Practice Essentials
Classification of nerve injury is based on the damage sustained by the nerve components, nerve functionality, and the ability for spontaneous recovery. [1, 2, 3] Classification systems for nerve injuries were established by Seddon in 1943 and Sunderland in 1951. [3] Seddon created a 3-grade classification, and Sunderland categorized nerve injuries into 5 grades. Neurapraxia is a reduction or complete block of conduction across a segment of a nerve with axonal continuity conserved. [1, 4, 5] Axonotmesis is a more severe grade of nerve injury than neurapraxia, and neurotmesis is the most severe grade of peripheral nerve injury. [6]
Mechanisms of injury include mechanical, crush/percussion, blunt, penetrating, stretch, high velocity (motor vehicle, gunshot), and cold leading to necrosis. Trauma to peripheral nerves is relatively common. The most common injury is from blunt trauma or from penetrating missiles, such as bullets or other objects. [4] An important quality of the peripheral nervous system, as compared to the central nervous system, is its remarkable ability to recover after an injury through remyelination and regeneration of the axon. [1]
Peripheral nerve dysfunction can be debilitating, because peripheral nerves generate the signals that govern both pain and peripheral motor function. An untreated acute injury to a nerve can progress to chronic nerve injury. [7, 8] Acute nerve injuries are common in patients with multisystem trauma. Once a nerve injury occurs, effects on the nerve, neuromuscular junction, and muscle begin to occur. Such effects may be irreversible 18-24 months after denervation. Therefore, appropriate acute management without delay is important. [9, 6]
Differentiating between a peripheral nerve problem and an injury involving the spinal cord, brain, bone, or soft tissue is crucial. After establishing baseline physical examination findings, the physician must answer the following questions [1] :
-
Do the symptoms and findings localize to a lesion in the central or peripheral nervous system?
-
Are the symptoms and findings consistent with a focal or a diffuse type of peripheral nerve problem?
-
Is the nerve injury complete or incomplete?
-
What is the grade of the peripheral nerve injury?
-
Does clinical evidence indicate recovery or further neurological deterioration?
(See the image below.)
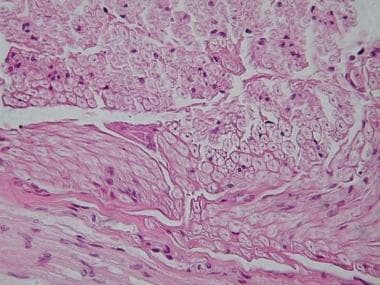 Peripheral nerve, cross-section. Image courtesy of the Department of Histology, Jagiellonian University Medical College.
Peripheral nerve, cross-section. Image courtesy of the Department of Histology, Jagiellonian University Medical College.
Diagnosis
The workup of every patient with acute nerve injury begins with a complete history and a physical examination. The site of injury can be accurately localized from a precise neurologic examination. [2] The strength of individual muscles or of muscle groups is graded, and a sensory examination is performed, which includes testing for light touch, pinprick, 2-point discrimination, vibration, and proprioception. [1] Imaging techniques, such as radiography, computed tomography (CT) scan, and magnetic resonance imaging (MRI) are valuable diagnostic tools for evaluating a peripheral nerve lesion. [1] To rule out bony and ligamentous injuries, all patients with axillary nerve injury should have radiographs taken of the shoulder and cervical spine. [10]
CT scan and traditional MRI have certain limitations in distinguishing peripheral nerves from the surrounding structures, in which case magnetic resonance neurography (MRN) can help visualize both normal and abnormal peripheral nerves. [11]
A nerve conduction study (NCS) can be effective in identifying peripheral nerve injury. [12] Peripheral nerves are stimulated by somatosensory evoked potentials (SSEPs), and if signal conduction is disrupted along any segment of the circuit, an evoked potentiation is not produced. [1, 5] [1]
Treatment
Technological advances in neurosurgical instrumentation and diagnostic imaging have led to great results in the repair of acute nerve injury. [5, 13] Accurate grading of an acute traumatic injury is necessary because surgery is indicated for patients with neurotmesis (Sunderland grade III-V). [3] Surgical intervention is based on the extent of nerve damage and functional viability of the nerve. Surgeons need to consider injury location, extent of injury, age, and medical condition. It is important to determine whether function can be restored and whether benefit outweighs risk. [14]
Primary repair is direct reconnection of the nerve immediately after injury. In an epineurial repair, the epineuriums of the separated nerve endings are sutured together [15] Best results occur when the nerves are either purely sensory or purely motor and when the intraneural connective tissue component is small and the fascicles have been clearly aligned.
