Practice Essentials
Spinal stenosis (progressive narrowing of the spinal canal) is part of the aging process, and predicting who will be affected is not possible. No clear correlation is noted between the symptoms of stenosis and race, occupation, sex, or body type. Treatment in spinal stenosis can be conservative or surgical. While the degenerative process can be managed, it cannot be prevented by diet, exercise, or lifestyle.
Acute and chronic neck and lower back pain represent major health care problems in the United States. An estimated 75% of all people will experience back pain at some time in their lives. Most patients who present with an acute episode of back pain recover without surgery, while 3-5% of patients presenting with back pain have a herniated disc, and 1-2% have compression of a nerve root. Older patients present with more chronic or recurrent symptoms of degenerative spinal disease. (See Epidemiology.)
Progressive narrowing of the spinal canal may occur alone or in combination with acute disc herniations. Congenital and acquired spinal stenoses place the patient at a greater risk for acute neurologic injury. Spinal stenosis is most common in the cervical and lumbar areas. [1, 2, 3, 4] (See the images below.)
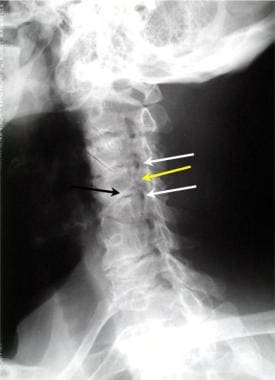 Oblique view of the cervical spine demonstrates 2 levels of foraminal stenosis (white arrows) resulting from facet hypertrophy (yellow arrow) and uncovertebral joint hypertrophy.
Oblique view of the cervical spine demonstrates 2 levels of foraminal stenosis (white arrows) resulting from facet hypertrophy (yellow arrow) and uncovertebral joint hypertrophy.
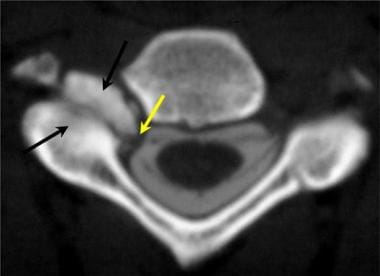 Axial cervical CT myelogram demonstrates marked hypertrophy of the right facet joints (black arrows), which results in tight restriction of the neuroforaminal recess and lateral neuroforamen.
Axial cervical CT myelogram demonstrates marked hypertrophy of the right facet joints (black arrows), which results in tight restriction of the neuroforaminal recess and lateral neuroforamen.
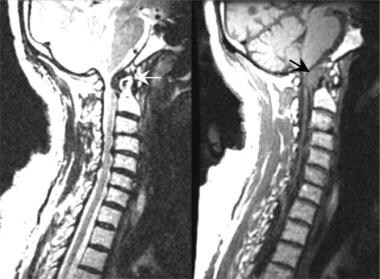 Short recovery time T1-weighted spin-echo sagittal MRI scan demonstrates marked spinal stenosis of the C1/C2 vertebral level cervical canal resulting from formation of the pannus (black arrow) surrounding the dens in a patient with rheumatoid arthritis. Long recovery time T2*-weighted fast spin-echo sagittal MRI scans better define the effect of the pannus (yellow arrow) on the anterior cerebrospinal fluid space. Note the anterior displacement of the upper cervical cord and the lower brainstem.
Short recovery time T1-weighted spin-echo sagittal MRI scan demonstrates marked spinal stenosis of the C1/C2 vertebral level cervical canal resulting from formation of the pannus (black arrow) surrounding the dens in a patient with rheumatoid arthritis. Long recovery time T2*-weighted fast spin-echo sagittal MRI scans better define the effect of the pannus (yellow arrow) on the anterior cerebrospinal fluid space. Note the anterior displacement of the upper cervical cord and the lower brainstem.
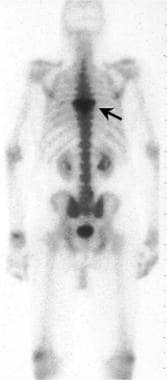 Posterior view from a radionuclide bone scan. A focally increased uptake of nuclide (black arrow) is demonstrated within the mid-to-upper thoracic spine in a patient with Paget disease.
Posterior view from a radionuclide bone scan. A focally increased uptake of nuclide (black arrow) is demonstrated within the mid-to-upper thoracic spine in a patient with Paget disease.
Lumbar spinal stenosis (LSS) implies spinal canal narrowing with possible subsequent neural compression. Although the disorder often results from acquired degenerative changes (spondylosis), spinal stenosis may also be congenital in nature (see Etiology). In some cases, the patient has acquired degenerative changes that augment a congenitally narrow canal. The canal components that contribute to acquired stenosis include the facets (hypertrophy, arthropathy), ligamentum flavum (hypertrophy), posterior longitudinal ligament (ossification of posterior longitudinal ligament [OPLL]), vertebral body (bone spurs), intervertebral disk, and epidural fat. Congenital stenosis may predispose an individual with mild degenerative changes to become symptomatic earlier in life. (See the images below.)
