Background
The flow-directed balloon-tipped pulmonary artery catheter (PAC) (also known as the Swan-Ganz or right heart catheter) was first described in the medical literature in 1970. [1] Initially developed for the management of acute myocardial infarction (AMI), it gained widespread use in the management of a variety of critical illnesses and surgical procedures. This article covers both the clinical and technical aspects of its use.
Settings in Which Use of a PAC Is Appropriate
In 2012, the American College of Cardiology Foundation (ACCF) published guidelines on the appropriate use of diagnostic pulmonary artery catheterization. [2]
Diagnostic
According to the ACCF guidelines, pulmonary artery catheterization is appropriate in the following diagnostic settings [2] :
-
Suspected acute coronary syndrome
-
Suspected coronary artery disease (CAD)
-
On stress testing with imaging, in high-risk findings in patients who are either symptomatic or asymptomatic, or in intermediate-risk findings, discordant findings, or equivocal/uninterpretable findings in patients who are symptomatic
-
On echocardiography, in findings of newly recognized, symptomatic, left ventricular (LV) dysfunction; in symptomatic, new regional wall-motion abnormality of unknown etiology; or in suspected significant ischemic complications related to CAD
-
On computed tomography (CT) angiography, for symptomatic lesions of 50% of greater, left main or non-left main; for symptomatic lesions of 50% or greater in more than one coronary territory; for symptomatic lesions of unclear severity, possibly obstructive (non-left main); and for symptomatic or asymptomatic, possibly obstructive non-left main lesions
Therapeutic
According to the ACCF guidelines, pulmonary artery catheterization is appropriate for aspiration of air emboli. [3]
Settings in Which Use of a PAC Is Inappropriate
According to the 2012 guidelines from the ACCF, pulmonary artery catheterization is inappropriate in the following settings [2] :
-
When CAD is suspected in an asymptomatic patient with low or intermediate global risk for CAD, in the absence of noninvasive stress imaging showing stenosis of 50% or greater; when CAD is suspected in a symptomatic patient with low pretest probability
-
As adjunctive testing in patients undergoing diagnostic coronary angiography, pulmonary artery catheterization is inappropriate in patients who have nonobstructive disease (non–left main) with stenosis of less than 50% in whom there are unexpected angiographic findings or findings of ischemia, or in the absence of noninvasive testing
-
In cases of arrhythmia, pulmonary artery catheterization is inappropriate in patients at low risk for syncope, in patients at low or intermediate risk for new-onset atrial fibrillation or flutter, and in patients with heart block or symptomatic bradyarrhythmia
-
In preoperative coronary evaluation, pulmonary artery catheterization is inappropriate for patients undergoing low-risk surgery, in patients with 4 or more METS (metabolic equivalents of functional capacity), in patients with no risk factors who are to undergo intermediate-risk surgery or vascular surgery, and in patients with 1 or 2 risk factors who are to undergo intermediate-risk surgery
-
Pulmonary artery catheterization is inappropriate in patients with mild or moderate mitral stenosis, mild or moderate mitral regurgitation, mild or moderate aortic stenosis, or mild or moderate aortic regurgitation
-
In patients with chronic native or prosthetic valvular disease who have symptoms related to their valvular disease, pulmonary artery catheterization is inappropriate for the following, provided that the patient's symptoms are concordant with the clinical impression of severity: mild, moderate, or severe mitral stenosis; mild, moderate, or severe mitral regurgitation; mild, moderate, or severe aortic stenosis; and mild or moderate aortic regurgitation
Best Practices
Passage of the PAC is difficult in certain disease states. When right-sided pressures are elevated, the air-filled balloon actually may hinder proper positioning. In such cases, filling the balloon with 1 mL of sterile saline and placing the patient in a more upright position allows gravity to cause the PAC to fall into position. Once the catheter is in position, aspirate the saline and replace it with air to ensure reproducible PCWP tracings. Insertion with a noninflated balloon may also allow passage into the PA. Neither of these techniques is advocated by PAC manufacturers and the techniques have the potential for adverse events. These techniques should only be used in extenuating circumstances and should only be attempted by experienced practitioners under the guidance of fluoroscopy.
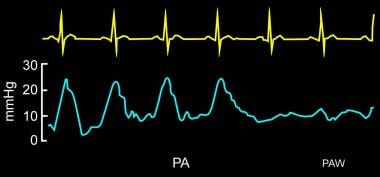
The presence of large V waves can make discriminating a PA tracing from a PCWP tracing difficult. Look for subtle signs of waveform differences, such as loss of the dicrotic notch in the PA tracing (see image below).
Determining the oxygen saturation of a blood sample obtained from the distal lumen while the balloon is inflated also can confirm that the waveform is a true PCWP. After aspirating enough volume (5-7 mL) to clear the blood from the PA distal to the inflated balloon, the oxygen saturation should be similar to that measured by arterial blood gas or pulse oximetry, thus confirming that the catheter is in the correct position to measure PWCP.