Secondary repairs are delayed repairs. Many surgeons prefer delayed suture because it allows the wound to heal and decreases the risk of infection. Also,, during a delayed repair, scarred ends of the nerve can be defined more accurately and trimmed back to normal fasciculi. [4]
In general, contraindications to surgery usually result when the risks of surgery outweigh the benefits. Surgery should not be performed when a poor outcome is expected. Some surgical repairs initially contraindicated may be performed later. If a swollen and discolored peroneal nerve is encountered during an acute knee repair, it should not be resected; rather, waiting several months is better. [16, 17]
In traumatic peripheral nerve injuries, the rule of 3 may be applied to surgical timing: immediate surgery within 3 days for clean and sharp injuries; early surgery within 3 weeks for blunt/contusion injuries; and delayed surgery 3 months after injury for closed injuries. [18]
Problem
Definition of nerve injury
Classification of nerve injury is based on the damage sustained by the nerve components, nerve functionality, and the ability for spontaneous recovery. [1, 2, 3] Seddon (1943) published his classification of nerve injuries, and Sunderland (1951) expanded this grading system. [3] The significance of Seddon's 3-grade classification system is its clinical relevance in predicting functional outcome and formulating an appropriate treatment plan. [1]
Nerve injury produces a long-lasting neuropathic pain, manifested as allodynia, a decrease in pain threshold and hyperplasia, and an increase in response to noxious stimuli. The mechanism underlying the lasting abnormal pain is not well understood. [19]
The mildest grade is called neurapraxia. [20, 21] Neurapraxia is a reduction or complete block of conduction across a segment of a nerve with axonal continuity conserved. [4, 1, 5] More specifically, it is dysfunction and/or paralysis without loss of nerve sheath continuity and peripheral wallerian degeneration. [3, 14] Nerve conduction is preserved both proximal and distal to the lesion but not across the lesion. [1] A person's foot "falling asleep" after his legs have been crossed is an example of a functional loss without abnormal change. [2]
Axonotmesis is a more severe grade of nerve injury than neurapraxia. Axonotmesis is a result of damage to the axons, with preservation of the neural connective tissue sheath (endoneurium), epineurium, Schwann cell tubes, and other supporting structures. [4, 1, 5] Thus, the internal architecture is relatively preserved. [14] This can guide proximal axonal regeneration to reinnervate distal target organs. [4, 2] Distal wallerian degeneration occurs in axonotmesis. [3]
Neurotmesis is the most severe grade of peripheral nerve injury. It occurs when the axon, myelin, and connective tissue components are damaged and disrupted or transected. [2, 3, 14] Recovery through axonal regeneration cannot occur. This grade of injury includes nerve lesions in which external continuity is preserved but intraneural fibrosis occurs and blocks axonal regeneration. [1, 14, 22]
Sunderland categorized nerve injuries into 5 grades. Grades I and II correspond to Seddon's neurapraxic and axonotmetic grades of injury. Sunderland further divided Seddon's category of neurotmesis injuries into grades III, IV, and V based on the extent of damage to the axonal supporting structures. [1]
In grade III injuries, axon continuity is disrupted by loss of endoneurial tubes (the neurolemmal sheaths), but the perineurium is preserved. Thus, when the axons regenerate, they may enter an incorrect nerve sheath, resulting in abnormal regeneration. [1, 3] Accompanying the loss of the nerve sheath is intraneural scarring, which further obstructs axonal regrowth through the site of injury. [4] In grade IV injuries, nerve fasciculi (ie, axon, endoneurium, perineurium) are damaged but nerve sheath continuity is preserved. [4, 1, 3] Nevertheless, intraneural scarring occurs. [4, 5, 8]
In grade V injuries, the endoneurium, perineurium, and epineurium, which make up the entire nerve trunk, are completely divided. [1, 5, 3] Substantial perineural hemorrhage and scarring occur in this grade of injury. [4] Grade V corresponds to Seddon's classification of a neurotmesis lesion. Mackinnon and Dellon presented a grade VI injury that represented a complex peripheral nerve injury. This grade involved combinations of Sunderland's grades of injury. [1, 8]
Epidemiology
Frequency
Trauma to peripheral nerves is relatively common. The most common injury is from blunt trauma or from penetrating missiles, such as bullets or other objects. [4] In the vast majority of gunshot wounds, the nerve is not divided. Occasionally, a complete or partial transection of the nerve develops. [4] Luce and Griffin reported that approximately 50% of nerve deficits after high-velocity shotgun injuries to the upper extremity involve complete transection. [3] Injuries from stab wounds or foreign bodies (eg, glass, sheet metal) resulting in clean lacerations of nerves are not common. However, this form of injury is significant because it presents an opportunity for primary repair of a peripheral nerve immediately after an injury. [4]