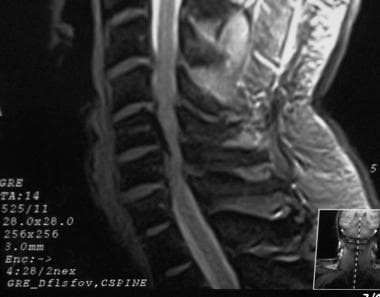 T2-weighted sagittal MRI of the cervical spine demonstrating stenosis from ossification of the posterior longitudinal ligament, resulting in cord compression.
T2-weighted sagittal MRI of the cervical spine demonstrating stenosis from ossification of the posterior longitudinal ligament, resulting in cord compression.
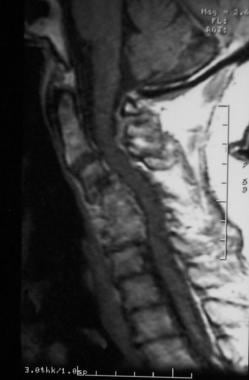 Severe cervical spondylosis can manifest as a combination of disk degeneration, osteophyte formation, vertebral subluxation, and attempted autofusion as depicted in this sagittal MRI. Also, note the focal kyphosis, which is typical in severe forms.
Severe cervical spondylosis can manifest as a combination of disk degeneration, osteophyte formation, vertebral subluxation, and attempted autofusion as depicted in this sagittal MRI. Also, note the focal kyphosis, which is typical in severe forms.
LSS is classified by anatomy or etiology. Anatomic subclassifications include central canal and lateral recess stenosis. The classification of lumbar stenosis is important because of the implications of the underlying etiology and because it affects the therapeutic strategy, specifically the surgical approach.
Stenosis of the central cervical and thoracic spine may result in myelopathy from cord compression. [5, 6] Canal stenosis in the lumbosacral region often results in radicular pain, neurogenic claudication, or both. (See Clinical Presentation.)
Lateral canal stenosis at any region of the spine may lead to nerve root compression. The patients may experience radicular pain, weakness, and numbness along the distribution of the affected spinal nerve. Lateral recess syndrome in the lumbar spine is a result of such focal stenosis.
Signs and symptoms of spinal stenosis
The primary clinical manifestation of spinal stenosis is chronic pain. In patients with severe stenosis, weakness and regional anesthesia may result. Among the most serious complications of severe spinal stenosis is central cord syndrome, which is the most common incomplete cord lesion. The presentation commonly is associated with an extension injury in a patient with an osteoarthritic spine. In hyperextension injury, the cord is injured within the central gray matter, which results in proportionally greater loss of motor function in the upper extremities than in the lower extremities, with variable sensory sparing.
Spinal stenosis of the cervical and thoracic regions may contribute to neurologic injury, such as development of a central spinal cord syndrome following spinal trauma. Spinal stenosis of the lumbar spine is associated most commonly with midline back pain and radiculopathy. In cases of severe lumbar stenosis, innervation of the urinary bladder and the rectum may be affected, but lumbar stenosis most often results in back pain with lower extremity weakness and numbness along the distribution of nerve roots of the lumbar plexus.
Diagnosis and management of spinal stenosis
Useful neuronal studies include the following:
-
Needle electromyography - Can help to diagnose lumbosacral radiculopathy with axonal loss
-
Nerve conduction studies - Can help to differentiate lumbar spinal stenosis (LSS) from other confounding neuropathic conditions (eg, lumbosacral plexopathy, generalized peripheral neuropathy, tarsal tunnel syndrome, other mononeuropathies)
-
Somatosensory evoked potentials - Are useful in the diagnosis of central nervous system (CNS) pathology and are also used intraoperatively during decompressive surgery to assist the physician in dynamically identifying any iatrogenic changes to the sensory pathways
The goal of spinal imaging is to localize the site and level of disease. It also is used to help differentiate conditions for which patients require surgery and conditions for which patients can recover with conservative treatment. Imaging studies used in LSS include standard radiography, magnetic resonance imaging (MRI), computed tomography (CT) scanning, nuclear imaging, and angiography (rarely).
Treatment in spinal stenosis can be conservative or surgical. The modes of conservative therapy include rest, physical therapy with strengthening exercises for paraspinal musculature, bracing, use of optimal postural biomechanics, nonsteroidal anti-inflammatory medications, analgesics, and antispasmodics. (See Treatment and Management.) Surgical decompression is indicated in persons who experience incapacitating pain, claudication, neurologic deficit, or myelopathy. [7, 8] Concomitant stabilization is reserved for individuals in whom segmental instability is suspected (ie, patients with spondylolisthesis showing abnormal movement on dynamic studies).