Fluoroscopy may be required for proper placement of the PAC in difficult situations.
Potential minimally invasive and noninvasive measures of hemodynamic profile
When pulmonary artery catheterization is not available in patients with a hyperdynamic condition (eg, cirrhotic patients with reduced SVR and increased cardiac output), minimally invasive indicators such as diastolic reflected waveform characteristics may potentially be used to predict high cardiac index and low SVR. [4]
Transthoracic echocardiography appears to be feasible and accurate for evaluating the hemodynamic profile in patients with decompensated heart failure. [5] As with pulmonary artery catheterization, this imaging modality is also able to measure central venous pressure, pulmonary arterial pressure, pulmonary capillary wedge pressure, pulmonary vascular resistance, stroke volume and cardiac output, and SVR. Transesophageal echocardiography potentially could have similar utlity. [5]
Controversies
Over 1 million PACs are used annually in North America. Given the frequency and duration of their use, it is surprising that only recently were quality randomized clinical trials published. Initially, observational studies from the 1980s and 1990s indicated a greater mortality rate in patients who underwent placement of a PAC than in those who did not. The major criticism of these studies is that the more acutely ill (and therefore at greatest risk of death initially) are more likely to receive a PAC.
However, since then a number of randomized clinical trials were published.
Sandham et al enrolled nearly 2000 surgical patients (ASA class 3 or 4) aged 60 years or older. The treatment group received a PAC and a guided therapy protocol while the control group received a central venous catheter and therapy based on physician discretion. No difference was noted in the 2 groups in mortality rate (8%), length of stay, or organ dysfunction. [6]
Richard et al randomized almost 700 patients with early shock, ARDS, or both to use of PAC or not. Treatment was left to physician discretion. No significant difference in mortality or morbidity was noted. [7]
Rhodes et al randomized 201 patients and found no difference in 28-day mortality, ICU, or hospital length of stay in patients with or without a PAC. A formal management protocol was not used. [8]
The PACMAN trial enrolled more than 1000 patients to management with or without a PAC. The timing of insertion and management were at the discretion of the treating physician. No difference in hospital mortality (primary outcome) was noted. [9]
The ESCAPE trial enrolled more than 400 patients who were admitted with severe heart failure. Patients were randomized to having therapy guided by clinical assessment and PAC or clinical assessment alone. No difference was noted in overall mortality or hospitalization. [10]
Shah et al published a large meta-analysis that consisted of 13 randomized clinical trials and more than 5000 patients (including the above mentioned trials). Neither an increase in mortality or length of hospital stay nor a significant benefit could be attributed to the use of a PAC. [11]
In 2006, the ARDSNET group looked at the role of the PAC in acute lung injury patients. Patients were randomized to protocolized hemodynamic management with either a PAC or a central venous catheter (CVC). No difference in mortality (primary outcome), ICU length of stay, or lung function was appreciated. [12] As well, economic analysis follow-up confirmed increased cost associated with PAC use. [13]
As well, even in the hallowed ground of PAC use in cardiac surgery patients, Djaiani et al showed that the use of PAC derived data lead to more interventions but no overall clinical benefit. [14]
Two observational, propensity-matched analyses in both trauma and cardiac surgery populations failed to show any benefit for the use of the PAC. In fact, these studies showed increased mortality and morbidity in the PAC use groups. [15, 16]
Unfortunately, a Cochrane Review of 12 studies was only able to support the need for "efficacy studies... to determine optimal management protocols and patient groups who could benefit from management with a PAC." [17] A more recent updated Cochrane Review included the ARDSNET trial but essentially came to similar conclusions. [18]
Data from the Acute Decompensated Heart Failure Syndromes (ATTEND) Registry involving 4842 patients showed appropriate PAC use reduced in-hospital mortality in patients with acute heart failure syndromes, particularly those with lower systolic blood pressure or who received inotropic therapy. [19] In the study, 16.8% patients (n = 813) were managed with PACs, of which 502 were propensity score-matched with 502 control subjects. Patients in the PAC group had lower all-cause mortality than those in the control group. [19]
Overall, the literature does not show a positive effect on patient outcome with PAC use. However, a criticism of the current available research is that patient groups potentially benefit from the use of a PAC but this effect is lost in studies that also include patient groups that gain little or no benefit. Chittock et al published an observational cohort study showing that PAC use was associated with increased mortality in less acutely ill patients but associated with decreased mortality in more acutely ill patients. [20]
As well, the use of a monitoring tool such as the PAC itself is unlikely to show a significant treatment effect. Some criticize the current literature because a number of studies did not use a predefined treatment protocol. The lack of defined specific treatment based on measured PAC-derived variables could contribute more to patient outcome than merely the presence or absence of a PAC.