Nerve injury can be associated with fractures and fracture-dislocations. The probability of nerve injury is doubled with fracture associated with shoulder dislocation. [23] Approximately 95% of peripheral nerve injuries associated with a fracture occur in the upper extremity. The most common form is radial nerve injuries associated with humeral fractures. [4] The most common injury resulting from dislocations or fracture-dislocation of the elbow seems to be ulnar nerve neurapraxia, which spontaneously resolves after a closed reduction. Radial nerve palsy after fracture of the humerus is the most common nerve lesion in long bone fractures. [24]
The average incidence of radial nerve lesions is approximately 11%. Most reported series of radial nerve lesions are associated with fractures of the middle third of the humerus. A review of the literature demonstrated that 60% of radial nerve lesions were found in midshaft fractures and that 28% were found in fractures in the distal one third. However, Pollack et al and others reported that 63% of radial nerve lesions were found in fractures of the distal one third of the humerus.
The reported incidence rate of nerve injury with supracondylar fractures in children ranges from 12 to 16%. Posterior, medially displaced fractures are more likely to be associated with neural compromise. Several studies suggest that 86 to 100% of these injuries are neurapraxias. A greater incidence of high-grade nerve injuries occurs with open fractures. Foster et al found that in 64% of open humeral shaft fractures, nerves were either lacerated or interposed between fracture fragments. [3]
Neural injury is more common with dislocations, which result in stretch injuries to the nerve, occurring in 18% or more of knee dislocations and more than 13% of posterior hip dislocations. [4] Nerve injury is common in dislocations of the shoulder, with an incidence rate of 48%. [23]
Bado reported a 20% incidence rate of radial nerve palsy with lateral radial head dislocations. [3] A 34% risk of nerve injury exists with isolated greater tuberosity fracture dislocation. [25] The literature reveals that in nerve injury from traumatic dislocation and fracture-dislocation of the hip, the incidence rate is approximately 10% in adults and 5% in children. A possible explanation for the low incidence of nerve injury in children is that younger children are more susceptible to dislocation after relatively low-energy trauma. However, in high-energy trauma (eg, motor vehicle accidents), considerably higher incidence of nerve injury would be reported in children. [26] Axonal loss in anterior dislocation of the shoulder was observed in 48% of 77 patients. Of these patients, 51% had a solitary nerve lesion and the axillary nerve was involved most frequently in 42% of the patients. [23]
Upper extremity nerve injury commonly results from impact to the neck and shoulder. One such injury, a burner (also called a stinger), is characterized by pain that radiates down one upper extremity. Numbness, paresthesias, or weakness can develop and commonly recur, leading to further disability and occasionally chronic syndromes. Burners are usually brief and self-limited, although recovery can take weeks or months in some cases. [27]
Iatrogenic etiology is another possible cause of injury. The incidence of nerve injuries varies from 1 to 10% in patients with closed forearm fractures treated by plating. However, determining whether the injury resulted from fracture or operative intervention often is difficult. [3] The reviews by Small and the Arthroscopy Association of America have demonstrated a low incidence of nerve injury after shoulder arthroscopy. [10] Associated trauma can increase the probability of nerve injury. For example, a hematoma formation at the site of injury increases the probability of nerve injury 4.4-fold. The sex of the individual, the cause of trauma, the position of the arm during the fall, the side of the body on which the injury occurs, dominance of the right or left side, the presence of diabetes mellitus, or the use of an anticoagulant does not influence the frequency of nerve damage. [23]
Etiology
Dysfunction of peripheral nerves results from damage to the neuron, to the Schwann cells, or to the myelin sheath. Damaged nerves cannot transmit impulses in normal fashion. [28] Many mechanisms of injury to peripheral nerves exist. Each mechanism of injury can cause specific nerve damage. The force and level of injury to the shoulder plays an important role in the type of nerve injury sustained. [29]
The first mechanism of injury is mechanical injury. Two examples of mechanical injury are "Saturday-night paralysis" of the radial nerve and tourniquet paralysis. [30] Kurihara and Goto reported radial nerve paralysis in 2 patients with previous humeral fractures after tourniquet use. [3] Mechanical injury results in focal conduction block related to an acute compression injury. [30]
The second mechanism of injury is crush and percussion injury. This injury can be described through an animal model. With this model, the usual method is squeezing the nerve with smooth-tipped forceps, which induces focal compression injury. [30] In humans, compression injuries can be induced by fractures, hematomas, and compartment syndrome. Proximal venous ligation, direct trauma to muscle, infection, burns, and localized pressure by casts or by circular dressings also are causes of compartment syndrome.