Anatomy
Central canal stenosis, commonly occurring at an intervertebral disk level, defines midline sagittal spinal canal diameter narrowing that may elicit neurogenic claudication (NC) or pain in the buttock, thigh, or leg. Such stenosis results from ligamentum flavum hypertrophy, inferior articulating process (IAP), facet hypertrophy of the cephalad vertebra, vertebral body osteophytosis, vertebral body compression fractures, and herniated nucleus pulposus (HNP). Abnormalities of the disk usually do not cause symptoms of central stenosis in a normal-sized canal. In developmentally small canals, however, a prominent bulge or small herniation can cause symptomatic central stenosis. Large disk herniations can compress the dural sac and compromise its nerves, particularly at the more cephalad lumbar levels where the dural sac contains more nerves. (See the images below.)
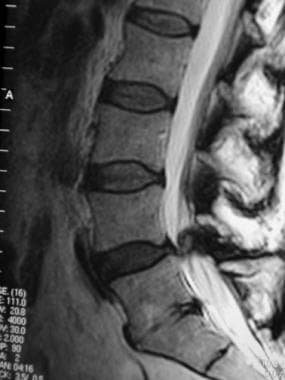 Lateral T2-weighted magnetic resonance imaging (MRI) scan demonstrating narrowing of the central spinal fluid signal (L4-L5), suggesting central canal stenosis.
Lateral T2-weighted magnetic resonance imaging (MRI) scan demonstrating narrowing of the central spinal fluid signal (L4-L5), suggesting central canal stenosis.
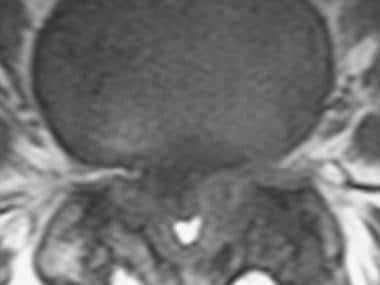 Axial T2 magnetic resonance imaging (MRI) scan (L4-L5) in the same patient as in the above image, confirming central canal stenosis.
Axial T2 magnetic resonance imaging (MRI) scan (L4-L5) in the same patient as in the above image, confirming central canal stenosis.
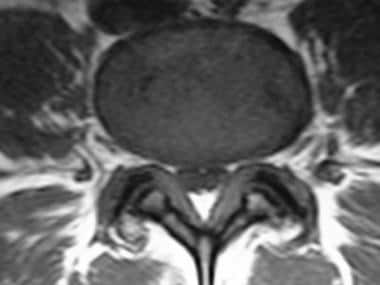 Trefoil appearance characteristic of central canal stenosis due to a combination of zygapophysial joint and ligamentum flavum hypertrophy.
Trefoil appearance characteristic of central canal stenosis due to a combination of zygapophysial joint and ligamentum flavum hypertrophy.
Lateral recess stenosis (ie, lateral gutter stenosis, subarticular stenosis, subpedicular stenosis, foraminal canal stenosis, intervertebral foramen stenosis) is defined as narrowing (less than 3-4 mm) between the facet superior articulating process (SAP) and the posterior vertebral margin. Such narrowing may impinge the nerve root and subsequently elicit radicular pain. This lateral region is compartmentalized into entrance zone, mid zone, exit zone, and far-out stenosis. Amundsen and colleagues found concomitant lateral recess stenosis in all cases of central canal stenosis. [9] (See the image below.)
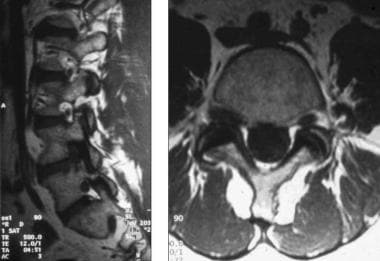 Lateral and axial magnetic resonance imaging (MRI) scan demonstrating right L4 lateral recess stenosis secondary to combination of far lateral disk protrusion and zygapophysial joint hypertrophy.
Lateral and axial magnetic resonance imaging (MRI) scan demonstrating right L4 lateral recess stenosis secondary to combination of far lateral disk protrusion and zygapophysial joint hypertrophy.
The entrance zone lies medial to the pedicle and SAP and, consequently, arises from facet joint SAP hypertrophy. Other causes include developmentally short pedicle and facet joint morphology, as well as osteophytosis and HNP anterior to the nerve root. The lumbar nerve root compressed below SAP retains the same segmental number as the involved vertebral level (eg, L5 nerve root is impinged by L5 SAP).
The mid zone extends from the medial to the lateral pedicle edge. Mid-zone stenosis arises from osteophytosis under the pars interarticularis and bursal or fibrocartilaginous hypertrophy at a spondylolytic defect.
Exit-zone stenosis involves an area surrounding the foramen and arises from facet joint hypertrophy and subluxation, as well as superior disk margin osteophytosis. Such stenosis may impinge the exiting spinal nerve.
Far-out (extracanalicular) stenosis entails compression lateral to the exit zone. Such compression occurs with far lateral vertebral body endplate osteophytosis and when the sacral ala and L5 transverse process impinge on the L5 spinal nerve.
