the posterior left atrium, illustrating the pulmonary veins: right superior pulmonary vein (RSPV), right inferior pulmonary vein (RIPV), left superior pulmonary vein (LSPV), and left inferior pulmonary vein (LIPV). Red circles represent discrete radiofrequency (RF) applications, predominantly delivered in a circumferential pattern around the pulmonary veins. This ablation strategy can isolate foci in or near the pulmonary vein opening that initiate atrial fibrillation (AF) or alter the substrate of the left atrium to inhibit fibrillatory activity due to reentry. Image courtesy of American College of Cardiology Foundation.
No consensus exists on the optimal left atrial ablation technique, beyond robust pulmonary vein isolation and ablation of non-pulmonary vein triggers. [15] Nor is there a consensus on what constitutes a clinically successful procedure. Nevertheless, catheter ablation for atrial fibrillation is generally more effective than antiarrhythmic drug therapy, [16, 17] especially for patients in whom antiarrhythmic pharmacotherapy has already failed. Guidelines for the management of atrial fibrillation list catheter ablation as a reasonable strategy for selected patients with atrial fibrillation even in the absence of attempted antiarrhythmic drug therapy. [4, 5, 6, 7, 11]
The complete surgical Maze procedure (incisions in both atria with or without transmural RF lesions) remains the most effective technique for potentially curing atrial fibrillation in all comers, regardless of chronicity or whether structural heart disease is present. The best success rates with left atrial catheter ablation are in patients with paroxysmal atrial fibrillation and hearts that are not too structurally abnormal.
Atrial flutter
Atrial flutter is most commonly due to a large reentrant circuit in the right atrium, involving an isthmus of tissue between the tricuspid valve annulus and the inferior vena cava. Most commonly, reentry proceeds counterclockwise up the atrial septum and down the lateral wall of the right atrium, inscribing inverted (ie, "sawtooth") flutter waves in the inferior leads and upright P waves in V1 (see the images below).
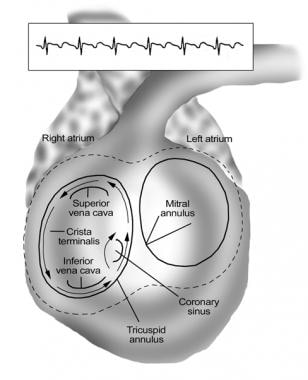 Catheter Ablation. Schema of the common variety of atrial flutter (counterclockwise cavotricuspid isthmus flutter). The reentry circuit is confined to the right atrium and circulates as a counterclockwise macroreentrant circuit proceeding superiorly over the atrial septum and inferiorly over the lateral atrial wall. The wave front circulates through a narrow isthmus of tissue between the tricuspid valve annulus and the inferior vena cava. Linear ablation across this isthmus (cavotricuspid isthmus) cures this common form of atrial flutter.
Catheter Ablation. Schema of the common variety of atrial flutter (counterclockwise cavotricuspid isthmus flutter). The reentry circuit is confined to the right atrium and circulates as a counterclockwise macroreentrant circuit proceeding superiorly over the atrial septum and inferiorly over the lateral atrial wall. The wave front circulates through a narrow isthmus of tissue between the tricuspid valve annulus and the inferior vena cava. Linear ablation across this isthmus (cavotricuspid isthmus) cures this common form of atrial flutter.
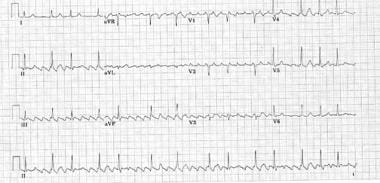 Catheter Ablation. Typical counterclockwise cavotricuspid isthmus atrial flutter (most common form of atrial flutter in patients who have not had prior catheter ablation or atrial surgery). Cardinal features are a perfectly regular atrial rhythm with inverted P waves inferiorly that have a positive overshoot, upright P waves in V1, and inverted P waves in V6.
Catheter Ablation. Typical counterclockwise cavotricuspid isthmus atrial flutter (most common form of atrial flutter in patients who have not had prior catheter ablation or atrial surgery). Cardinal features are a perfectly regular atrial rhythm with inverted P waves inferiorly that have a positive overshoot, upright P waves in V1, and inverted P waves in V6.
Clockwise reentry using this same circuit can also occur, producing upright P waves inferiorly and inverted P waves in V1. Linear ablation of the cavotricuspid isthmus cures these common forms of atrial flutter. (See the video below.)