Compartment syndrome injuries cause high pressure in the surrounding tissue. Pressure compresses the arterial blood supply of the nerve, predisposing the nerve to ischemic cell damage and cell death. [4] Although the peripheral nervous system is relatively resistant to ischemia, long periods of stretch and compressive force can cause vascular compromise and neuronal ischemia. [1] Delays in assessment and treatment of compartment syndrome can lead to nerve injury in the forearm. [3] Concussion or compression of the nerve causes neurapraxia. [4] Other causes of neurapraxia include ischemia secondary to vascular compromise, metabolic derangement, and diseases or toxins causing demyelination of the nerve. [1]
A third mechanism of injury is laceration injury caused by blunt or penetrating trauma. The nerves in these injuries are not cleanly sectioned but are damaged in an irregular pattern. [4]
The fourth mechanism of injury is penetrating trauma, whereby peripheral nerves are partially or completely severed. Penetrating trauma is caused by stab wound lacerations and by glass and surgical incisions. [2, 30]
The fifth mechanism of injury is stretch injury. The internal anatomy of nerves permits the nerve to stretch approximately 10-20% before structural damage occurs. [1] Stretch injuries or severe blows to a nerve cause axonotmesis. In axonotmesis caused by stretch injuries, axons over long segments of nerve are disrupted. In axonotmesis caused by severe blows, axons are disrupted only at the site of impact. [4]
Stretch injuries can be induced by traction. For example, forcible depression of the shoulder or traction of the arm can injure the brachial plexus. This results in a stretch injury. Traction injuries to the peripheral nerve trunk limb girdle plexuses or to spinal nerve roots can occur in a number of ways. Injuries to the lumbosacral plexus or to the lumbosacral roots commonly are associated with fractures of the pelvis. Displacement of fractures and joint dislocations can result in stretch injury to peripheral nerves. Stretch injury to peripheral nerves may occur during operative or other surgical procedures. [30] Stretching of the nerve around the radial neck during a closed reduction is an example of procedural etiology for stretch injury. [3]
A sixth mechanism of injury is high-velocity trauma caused by motor vehicle accidents and gunshot wounds. Gunshot wounds can cause direct nerve damage by avulsing soft tissue, causing severe tissue destruction. [2, 30] Open and closed fractures of the radius, gunshot wounds, and contusions also cause traumatic posterior interosseous nerve injury around the proximal forearm. [3] These types of fractures can occur in motor vehicle accidents. Nerves not directly damaged can be affected secondary to rapid tissue expansion in the track of a missile wound. [30]
Motorcycle accidents cause the majority of blunt traumatic brachial plexus injuries (BTBPI). Rural areas with colder climates also report increased risk for BTBPIs in snowmobile crashes. BTBPIs are severe injuries that result from traction or stretching of the brachial plexus. The sequela ranges from temporary neuroproxia due to a mild stretch to permanent neurological disability and severe chronic pain in cases of nerve avulsion. [31]
A seventh mechanism of injury is cold injury. Frostbite leads to necrosis of all involved tissues, including the peripheral nerves. Several hours of nonfreezing exposure with a temperature above -2.5°C and below 10°C damages peripheral nerves because they are more vulnerable than the surrounding tissue. [30]
Other contributing factors explain the statistical probability in the distribution of acute nerve injury. The anatomic relationship between the peripheral nerves and surrounding tissues causes certain nerves to be vulnerable to injury. The peroneal division of the sciatic nerve is affected more commonly in traumatic hip dislocations because the peroneal division is tethered at the fibular neck and at the sciatic notch and the tibial division is tethered only at the sciatic notch. This difference allows the tibial division a greater length over which to dissipate stresses. The funiculi of the peroneal division are fewer, larger, and protected by less connective tissue than those in the tibial division. Therefore, at the time of injury, a dislocated femoral head or displaced acetabular fragments can directly injure the sciatic nerve.