Cervical stenosis
The anteroposterior (AP) diameter of the normal adult male cervical canal has a mean value of 17-18 mm at vertebral levels C3-5. The lower cervical canal measures 12-14 mm. Cervical stenosis is associated with an AP diameter of less than 10 mm, while diameters of 10-13 mm are relatively stenotic in the upper cervical region. (See image below.)
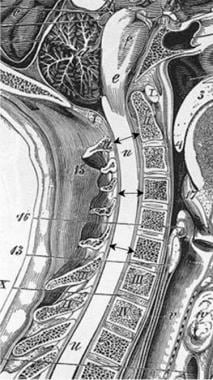 Sagittal measurements taken of the anteroposterior diameter of the cervical spinal canal are highly variable in otherwise healthy persons. An adult male without spinal stenosis has a diameter of 16-17 mm in the upper and middle cervical levels. Magnetic resonance imaging (MRI) scans and reformatted computed tomography (CT) images are equally as effective in obtaining these measurements, while radiography is not accurate.
Sagittal measurements taken of the anteroposterior diameter of the cervical spinal canal are highly variable in otherwise healthy persons. An adult male without spinal stenosis has a diameter of 16-17 mm in the upper and middle cervical levels. Magnetic resonance imaging (MRI) scans and reformatted computed tomography (CT) images are equally as effective in obtaining these measurements, while radiography is not accurate.
Movement of the cervical spine exacerbates congenital or acquired spinal stenosis. In hyperextension, the cervical cord increases in diameter. Within the canal, the anterior roots are pinched between the annulus margins and spondylitic bony bars. In the posterior canal, hypertrophic facet joints and thickened infolded ligamentum flavum compress the dorsal nerve roots. In hyperflexion, neural structures are tethered anteriorly against the bulging disc annulus and spondylitic bars. In the event of a vertebral collapse, the cervical spine loses its shape, which may result in anterior cord compression.
In the central cervical spinal region, hypertrophy of the ligamentum flavum, bony spondylitic hypertrophy, and bulging of the disc annulus contribute to development of central spinal stenosis. In each case, the relative significance of each structure contributing to the stenotic pattern is variable.
Congenital stenosis of the cervical spine may predispose an individual to myelopathy as a result of minor trauma or spondylosis. [5, 6, 10, 11, 12, 13, 14]
Cervical spondylosis refers to age-related degenerative changes of the cervical spine. These changes, which include intervertebral disk degeneration, disk space narrowing, spur formation, and facet and ligamentum flavum hypertrophy, can lead to the narrowing of the cervical spinal canal. Cervical spondylotic myelopathy (CSM) refers to the clinical presentation resulting from these degenerative processes. CSM is the most common cause of spinal cord dysfunction in adults older than 55 years. Degenerative changes of the cervical spine have been observed in as many as 95% of asymptomatic individuals older than 65 years. Myelopathy is believed to develop in up to 20% of individuals with evidence of spondylosis. [5, 11, 13, 14, 15, 16, 17]
Lateral cervical stenosis results from encroachment on the lateral recess and the neuroforamina of the cervical region, primarily as a result of hypertrophy of the uncovertebral joints, lateral disc annulus bulging, and facet hypertrophy.
Thoracic spinal stenosis
The thoracic spinal canal varies from 12 to 14 mm in diameter in the adult. Thoracic spinal stenosis is often associated with focal disease of a long-standing nature. It may be associated with disk bulging or herniation, hypertrophy of the posterior elements (namely, the facet and ligamentum flavum), and, occasionally, calcification of ligamentum flavum. Primary central thoracic spinal stenosis is rare. In some cases, hypertrophy or ossification of the posterior longitudinal ligament results in central canal stenosis. [6] Lateral thoracic stenosis may result from hypertrophy of facet joints with occasional synovial cyst encroachment.
Lumbar spinal stenosis
The diameter of the normal lumbar spinal canal varies from 15 to 27 mm. Lumbar stenosis results from a spinal canal diameter of less than 12 mm in some patients; a diameter of 10 mm is definitely stenotic.
Keim and colleagues present the following lumbar spinal stenosis (LSS) anatomical classification scheme [18] :
-
Lateral, secondary to superior articulating process (SAP) hypertrophy
-
Medial, secondary to inferior articulating process (IAP) hypertrophy
-
Central, due to hypertrophic spurring, bony projection, or ligamentum flavum/laminar thickening
-
Fleur de lis (clover leaf), from laminar thickening with subsequent posterolateral bulging
A study by Abbas et al indicated that persons with degenerative lumbar spinal stenosis tend to have wider pedicles at all lumbar levels than do members of the general population. In addition, females in the study’s stenosis group were found to have much smaller pedicle heights at the L4 and L5 levels than did females in the control group. [19]
Pathophysiology
The pathophysiology of spinal stenosis is related to cord dysfunction elicited by a combination of mechanical compression and degenerative instability. With aging, the intervertebral disk degenerates and collapses, leading to spur formation. This most commonly occurs at C5-6 and C6-7. A relative decrease in spinal motion occurs at these levels with a concomitant increase in spinal motion at C3-4 and C4-5. The spine responds to physiological stresses with bone growth at the superior and inferior margins of the vertebral body (osteophytes). Osteophytes can form anteriorly or posteriorly. Posterior osteophytes narrow the intraspinal diameter and also cause lateral recess stenosis. This results in spinal cord or nerve root impingement. Furthermore, arthritic degeneration causes formation of synovial cysts and hypertrophy of the facet joints, which further compromise the patency of the spinal canal and the neural foramina.