Non–isthmus-dependent flutters can occur elsewhere in the right atrium, as well as in the left atrium. Left atrial flutters are common after catheter ablation for atrial fibrillation, may be difficult to ablate, and generally require a three-dimensional mapping system to facilitate the procedure.
Atrioventricular nodal reentrant tachycardia
In the common form of AV nodal reentrant tachycardia (AVNRT), the inferior atrionodal input to the AV node serves as the anterograde limb (ie, the slow pathway) of the reentry circuit, and the superior atrionodal input serves as the retrograde limb (ie, the fast pathway). Typically, AVNRT can be cured by targeting the slow pathway near the inferior tricuspid valve annulus at the level of the coronary sinus os or somewhat higher.
The risk of iatrogenic heart block with ablation in this region is quite low (1-2%) when RF energy is used and close to zero when cryoablation is performed. Targeting the slow pathway is safer than targeting the fast pathway, which is located closer to the compact AV node. (See the images below.)
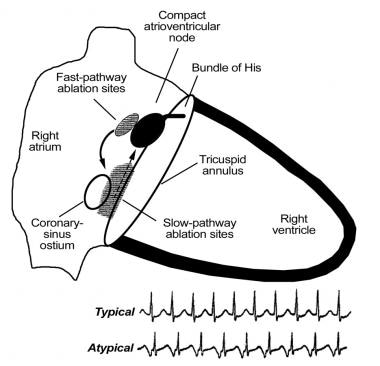 Catheter Ablation. Diagrammatic schema of the typical type of atrioventricular (AV) nodal reentrant tachycardia (AVNRT). The slow pathway (dashed arrow) is the usual anterograde limb of the reentry circuit and the usual ablation target area (shaded). The fast pathway (solid arrow) is the usual retrograde limb of the reentry circuit and commonly activates the atria simultaneously with ventricular activation, producing the typical electrocardiographic (ECG) finding of AVNRT shown below the figure in the first rhythm strip. The P wave is not visible, because it is buried in the QRS complex. Infrequently, the reentry circuit is reversed, with anterograde conduction over the fast pathway and retrograde conduction over the slow pathway, producing an atypical ECG finding of AVNRT as shown in the lower rhythm strip below the figure (ie, long R-P tachycardia, in which the interval between the QRS complex and the retrograde P wave is longer than the subsequent P-R interval, and the P wave is in second half of R-R interval). The fast pathway is close to the compact AV node, and ablation in this area is avoided if possible because of the risk of iatrogenic heart block.
Catheter Ablation. Diagrammatic schema of the typical type of atrioventricular (AV) nodal reentrant tachycardia (AVNRT). The slow pathway (dashed arrow) is the usual anterograde limb of the reentry circuit and the usual ablation target area (shaded). The fast pathway (solid arrow) is the usual retrograde limb of the reentry circuit and commonly activates the atria simultaneously with ventricular activation, producing the typical electrocardiographic (ECG) finding of AVNRT shown below the figure in the first rhythm strip. The P wave is not visible, because it is buried in the QRS complex. Infrequently, the reentry circuit is reversed, with anterograde conduction over the fast pathway and retrograde conduction over the slow pathway, producing an atypical ECG finding of AVNRT as shown in the lower rhythm strip below the figure (ie, long R-P tachycardia, in which the interval between the QRS complex and the retrograde P wave is longer than the subsequent P-R interval, and the P wave is in second half of R-R interval). The fast pathway is close to the compact AV node, and ablation in this area is avoided if possible because of the risk of iatrogenic heart block.
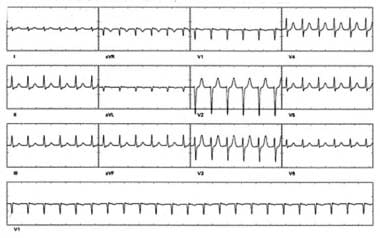 Catheter Ablation. The pseudo S waves inferiorly are retrograde P waves. This short interval (no isoelectric baseline) between the QRS complex and the retrograde P wave is highly specific for atrioventricular (AV) nodal reentrant tachycardia (AVNRT). A pseudo R wave in V1 may also be observed but is not seen here. More commonly, the retrograde P wave occurs during QRS activation and is not observed; this "no-P-wave" tachycardia also suggests AVNRT.