Operative findings establish that in traumatic hip injuries, the sciatic nerve can be compromised in a number of ways. These include direct compression of the sciatic nerve by the dislocated femoral head, compression by acetabular fragments, laceration or puncture by the acetabular fragments, or entrapment in the acetabular fragments. [26, 32]
Anatomic positions of the radial, median, and ulnar nerves, including their branches, predispose them to injury with specific types of fractures. Examples include radial nerve palsies with a Holstein-Lewis distal one third humeral shaft fracture, posterior interosseous nerve injury with a Monteggia fracture-dislocation, ulnar nerve and/or anterior interosseous nerve injuries with elbow dislocations, median nerve and/or radial nerve injuries with supracondylar and medial epicondyle fracture in children, and ulnar nerve injury at the cubital tunnel. [3] Because of the construction of the human shoulder, a direct blow to the anterolateral deltoid muscle can damage the axillary nerve as it travels through the deep subfascial surface and then through the deltoid muscle. A glenohumeral dislocation, a proximal humerus fracture, or a direct blow to the deltoid muscle is the usual mechanism of injury to the axillary nerve. [10, 24]
Injury to nerves also can occur from physiologic healing processes. For example, the sciatic nerve can be compressed from scar formation and massive heterotopic ossification after a hip trauma. [26] After elbow trauma, scarring can jeopardize the normal gliding of the ulnar nerve in the elbow because of adherence to scar tissue, fracture callus, or heterotopic bone. [3]
Iatrogenic injury is another possible cause of acute nerve injury. Iatrogenic injury to the axillary nerve can occur in shoulder instability surgery. Here, the axillary nerve injury can be secondary to tension, suture compression, or iatrogenic laceration. Furthermore, in rotator cuff surgery, overzealous muscle splitting places the axillary nerve at risk for injury. [10] Posterior interosseous and median nerve injury can occur during elbow arthroscopy. Iatrogenic causes of traumatic posterior interosseous nerve injury around the proximal forearm have been documented. [3]
Perioperative to brachial plexus, ulnar, radial, sciatic, femoral, and peroneal (fibular) nerves has been reported. [33]
Pathophysiology
A stimulus to the human body elicits a response—appropriate or not. If the stimulus is injury to human tissue, the body's response is repair. Repair is a process of degeneration followed by regeneration. Wallerian degeneration occurs in peripheral nerves. It is a process by which the damaged segment of a nerve is phagocytosed, beginning at the first intact node of Ranvier. The Schwann cell tubes also are phagocytosed to prevent obstruction of the regenerating axon. Many different growth factors and cytokines affect this process of degeneration-regeneration. Nerve growth factor (NGF) has sparked much interest among researchers because of its ability to stimulate the wallerian degeneration and regeneration of sensory axons. [5]
Regeneration of a peripheral nerve occurs at rate of approximately 1 mm/day. [4, 1, 2, 3] In injuries that are more proximal, improvement may not be obvious for many months. [1, 2] For example, injuries to the midshaft level of the humerus may have to cross as much as 16 cm (>5 mo) before innervating the brachioradialis or wrist extensors. [3] Furthermore, Perlmutter reported that because of the distance from the location of nerve injury to the deltoid muscle, signs of reinnervation of the axillary nerve would be observed 3-4 months after injury. [10, 34]
At the beginning stage of regeneration, the proximal axon stump sprouts buds that comprise the nerve growth cone. [5] Axonal regeneration is guided toward the distal end of the nerve by a gradient of diffusible substances. This process is called neurotropism. [5] Because of the constricting effect of intraneural and extraneural scar tissue, axonal regrowth can be blocked at the site of the lesion. [2] NGF affects the sensory regeneration but does not directly guide the regenerating axon. The axonal buds preferentially move toward neural tissue. However, they cannot differentiate between sensory or motor fascicles. The size of the distal fascicle appears to be the most significant factor in determining the target of the regenerating growth cone. Buds are more likely to find and attach to bigger fascicles. [5]