Spinal stenosis results from progressive narrowing of the central spinal canal and the lateral recesses. The essential content of the spinal canal includes the spinal cord, the cerebrospinal fluid (CSF) of the thecal sac, and the dural membranes that enclose the thecal sac. In the absence of prior surgery, tumor, or infection, the spinal canal may become narrowed by bulging or protrusion of the intervertebral disc annulus, herniation of the nucleus pulposus posteriorly, thickening of the posterior longitudinal ligament, hypertrophy of the facet joints, hypertrophy of the ligamentum flavum, epidural fat deposition, spondylosis of the intervertebral disc margins, uncovertebral joint hypertrophy in the neck, or a combination of 2 or more of the above factors. [20]
The resultant degeneration and abnormal motion lead to instability with anterolisthesis or retrolisthesis (subluxation of vertebral bodies out of the normal cervical alignment). Therefore, the cord tends to be compressed from spur formation at C5-6 and C6-7 and compressed from listhesis at C3-4 and C4-5. Often, this is accompanied by posterior canal compromise from ligamentum flavum hypertrophy. [6, 21]
The cord is subject to further injury from repetitive dynamic injury during normal neck movements. These static and dynamic compressive forces on the cord lead to spinal cord injury and the clinical myelopathic syndrome. [6]
Disk desiccation and degenerative disk disease (DDD) with resulting loss of disk height may induce segmental instability. Such instability incites vertebral body and facet joint hypertrophy. Cephalad vertebral body IAP hypertrophy promotes central spinal canal stenosis. Further canal volume loss results from HNP, ligamentum flavum hypertrophy, and disk space narrowing.
Alternatively, the caudal vertebral body superior articulating process (SAP) contributes to lateral recess and foraminal stenosis (see the image below). Indeed, facet hypertrophy between L4 and L5 vertebrae may impinge the L4 nerve root in the foramen and the L5 proximal nerve root sheath in the lateral recess. The 2 lower motion segments (L3-L4, L4-L5) are most commonly affected by degenerative stenosis. These segments are in a transition zone from the rigid sacrum to the mobile lumbar spine. In addition, the posterior joints in this area have less of a sagittal orientation, which affords more rotation and are therefore more vulnerable to rotatory strains.
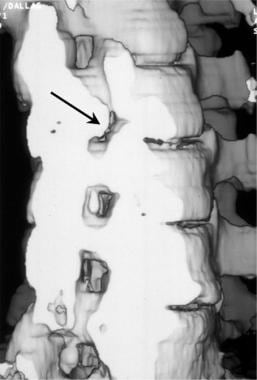 Oblique 3-dimensional shaded surface display CT reconstruction of right foraminal stenosis resulting from unilateral facet hypertrophy (black arrow). The volume of the reconstruction has been cut obliquely across the neuroforaminal canal.
Oblique 3-dimensional shaded surface display CT reconstruction of right foraminal stenosis resulting from unilateral facet hypertrophy (black arrow). The volume of the reconstruction has been cut obliquely across the neuroforaminal canal.
Jenis and An eloquently describe foraminal stenosis pathoanatomy, characterized by disk desiccation and DDD, which narrows disk height, permitting the caudad SAP to sublux anterosuperiorly. [22] Such subluxation decreases foraminal space. Continued subluxation with resulting biomechanical disruption provokes osteophytosis and ligamentum flavum hypertrophy, further compromising foraminal volume. Anteroposterior (transverse) stenosis ultimately results from narrow disk height and hypertrophy anterior to the facet; specifically, the SAP and posterior vertebral body transversely trap the nerve root. Furthermore, in vertical (craniocaudal) stenosis, posterolateral vertebral endplate osteophytes and a lateral HNP may impinge the spinal nerve against the superior pedicle.
Dynamic foraminal stenosis implies intermittent lumbar extension-provoked nerve root impingement from HNP, osteophytosis, and vertebral body slippage. Such dynamic stenosis with associated intermittent position-dependent symptoms may not manifest on imaging studies, thereby confounding diagnosis. Other factors promoting development of LSS include shortened gestational age and synovial facet joint cysts with resulting radicular compression. Adult degenerative scoliosis, secondary to DDD-induced instability with subsequent vertebral rotation and asymmetric disk space narrowing, promotes facet hypertrophy and subluxation in the curve concavity. Degenerative spondylolisthesis, when combined with facet hypertrophy, causes central canal and lateral recess stenosis.