Catheter Ablation. The pseudo S waves inferiorly are retrograde P waves. This short interval (no isoelectric baseline) between the QRS complex and the retrograde P wave is highly specific for atrioventricular (AV) nodal reentrant tachycardia (AVNRT). A pseudo R wave in V1 may also be observed but is not seen here. More commonly, the retrograde P wave occurs during QRS activation and is not observed; this "no-P-wave" tachycardia also suggests AVNRT.
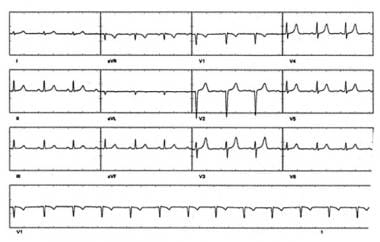 Catheter Ablation. Sinus rhythm in the same patient with atrioventricular (AV) nodal reentrant tachycardia (AVNRT) as in the preceding electrocardiogram. Note that pseudo S waves are no longer visible.
Catheter Ablation. Sinus rhythm in the same patient with atrioventricular (AV) nodal reentrant tachycardia (AVNRT) as in the preceding electrocardiogram. Note that pseudo S waves are no longer visible.
Orthodromic reciprocating tachycardia
In orthodromic reciprocating tachycardia (ORT), the AV node serves as the anterograde limb, and an accessory AV connection (typically called an accessory pathway) serves as the retrograde limb (see the images below).
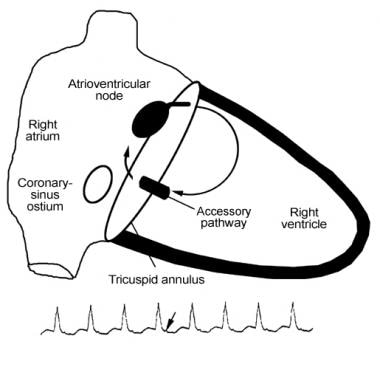 Catheter Ablation. Schema of orthodromic reciprocating tachycardia (ORT). The atrioventricular (AV) node serves as the anterograde limb, whereas an accessory pathway (AV connection) serves as the retrograde limb. Ventricular activation is necessary prior to retrograde activation of the accessory pathway. Therefore, the retrograde P wave (arrow in rhythm strip below the figure) occurs after the QRS complex.
Catheter Ablation. Schema of orthodromic reciprocating tachycardia (ORT). The atrioventricular (AV) node serves as the anterograde limb, whereas an accessory pathway (AV connection) serves as the retrograde limb. Ventricular activation is necessary prior to retrograde activation of the accessory pathway. Therefore, the retrograde P wave (arrow in rhythm strip below the figure) occurs after the QRS complex.
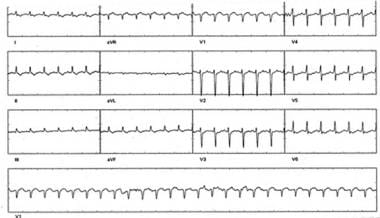 Catheter Ablation. Supraventricular tachycardia (SVT) in a patient with orthodromic reciprocating tachycardia (ORT) due to a concealed pathway. Note the retrograde P wave, seen best in lead V2, separated from the QRS complex by isoelectric baseline. This pattern of "short R-P tachycardia" (in which the interval between the QRS complex and the retrograde P wave is shorter than the subsequent P-R interval and the P wave is in first half of the R-R interval) suggests SVT incorporating an accessory pathway. A minority of AVNRT cases can also demonstrate a short-RP tachycardia (slow-slow AVNRT).
Catheter Ablation. Supraventricular tachycardia (SVT) in a patient with orthodromic reciprocating tachycardia (ORT) due to a concealed pathway. Note the retrograde P wave, seen best in lead V2, separated from the QRS complex by isoelectric baseline. This pattern of "short R-P tachycardia" (in which the interval between the QRS complex and the retrograde P wave is shorter than the subsequent P-R interval and the P wave is in first half of the R-R interval) suggests SVT incorporating an accessory pathway. A minority of AVNRT cases can also demonstrate a short-RP tachycardia (slow-slow AVNRT).
If an accessory pathway only conducts retrograde, it is termed a concealed accessory pathway because it is not identifiable on an electrocardiogram (ECG) in sinus rhythm (compare with Wolff-Parkinson-White [WPW] syndrome below). Typically, ORT can be cured by targeting the accessory pathway as it crosses the mitral or tricuspid valve annulus.
Wolff-Parkinson-White syndrome
Whereas ORT uses an accessory pathway in the retrograde direction, WPW syndrome by definition indicates an accessory pathway capable of anterograde conduction and is manifested by preexcitation (delta waves) on the sinus rhythm ECG.