Misdirected axonal buds can result in abnormal nerve connections. Abnormal motor nerve innervations can cause jerky or awkward movement. Abnormal sensory nerve innervations can cause misperception of the location of touch or pain. [28] Motor endplates must be reinnervated within 18 months of trauma for function to be resumed. [14] Prevention of motor endplate degradation is important to ensure motor functionality after regeneration is complete. Leupeptin inhibits calcium-activated neuronal protease (calpain), which disrupts the cytoskeletal elements of the motor endplates. This has been demonstrated in laboratory animals as a means of enhancing nerve regeneration by preventing digestion of the receptors in skeletal muscle. [5]
In general, most traumatic nontransecting nerve injuries result in increased nerve swelling and pressure caused by endoneurial edema within a noncompliant perineurium. Epineurial vascular stripping (ie, epineurectomy) in rat sciatic nerve, which interrupts the vasa nervorum, reproduces injuries that result in increased epineurial edema and pressure. This proves dependence of the subperineurial axonal population on an intact vasa nervorum. Studies have verified that ischemic reperfusion type injuries, similar to those observed in muscle and skin, are a significant cause of nerve injury. [35]
Each of the mechanisms of injury causes specific damage to a nerve. The first mechanism of injury is mechanical injury, which can be described from analysis of animal experiments. In cats, studies with pressure cuffs demonstrate that focal demyelination causes the conduction block associated with this type of injury. Recovery occurs after remyelination of the axon. Studies with baboons by Ochoa and Fowler and Gilliatt illustrate that the demyelination is related to displacement of the axoplasm to either side from under the compressed region. Outward dislocation of myelin internodes and their invagination into adjacent myelin internodes accompanies the displacement. This process obliterates the node of Ranvier. The demyelination occurs at the displaced nodes and is recovered by remyelinating those myelin sheaths. [30]
The second mechanism of injury, crush and percussion injury, has been the result of axonal interruption within the intact Schwann cell basal laminal tubes. [30] Santos discovered that crush injuries do not preserve the basal lamina of all myelinated axons due the presence of axonal sprouting and regeneration. [35] After the injury, Schwann cells quickly invade the region, and regenerating axons cross within a few days. The surviving basal laminal tubes guide most of the regenerating axons to their former peripheral connections. This pathophysiology is borrowed from information obtained through animal studies because no information on percussion nerve injury is available from human studies. Experimental studies by Denny-Brown and Brenner and by Richardson and Thomas have indicated a combination of segmental demyelination, periaxonal and intramyelinic edema, and axonal interruption of nerves damaged from compression injury. [30]
In penetrating wounds, 2 possible injury outcomes exist: complete division and partial severance. With complete severance of the nerve, the severed nerve ends draw back because of their inherent elasticity. [30] For this reason, transected stumps are tacked down firmly to surrounding tissues during surgery. [16]
Both the proximal stump and the distal stump have outgrowths. The outgrowth on the proximal stump consists of groups of intertwined regenerating axon buds and associated Schwann cells, each surrounded by a perineural sheath, resulting in a multicompartment arrangement. According to Thomas, the outgrowth on the distal stump consists of interlacing columns of Schwann cells, Bungner bands, and associated connective tissue of the endoneurial type. [30] Bungner bands are longitudinal conduits formed by the proliferating Schwann cells, and axons can regenerate through them. [1] In a penetrating wound with partial severance or following a nerve suture, the regenerating axons are able to reconnect with the distal portion and invade the Schwann cell Büngner bands. [30]
The fourth injury, stretch injury, can cause a mild conduction block. Why stretch injury paralysis, which recovers within a couple of hours, occurs is uncertain. The time to recovery is too short for demyelination and remyelination. According to Highet and Holmes more severe degrees of stretch injury usually result in damage to a significant length of nerve with interruption of axons, disruption of neural connective tissues, and intraneural hemorrhage. [30]
The fifth type of injury is lacerating injuries. This type of injury is localized within a few millimeters beyond the divided ends of the nerve. [4]