Proposed mechanisms for development of neurogenic claudication (NC) include cauda equina microvascular ischemia, venous congestion, axonal injury, and intraneural fibrosis. Ooi and colleagues myeloscopically observed ambulation-provoked cauda equina blood vessel dilation with subsequent circulatory stagnation in patients with LSS who have NC. [23] They proposed that ambulation dilates the epidural venous plexus, which, amidst narrow spinal canal diameter, increases epidural and intrathecal pressure. Such elevation of pressure ultimately compresses the cauda equina, compromises its microcirculation, and causes pain.
Another pain generator may be the dorsal root ganglion (DRG), which contains pain-mediating neuropeptides, such as substance P, that possibly increase with mechanical compression. The DRG varies spatially within the lumbosacral spine, with L4 and L5 DRG in an intraforaminal position and S1 DRG located intraspinally. Such foraminal placement may predispose to stenotic compression with subsequent radicular symptomology.
Etiology
Primary stenosis is uncommon, occurring in only 9% of cases. Congenital malformations include the following:
-
Incomplete vertebral arch closure (spinal dysraphism)
-
Segmentation failure
-
Achondroplasia
-
Osteopetrosis
Developmental flaws include the following:
-
Early vertebral arch ossification
-
Shortened pedicles
-
Thoracolumbar kyphosis
-
Apical vertebral wedging
-
Anterior vertebral beaking (Morquio syndrome)
-
Osseous exostosis
Secondary (acquired) stenosis arises from degenerative changes, iatrogenic causes, systemic processes, and trauma. Degenerative changes include central canal and lateral recess stenosis from posterior disk protrusion, zygapophyseal joint and ligamentum flavum hypertrophy, and spondylolisthesis. Iatrogenic changes result following surgical procedures such as laminectomy, fusion, and diskectomy. Systemic processes that may be involved in secondary stenosis include Paget disease, fluorosis, acromegaly, neoplasm, and ankylosing spondylitis. (See the image below.)
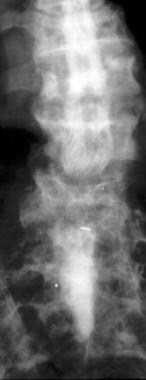 Anterior view of a lumbar myelogram demonstrates stenosis related to Paget disease. Myelography is limited because of the superimposition of multiple spinal structures that contribute to the overall pattern of stenosis.
Anterior view of a lumbar myelogram demonstrates stenosis related to Paget disease. Myelography is limited because of the superimposition of multiple spinal structures that contribute to the overall pattern of stenosis.
The central canal and the neurorecess may be compromised by tumor infiltration, such as metastatic disease of the spine, or by infectious spondylitis. An abscess may directly compress the spinal cord if it is contained in the epidural space, while discitis and vertebral osteomyelitis may compress the canal following vertebral collapse. Paget disease results in spinal stenosis as a result of enlargement of the vertebral body, while idiopathic ossification of the posterior longitudinal ligament directly narrows the central spinal canal most often in the cervical or thoracic regions.
Skeletal conditions that predominantly lead to stenosis or deformity of the cervical spinal canal include rheumatoid arthritis, ankylosing spondylitis, and ossification of posterior longitudinal ligament (OPLL). Genetic factors play a major role in the geographic prevalence of these conditions.
Epidemiology
Approximately 250,000-500,000 US residents have symptoms of spinal stenosis. This represents about 1 per 1000 persons older than 65 years and about 5 of every 1000 persons older than 50 years. About 70 million Americans are older than 50 years, and this number is estimated to grow by 18 million in the next decade alone, suggesting that the prevalence of spinal stenosis will increase. Lumbar spinal stenosis (LSS) remains the leading preoperative diagnosis for adults older than 65 years who undergo spine surgery. The incidence of lateral nerve entrapment is reportedly 8-11%. Some studies implicate lateral recess stenosis as the pain generator for 60% of patients with symptomatology of failed back surgery syndrome.
As many as 35% of persons who are asymptomatic and aged 20-39 years demonstrate disc bulging. CT scanning and MRI studies in patients who are asymptomatic and younger than 40 years demonstrate a 4-28% occurrence of spinal stenosis. Most persons older than 60 years have spinal stenosis to some degree. Because most patients with mild spinal stenosis are asymptomatic, the absolute frequency can only be estimated. [4]
Incidence of foraminal stenosis increases in lower lumbar levels because of increased dorsal root ganglion (DRG) diameter with resulting decreased foramen (ie, nerve root area ratio). Jenis and An cite commonly involved roots as L5 (75%), L4 (15%), L3 (5.3%), and L2 (4%). [22] The lower lumbar levels maintain greater obliquity of nerve root passage, as well as higher incidence of spondylosis and DDD, further predisposing patients to L4 and L5 nerve root impingement.
Cervical stenosis resulting from ossification of the posterior longitudinal ligament is more common among Asians, and LSS occurs most frequently in males. Patients with LSS due to degenerative causes generally are aged at least 50 years; however, LSS may be present at earlier ages in cases of congenital malformations.