Atrial fibrillation in WPW syndrome may result in life-threateningly fast anterograde conduction over the accessory pathway, manifested by an irregular wide-complex (preexcited) tachycardia that can sometimes degenerate to ventricular fibrillation (VF). Atrial fibrillation in WPW syndrome may be triggered by ORT. Ablation of the accessory pathway cures WPW syndrome, eliminating ORT, as well as atrial fibrillation in the majority of patients.
Unifocal atrial tachycardia
Unifocal atrial tachycardia, which can arise from either atrium or the noncoronary cusp of the aorta, is somewhat more challenging to ablate than the more common forms of generic supraventricular tachycardia (SVT). For those tachycardias originating from the left atrium, transseptal catheterization via a patent foramen ovale or transseptal puncture is usually required.
Ventricular tachycardia
Idiopathic VT most commonly arises from the right ventricular outflow tract and less commonly originates in the inferoseptal left ventricle about two thirds of the way toward the apex from the base of the left ventricle. These forms of VT are amenable to catheter ablation, though success rates are somewhat lower than those for the common forms of SVT.
VT in structural heart disease is also amenable to ablation. Some form of three-dimensional electroanatomic mapping is employed for these complex ablations to identify the scar that contributes to the anatomic substrate for reentry. Intracardiac echocardiography during the procedure and preprocedural imaging with magnetic resonance imaging (MRI) or computed tomography (CT) are also used in some instances. Anatomy from such imaging can be integrated with the electroanatomic map if necessary. Some VT substrates, especially VT in nonischemic cardiomyopathy, are not reachable from the endocardium. In these instances, percutaneous epicardial mapping and ablation are necessary.
Complications
The radiation risk from catheter ablation is low, but it may exceed the risk from common radiologic procedures. The average risk of genetic defects has been computed at 1 case per million births. The average risk of fatal malignancies ranges from 0.3 to 2.3 deaths per 1000 cases for every 60 minutes of fluoroscopy. [18] Many ablation procedures require less than 60 minutes of fluoroscopy.
Major complications occur in up to 3% of patients who undergo ablation procedures, [19] including thromboembolism in fewer than 1% (higher in some atrial fibrillation ablation series) and death in 0.1-0.2% of all procedures. The incidence of cardiac complications varies according to the site and type of ablation. Cardiac complications include the following:
-
High-grade atrioventricular block
-
Coronary artery spasm/thrombosis
-
Pericarditis
-
Valve trauma
Vascular complications, which occur in approximately 2-4% of procedures, include the following:
-
Retroperitoneal bleeding
-
Hematoma
-
Vascular injury
-
Transient ischemic attack/stroke
-
Hypotension
-
Thromboembolism or air embolism
Pulmonary complications include the following:
-
Pulmonary hypertension, with or without hemoptysis (secondary to pulmonary vein stenosis)
-
Pneumothorax
Miscellaneous complications include the following:
-
Left atrial–esophageal fistula
-
Acute pyloric spasm/gastric hypomotility
-
Phrenic nerve paralysis
-
Radiation- or electricity-induced skin damage
-
Infection at access site
-
Inappropriate sinus tachycardia
-
Proarrhythmia
-
Stiff left atrial syndrome [22]
Outcomes
Atrial fibrillation
Radiofrequency catheter ablation (RFCA) of the atrioventricular (AV) junction results in excellent rate control, relieves palpitations, and improves functional capacity. However, patients who undergo this procedure require both permanent pacemaker implantation to manage the resulting AV block and anticoagulation therapy to prevent stroke, because the atrial fibrillation itself is not affected. AV nodal modification is less therapeutic than AV junction ablation and may result in late heart block.
Single-procedure success rates for curing atrial fibrillation with RFCA are as high as 80% for paroxysmal atrial fibrillatoin in the absence of structural heart disease, and as low as 55% (or less) in patients with persistent atrial fibrillation in the presence of structural heart disease and left atrial enlargement. [23, 24] Repeat procedures are typically needed in at least 25% of patients and result in an increase in these success rates.
Samuel et al investigated the long-term effectiveness of catheter ablation in a cohort of 451 patients with atrial fibrillation and heart failure. Patients who underwent catheter ablation had fewer rehospitalizations and all-cause mortality compared to patients who did not receive catheter ablation. [25]
Success rates for atrial fibrillation ablation have historically been based on patient symptoms and periodic electrocardiographic (ECG) monitoring. Success rates are lower if intensive ambulatory monitoring to detect asymptomatic atrial fibrillation recurrence is used, such as daily monitoring for a month with an auto-triggering event monitor. Some patients require the use of previously ineffective antiarrhythmic drugs to maintain success.