Cold injury is the sixth type of injury. Animal studies in nonfreezing cold injury showed a focal conduction block and cessation of axonal transport in the acute stage, with early recovery of function. However, after a few days, axonal degeneration occurs, particularly of large myelinated fibers, as described by Basbaum and by Nukada, Pollock, and Allpress. [30] The mechanism of delayed degeneration is uncertain. The blood-nerve barrier has been demonstrated to become damaged, and extensive endoneurial edema occurs. This is a progressive process, probably due to the lack of endoneurial lymphatics. The greater vulnerability of the peripheral nerve can be explained if the raised intraneural pressure, secondary to edema, is the mechanism of fiber degeneration. [30]
Presentation
Patients with acute peripheral nerve injury usually have nerve conduction defects that can manifest as motor impairment or sensory dysfunction. In quadrilateral space syndrome, compression of the axillary nerve and posterior humeral circumflex artery occurs. This compression produces poorly localized posterior shoulder pain, paresthesias over the lateral aspect of the shoulder and arm, and deltoid muscle weakness. [10] Acute nerve injury can cause temporary or persisting paralysis. [30] For example, presentation of acute axillary nerve injury is quite variable. Presentation can include weakness in shoulder elevation with abduction and numbness, and paresthesias throughout the lateral arm can occur. [10] Nerve injury from anterior shoulder dislocations can cause paresis and an inability to move the arm. [23] Studies have demonstrated that paresis of the deltoid muscle is the best indicator of nerve injury after one week. [23]
The sensory component and motor component must be evaluated separately to ensure accurate diagnosis. Visser et al reported that early-stage detection of nerve injury can be conducted through sensory testing of the axillary and musculocutaneous nerves. However, these results cannot provide a reliable indication of lesions of the motor nerves. [23]
In some cases, the injury does not match the clinical presentation. Operative findings of nerve injury may or may not match clinical findings. Data exist of gross operative evidence of nerve injury without clinical evidence of neurologic dysfunction, such as with sheath hematoma and compression by fracture fragments. Conversely, data exist of intraoperative grossly normal-appearing nerves with clinical paresis. [26] These deficits may develop after initially appearing with a normal neurologic examination.
More specifically, following a traumatic hip dislocation, some patients may develop sciatic nerve deficits even after an initial normal neurologic examination. [26] Hence, when a nerve is damaged, it may continue to appear normal in a neurologic examination. In truth, damage can be revealed only through diagnostic studies. Despite a normal neurologic examination after a shoulder dislocation, Toolanen et al reported electromyographic evidence of axillary nerve injury in 8 of 65 patients. [10]
In many instances, acute nerve injury associated with complex trauma complicates a thorough neurologic examination. For example, of the patients with traumatic hip dislocations who are treated in the emergency department, many also present with concomitant head, visceral, or skeletal injuries. With these cases, a thorough neurologic examination of the affected extremity becomes difficult to perform. [26] Furthermore, in many patients, nerve injury may remain undetected because joint and/or bony injury may dominate the clinical picture. [10]
Certain presenting traumas should alert the clinician to the possibility of associated nerve injury. For example, for patients with trauma and obvious skeletal deformities to the shoulder, such as glenohumeral dislocation and/or fracture, be aware of the potential for associated nerve injury. [10] Nerve injury may be apparent immediately after injury. For example, immediate paralysis of common peroneal nerve function and foot drop with loss of eversion of the foot usually are reported at the time of a stretch/contusion injury without fracture or dislocation at the knee level. [16] In an aggravated patient who is vigorously moving all extremities, a motionless upper extremity strongly suggests brachial plexus involvement. [26]
Indications
Surgical intervention for acute nerve injury is based on the extent of damage to the nerve and the nerve's functional viability. Consider each patient on an individual basis. When evaluating patients for surgery, surgeons should consider the location, the extent of the injury, the patient's age, and the patient's medical condition. Two important questions to consider before surgery are whether function can be obtained from the repaired nerve and whether the potential benefit to the patient outweighs the surgical risks, costs, and loss of productivity. [14] For example, adults older than 40 years rarely achieve a functional result from ulnar nerve repairs proximal to the elbow. [1] Consequently, these patients may not be candidates for surgery.