Prognosis
Spinal stenosis can result in significant morbidity. Severe disability and death may result from the association of cervical stenosis with even minor trauma resulting in the central cord syndrome. Both upper (cervical) and lower (lumbar) spinal stenosis may result in motor weakness and chronic pain. Severe lumbar stenosis is associated with cauda equina syndrome.
In the patient with spinal canal stenosis, flexion or marked hyperextension may result in further compromise of the spinal canal in the absence of a fracture. Anterior compression of the cord may result in a central spinal cord syndrome, and dorsal compression may result in a partial dorsal column syndrome.
Central spinal stenosis of the cervical or thoracic regions may result in neurosensory changes at the level of the spinal stenosis or may further compress the spinal cord, resulting in myelopathy. The effects of central spinal canal stenosis may result in lower extremity weakness and gait disturbance.
Lateral spinal stenosis generally results in symptoms that are directly related to compression of the nerve roots at the level of the stenosis. Both pain and muscular weakness may result from hypertrophy of the facet joints, spondylosis deformity, bulging of the disc annulus, or herniation of the nucleus pulposus. Although large central disc herniations occur, most extruded disc fragments migrate laterally, and some disc fragments move to a position that is superior or inferior to the interspace.
Many patients with lumbar spinal stenosis (LSS) show symptomatic and functional improvement or remain unchanged over time. [24] In one study 90% of 169 untreated patients with suspected lateral recess stenosis improved symptomatically after 2 years. [25] A 4-year study of 32 patients treated conservatively for moderate stenosis reported unchanged symptoms in 70% of patients, improvement in 15%, and worsening in 15%. Walking capacity improved in 37% of patients, remained unchanged in 33%, and worsened in 30%. [26]
The natural history of LSS is not well understood. A slow progression appears to occur in all affected individuals. Even with significant narrowing, such persons are very unlikely to develop an acute cauda equina syndrome in the absence of significant disk herniation. Slow progression of dysfunction in the lumbar spine often leads to a feeling of heaviness in the legs that is only relieved by periods of rest. Infrequently, a facet joint synovial cyst leads to severe canal stenosis and the development of subacute radiculopathy, often characterized by pain and mild weakness. This may develop as a result of trauma or arthritic changes in the facet joint. [10, 27]
Patient Education
Patients with lumbar spinal stenosis should be educated to avoid aggravating factors, such as excessive lumbar extension and downhill ambulation. Additionally, patients should be instructed on correct posture and should also receive instructions concerning a home exercise program (eg, flexion-biased lumbar stabilization, flexibility training, gluteal strengthening, aerobic conditioning).
For patient education information, see Back Pain, Lumbar Laminectomy, and Chronic Pain.
-
Oblique view of the cervical spine demonstrates 2 levels of foraminal stenosis (white arrows) resulting from facet hypertrophy (yellow arrow) and uncovertebral joint hypertrophy.
-
Axial cervical CT myelogram demonstrates marked hypertrophy of the right facet joints (black arrows), which results in tight restriction of the neuroforaminal recess and lateral neuroforamen.
-
Short recovery time T1-weighted spin-echo sagittal MRI scan demonstrates marked spinal stenosis of the C1/C2 vertebral level cervical canal resulting from formation of the pannus (black arrow) surrounding the dens in a patient with rheumatoid arthritis. Long recovery time T2*-weighted fast spin-echo sagittal MRI scans better define the effect of the pannus (yellow arrow) on the anterior cerebrospinal fluid space. Note the anterior displacement of the upper cervical cord and the lower brainstem.
-
Posterior view from a radionuclide bone scan. A focally increased uptake of nuclide (black arrow) is demonstrated within the mid-to-upper thoracic spine in a patient with Paget disease.
-
T2-weighted sagittal MRI of the cervical spine demonstrating stenosis from ossification of the posterior longitudinal ligament, resulting in cord compression.
-
Severe cervical spondylosis can manifest as a combination of disk degeneration, osteophyte formation, vertebral subluxation, and attempted autofusion as depicted in this sagittal MRI. Also, note the focal kyphosis, which is typical in severe forms.
-
Lateral T2-weighted magnetic resonance imaging (MRI) scan demonstrating narrowing of the central spinal fluid signal (L4-L5), suggesting central canal stenosis.
-
Axial T2 magnetic resonance imaging (MRI) scan (L4-L5) in the same patient as in the above image, confirming central canal stenosis.
-
Trefoil appearance characteristic of central canal stenosis due to a combination of zygapophysial joint and ligamentum flavum hypertrophy.
-
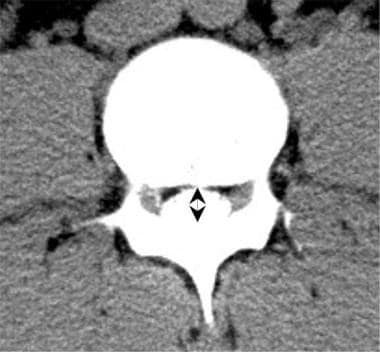
Lumbar computed tomography (CT) myelogram scan demonstrates a normal central canal diameter.