Supraventricular tachyarrhythmias
The common forms of supraventricular tachycardia (SVT) (eg, AV nodal reentrant tachycardia [NRT], SVT associated with an accessory pathway) are usually curable with a single procedure; the success rate is typically 90-95%. Cure rates for unifocal atrial tachycardia and common right atrial flutter are somewhat lower but still approach 90%. Recurrent tachyarrhythmias typically occur in the first few months after ablation and may be amenable to cure with a second procedure.
AVNRT is usually amenable to cure with a slow pathway ablation near the inferior atrial septum, where the risk of heart block is 1-2% with RF energy. In those uncommon cases where RF ablation near the compact AV node is required (eg, fast pathway ablation for AVNRT or an accessory pathway in a para-Hisian location), the risk of heart block may approach 5% or a little higher.
In a number of centers, catheter-based cryoablation, rather than RFCA, is used near the compact AV node to minimize the risk of heart block. With cryoablation, heart block is generally reversible with prompt rewarming. However, cryoablation appears to be slightly less effective than RF as an energy source, especially for deep accessory pathways.
Ventricular tachyarrhythmias
Idiopathic ventricular tachycardia (VT) is curable (success rate ~80%), provided that it is readily inducible during the electrophysiologic study. The most common location for these VTs is the right ventricular outflow tract. Because these VTs are usually not reentrant in nature, a significant percentage are not inducible. Some cannot be ablated because of their deep septal location or their epicardial location near a coronary artery.
Of patients with VT associated with structural heart disease, half to two thirds can obtain palliation with catheter ablation. Extensive scarring in these ventricles may limit the efficacy of the relatively small lesions made by RFCA, and multiple VT circuits may also contribute to this moderate success rate. In practice, many of these patients have implantable cardioverter-defibrillators (ICDs), and catheter ablation is used as adjunctive therapy for frequent device activations.
For patients with structural heart disease and stable VT, the potential benefit of catheter ablation before implantation of an ICD was demonstrated in the Ventricular Tachycardia Ablation in Coronary Heart Disease (VTACH) study. [26] This prospective, randomized, controlled international trial in 104 patients found that time to recurrence of VT or ventricular fibrillation (VF) was longer in the ablation group (median, 18.6 months) than in the control group (5.9 months). At 2 years, estimates for survival free from VT or VF were 47% in the ablation group and 29% in the control group. [26]
Future Directions
A curative procedure for atrial fibrillation is a major goal in clinical cardiac electrophysiology. Success has been achieved in patients with paroxysmal lone atrial fibrillation by eliminating conduction from the pulmonary veins to the left atrium, as many of these episodes are triggered by rapid electrical activity arising from tissue near the pulmonary vein ostia or from muscle sleeves surrounding the proximal veins. Other forms of atrial fibrillation may require some degree of substrate ablation (eg, linear transmural lesions in the left atrium).
Techniques are evolving to address the challenge of a catheter-based cure for all forms of atrial fibrillation. Three-dimensional electroanatomic maps, overlaid on magnetic resonance imaging (MRI) or computed tomography (CT) scans of the left atrium, can facilitate navigation of the catheter and mapping of the arrhythmogenic substrate. Intracardiac echocardiography may also help in avoiding collateral damage to the pulmonary veins or esophagus, ensuring adequate endocardial contact, and monitoring for complications (eg, pericardial effusion, thrombus development).
Evidence is accruing in support of radiofrequency catheter ablation (RFCA) of atrial fibrillation to reduce mortality and heart failure hospitalization in selected patients with left ventricular dysfunction. [16]
Alternative energy sources are being investigated in the ablation of atrial fibrillation (eg, laser, ultrasound) and ventricular tachycardia (external radiation). In addition, robotic catheter navigation is available to deliver RFCA.
Research is also focused on developing better methods and tools for catheter ablation of ventricular tachycardia (VT), and even ventricular fibrillation (VF), in patients with structural heart disease. Epicardial electrophysiology via subxiphoid pericardial puncture is a relatively new frontier; some tachyarrhythmia substrates (especially VT in nonischemic cardiomyopathy) cannot be reached from the endocardium.