Contention also exists that PACs do not harm people; people harm people. In other words, operator competence may be the root cause of the mortality difference. Reviews have shown deficiencies in both nursing-dependent information derived from the PAC as well as physician-dependent interpretation and subsequent management.
Nevertheless, the growing evidence of the limitations of PACs may be affecting clinical practice. A time-trend analysis on national estimates of PAC use in the United States from 1993–2004, using data from the Nationwide Inpatient Sample, found a 65% decrease in PAC use during that period; the most prominent decline, by 81%, was in use of PACs for myocardial infarction. [21] Canadian authors also found more than a 50% reduction in PAC use. [22]
The advent of newer noninvasive imaging modalities may also be making inroads into PAC use. For example, noninvasive devices have been developed that measure cardiac output using ultrasound. [23] As well, the measurement of arterial pressure waveforms have shown good correlation when compared to the PAC thermodilution technique. [24]
The increased availability of bedside echocardiography is likely contributing to the decline in PAC use, as was postulated in a recent observational study in patients with acute coronary syndromes. [25]
More recent reviews agree on the importance of a measured approach to PAC use; PACs remain an important tool, but they should be used only in selected patients and only by well-trained physicians. [26, 27, 28, 29, 30]
A concise review by Chatterjee offers some reasonable indications for ongoing PAC use; however, these unfortunately lack empiric evidence at present. [26]
-
A pulmonary artery catheter is shown here.
-
The balloon of the catheter should be checked prior to insertion.
-
Pulmonary artery catheter being introduced from pulmonary artery in to wedge position.
-
Normal hemodynamic parameters.
-
Central venous pressure (CVP) measured in superior vena cava (SVC) is identical to right atrial pressure (RAP).
-
Respiratory variation is easily identified on the right atrial waveform.
-
Various waveforms of central venous pressure (CVP) monitoring are shown here.
-
Pulmonary arterial pressure (Ppa) waveform.
-
Pulmonary artery wedge pressure (PAWP) waveform can be distinguished easily from the pulmonary arterial waveform in most clinical scenarios.
-
Pulmonary artery wedge pressure (PAWP) reflects left atrial pressure (LAP).
-
Inflated balloon obstructs arterial flow and reflects pressures at J point. Redrawn from Principles of Critical Care by Jesse B. Hall, Gregory A. Schmidt, Lawrence D. H. Wood, 2000, McGraw-Hill, Inc.
-
Having an inflated balloon in a proximal vessel is better because a vessel branch is likely to reflect left atrial pressure (LAP) accurately. Redrawn from Principles of Critical Care by Jesse B. Hall, Gregory A. Schmidt, Lawrence D. H. Wood, 2000, McGraw-Hill, Inc.
-
Right or left atrial pressure waveform.
-
Timing of the pulmonary artery waveforms in relation to electrocardiographic monitoring is shown here. An A wave follows the QRS wave on ECG, whereas V wave follows the T wave on ECG.
-
Physiologic lung zones. For pulmonary capillary wedge pressure (PCWP) to be reliable, the catheter tip must lie in zone 3. Pulmonary artery pressure (Ppa) is greater than pulmonary venous pressure (Ppv), which is greater than alveolar pressure (Palv) at end-expiration. In zones 1 and 2, Ppw reflects Palv if Palv is greater than Ppv. Redrawn from Principles of Critical Care by Jesse B. Hall, Gregory A. Schmidt, Lawrence D. H. Wood, 2000, McGraw-Hill, Inc.
-
Hemodynamic parameters in different pathologic states.
-
Tall V waves presented here on pulmonary arterial and wedge pressure waveforms are characteristic of severe mitral regurgitation.
-
Large V waves in a case of mitral regurgitation.
-
Simultaneous recording of ECG helps identify V waves in mitral valve regurgitation; V waves correspond to T waves on ECG.
-
Hemodynamic monitoring can confirm the diagnosis of pericardial tamponade. Equalization of diastolic pressures on the left and right sides of the heart, elevated right atrial pressure, and Kussmaul sign (ie, increase in right atrial pressure with inspiration) are noted.
-
In cardiac tamponade, systemic arterial pressure (Pa) reflects pulsus paradoxus. Right atrial pressure (RAP) is elevated. Pulmonary artery (PA) diastolic pressure equals mean right atrial (RA), right ventricular (RV) diastolic, and wedge pressures.
-
Simultaneous recordings of pulmonary capillary wedge pressure and left ventricular pressure waveforms in a patient with constrictive pericarditis. Note the equalization of diastolic pressures and "square root sign" or "dip and plateau sign" of the left ventricular waveforms, which are confirmatory of the diagnosis of constrictive pericarditis.
-
Right atrial pressure waveform of a patient with constrictive pericarditis. Please note rapid X and Y descents, and elevated A and V waves. This gives an impression of the letter "M" or "W" and is confirmatory of the diagnosis of constrictive pericarditis.
-
Principle of cardiac output measurement.