Deciding the timing of surgery is very important. In clean lacerating injuries in which the nerve ends are visible in the wound or when clinical examination reveals obvious motor and sensory deficits resulting from the injury at the laceration, immediate primary repair may be indicated. [4] In blunt transections resulting from lacerations, delayed repair has a better surgical result. [16] Injuries that do not demonstrate evidence of early spontaneous recovery, such as those caused by bullets, crushing blows, traction, fractures, or injections, are explored 2 months after the injury. For a nerve injury within 2-3 inches of recoverable muscle, 2 months is required for the growing axons to begin the process of muscle reinnervation. Therefore, an additional delay of 1 month is justified before surgical exploration. Brachial plexus stretches or contusions are observed for 4 months. If no evidence of recovery is present, the plexus is explored. [4]
Because nerves in the elbow are anatomically vulnerable, many researchers recommend early surgical exploration after fractures of the humerus. [3] Justification for late exploration is to allow sufficient time for spontaneous recovery. For example, for patients without functional recovery of the radial nerve, the time frame for late exploration ranges from 8 weeks to 5 months. This allows sufficient time for spontaneous recovery without jeopardizing the results of late repair. [3] Perlmutter mentioned that for injuries consistent with nerve rupture (eg, glenohumeral dislocation), a 3- to 6-month period without clinical or electrophysiologic evidence of recovery warrants surgery. This time frame is sufficient for neurapraxic and axonotmetic injuries to resolve but is not long enough to jeopardize results of subsequent axillary nerve surgical repair. [10]
In the case of traumatic hip dislocation with a successful closed reduction, a short period of conservative treatment is recommended before surgical exploration. [26] Different opinions exist with regard to how much time to allow for nerve function to return. More specifically, if nerve function does not return within 1 week, Nerubay et al advised surgical exploration. Stewart et al allowed 2 weeks, while Proctor allowed as many as 3 weeks for nerve function to return before performing exploratory surgery. On the other hand, Bromberg and Weiss recommended surgical exploration if nerve function did not return within 8 to 10 months. [26]
When certain cases are unresponsive to conservative treatment, surgery is the only alternative. For example, the pathogenesis of the quadrilateral space syndrome is poorly understood. Therefore, reserve surgery for patients in whom symptoms persist despite appropriate conservative treatment. [10] In cases of late sciatic nerve dysfunction from heterotopic ossification or scar tissue, a different controversy arises regarding surgical exploration. Several authors recommend surgical exploration as soon as the diagnosis becomes evident. [26] However, Proctor cautioned against late sciatic nerve exploration because of the probable presence of scar tissue and/or heterotopic ossification and the consequent risk of iatrogenic nerve injury. [26]
Surgery is indicated for patients with neurotmesis (Sunderland grade III-V). Therefore, accurate grading of an acute traumatic injury is essential. Accurate grading is necessary for identifying high-grade injuries that may benefit from early surgery and for preventing unnecessary early exploration of grade I and II lesions. [3]
Evolution of nerve injuries is important in indicating the need for open treatment. If nerve function is progressively deteriorating as per electrodiagnostic study findings, surgery may be indicated because the status of the connective tissue cannot be assessed without direct exploration. [3]
Relevant Anatomy
Peripheral nerves are composed of axons and associated Schwann cells enclosed in a basement membrane. [14] Schwann cells ensheathe individual axons in myelinated fibers and groups of axons in unmyelinated fibers. [1] The basement membrane is surrounded by thin collagen fibers called the endoneurium. The composite of axons and Schwann cells is called the endoneurial tube [14] or Schwann ubet [5] . Endoneurial tubes are grouped together, forming a variable number of fascicles.
Perineurium surrounds each fascicle. Perineurium is composed of collagen fibers and concentric layers of closely packed flattened cells, united by tight junctions. The perineurium creates a diffusion barrier against the surrounding environment, similar to the blood-brain barrier. The perineurium maintains a positive intrafascicular pressure and protects against infection.
Epineurium surrounds the layers of perineurium. The epineurium that fills the space between fascicles is called internal epineurium, and epineurium surrounding the nerve is termed external, or outer, epineurium. The internal epineurium functions as a cushion for the fascicles. Where peripheral nerves span joints and where greater compressive forces are applied, the thickness of the internal epineurium is increased. The outer epineurial layers are composed of collagen and some elastin fibers. [14]
Anatomic fascicular groups are formed by condensations of internal epineurium. Interconnections between fascicles form a fascicular plexus. [5] Although these fascicular plexuses are abundant in the proximal portions of peripheral nerves, few are located in the more distal part of peripheral nerves. [5, 15] The location and position of fascicles inside the nerve depend on the destination of the fascicle branch in the periphery. [14]
The blood supply to peripheral nerves usually is via the mesoneurium or suspending mesentery. [15] The vasa nervorum is a longitudinal system of vessels within the nerve that allows circulation to continue even after some freeing of surrounding tissue. [35, 15]
-
Peripheral nerve, cross-section. Image courtesy of the Department of Histology, Jagiellonian University Medical College.