-
Catheter Ablation. Diagrammatic schema of the typical type of atrioventricular (AV) nodal reentrant tachycardia (AVNRT). The slow pathway (dashed arrow) is the usual anterograde limb of the reentry circuit and the usual ablation target area (shaded). The fast pathway (solid arrow) is the usual retrograde limb of the reentry circuit and commonly activates the atria simultaneously with ventricular activation, producing the typical electrocardiographic (ECG) finding of AVNRT shown below the figure in the first rhythm strip. The P wave is not visible, because it is buried in the QRS complex. Infrequently, the reentry circuit is reversed, with anterograde conduction over the fast pathway and retrograde conduction over the slow pathway, producing an atypical ECG finding of AVNRT as shown in the lower rhythm strip below the figure (ie, long R-P tachycardia, in which the interval between the QRS complex and the retrograde P wave is longer than the subsequent P-R interval, and the P wave is in second half of R-R interval). The fast pathway is close to the compact AV node, and ablation in this area is avoided if possible because of the risk of iatrogenic heart block.
-
Catheter Ablation. The pseudo S waves inferiorly are retrograde P waves. This short interval (no isoelectric baseline) between the QRS complex and the retrograde P wave is highly specific for atrioventricular (AV) nodal reentrant tachycardia (AVNRT). A pseudo R wave in V1 may also be observed but is not seen here. More commonly, the retrograde P wave occurs during QRS activation and is not observed; this "no-P-wave" tachycardia also suggests AVNRT.
-
Catheter Ablation. Sinus rhythm in the same patient with atrioventricular (AV) nodal reentrant tachycardia (AVNRT) as in the preceding electrocardiogram. Note that pseudo S waves are no longer visible.
-
Catheter Ablation. Schema of orthodromic reciprocating tachycardia (ORT). The atrioventricular (AV) node serves as the anterograde limb, whereas an accessory pathway (AV connection) serves as the retrograde limb. Ventricular activation is necessary prior to retrograde activation of the accessory pathway. Therefore, the retrograde P wave (arrow in rhythm strip below the figure) occurs after the QRS complex.
-
Catheter Ablation. Supraventricular tachycardia (SVT) in a patient with orthodromic reciprocating tachycardia (ORT) due to a concealed pathway. Note the retrograde P wave, seen best in lead V2, separated from the QRS complex by isoelectric baseline. This pattern of "short R-P tachycardia" (in which the interval between the QRS complex and the retrograde P wave is shorter than the subsequent P-R interval and the P wave is in first half of the R-R interval) suggests SVT incorporating an accessory pathway. A minority of AVNRT cases can also demonstrate a short-RP tachycardia (slow-slow AVNRT).
-
Catheter Ablation. Schema of the common variety of atrial flutter (counterclockwise cavotricuspid isthmus flutter). The reentry circuit is confined to the right atrium and circulates as a counterclockwise macroreentrant circuit proceeding superiorly over the atrial septum and inferiorly over the lateral atrial wall. The wave front circulates through a narrow isthmus of tissue between the tricuspid valve annulus and the inferior vena cava. Linear ablation across this isthmus (cavotricuspid isthmus) cures this common form of atrial flutter.
-
Catheter Ablation. Typical counterclockwise cavotricuspid isthmus atrial flutter (most common form of atrial flutter in patients who have not had prior catheter ablation or atrial surgery). Cardinal features are a perfectly regular atrial rhythm with inverted P waves inferiorly that have a positive overshoot, upright P waves in V1, and inverted P waves in V6.
-
Catheter Ablation. Early version of an electroanatomic map of the posterior left atrium, illustrating the pulmonary veins: right superior pulmonary vein (RSPV), right inferior pulmonary vein (RIPV), left superior pulmonary vein (LSPV), and left inferior pulmonary vein (LIPV). Red circles represent discrete radiofrequency (RF) applications, predominantly delivered in a circumferential pattern around the pulmonary veins. This ablation strategy can isolate foci in or near the pulmonary vein opening that initiate atrial fibrillation (AF) or alter the substrate of the left atrium to inhibit fibrillatory activity due to reentry. Image courtesy of American College of Cardiology Foundation.
-
Catheter Ablation. Three-dimensional electroanatomic map of cavotricuspid isthmus flutter. Colors progress from red to purple and represent relative conduction time in the right atrium (early to late). An ablation line (red dots) has been created from the tricuspid annulus to the inferior vena cava. This interrupts the flutter circuit.