-
Lateral and axial magnetic resonance imaging (MRI) scan demonstrating right L4 lateral recess stenosis secondary to combination of far lateral disk protrusion and zygapophysial joint hypertrophy.
-
Sagittal measurements taken of the anteroposterior diameter of the cervical spinal canal are highly variable in otherwise healthy persons. An adult male without spinal stenosis has a diameter of 16-17 mm in the upper and middle cervical levels. Magnetic resonance imaging (MRI) scans and reformatted computed tomography (CT) images are equally as effective in obtaining these measurements, while radiography is not accurate.
-
Oblique 3-dimensional shaded surface display CT reconstruction of right foraminal stenosis resulting from unilateral facet hypertrophy (black arrow). The volume of the reconstruction has been cut obliquely across the neuroforaminal canal.
-
Anterior view of a lumbar myelogram demonstrates stenosis related to Paget disease. Myelography is limited because of the superimposition of multiple spinal structures that contribute to the overall pattern of stenosis.
-
Lateral view of a lumbar myelogram performed in a patient who has been fused across the L4-L5 and the L5-S1 vertebral interspaces using transpedicular screws. Treatment of lumbar spinal stenosis may include decompression laminectomies, followed by the placement of transpedicular screws (yellow arrows) with a posterior stabilization bar.
-
Sagittal view of a 3-dimensional volume image of the lumbar spine in a patient with a posterior fusion using transpedicular screws in L4 and L5. Note that an interposition graft has been placed between L4 and L5 to maintain satisfactory
-
Lateral swimmer's radiographic view demonstrates compression of the anterior contrast-filled cervical thecal sac. The defect helps localize the stenosis; however, the pattern does not reflect lateral disc herniation or spondylosis directly.
-
Axial T2-weighted gradient echo MRI scan. Note the high-grade spinal stenosis resulting in severe upper cervical cord compression (arrows). This patient presented with a central spinal cord syndrome that improved following surgical decompression.
-
Sagittal T2-weighted MRI image demonstrates severe stenosis. Spinal stenosis is demonstrated at several levels (white and yellow arrows) resulting from a combination of disc annulus bulging (white arrow) and epidural soft-tissue thickening (yellow arrow).
-
Superior-to-inferior view of 3-dimensional volume reconstruction of central canal spinal stenosis resulting from chronic disc herniation. The patient presented with lower extremity weakness and loss of bladder control.
-
: Sagittal T2 weighted fast spin-echo (FSE) MRI scan of a meningioma of the lower thoracic spine obtained without contrast enhancement. The effect of the mass is better seen because of the contrast between the mass and the cerebrospinal fluid (CSF). The anterior spinal canal is occupied by a mass that displaces and compresses the conus medullaris (C) at the T12 level. The mass (white arrow) is of intermediate increased signal brightness, compared to the normal spinal cord.
-
Sagittal T1-weighted spin-echo (SE) MRI scan of a meningioma of the lower thoracic spine obtained following IV gadolinium contrast enhancement. The mass is better seen because of the contrast enhancement within the meningioma (M). The anterior spinal canal is occupied by a mass that displaces and compresses (white arrows) the conus medullaris (C) at the T12 level. The mass (white arrow) is of intermediate increased signal brightness, compared to the normal spinal cord.
-
Normal findings in the thoracic spine as demonstrated by CT myelography. Note the detail of the spinal cord and the ventral and dorsal nerves surrounded by contrast.
-
nal-cut view of 3-dimensional reconstruction CT scan of the thoracic spine in tuberculosis spondylitis. Note the central spinal cavity (black arrow). The vertebral endplate has compressed downward (double blue arrows). The advantage of 3-dimensional reconstructions is the ability to better evaluate preoperatively the type of surgery needed to stabilize spinal compression fractures.
-
Paraspinal abscess aspiration biopsy. The stains were positive for mycobacteria (black arrows; acid-fast stain, magnification X100).
-
With the patient in a prone position and using CT localization, a bone biopsy and aspiration were performed from the area of greatest destruction within the vertebral endplate (arrow).
-
Aspergillosis organisms were recovered from a lumbar disc space abscess. The patient had received a renal transplant 12 months prior to the infection (hematoxylin and eosin, magnification X40).
-
Long recovery time T2*-weighted fat-suppressed sagittal MRI scan of the thoracic spine demonstrates subtle enlargement of a thoracic vertebral body (double white arrows) and a slightly increased degree of signal brightness within the vertebral body (yellow arrow).
-
Paget disease of the thoracic spine. Thoracic spinal CT scan demonstrates enlarged vertebral body endplates (black arrows). The axial image on the left demonstrates the characteristic thickening of the bony matrix of the vertebral body.
-
Axial lumbar CT scan demonstrates marked right-sided spinal canal stenosis (black arrow) resulting from advanced right-sided facet hypertrophy. Note the vacuum disc sign within the intervertebral disc (double yellow arrow). The vacuum disc sign is further indication of degenerative changes and spinal instability.
-
Pantopaque tracer in the epidural spaces. Pantopaque can remain in the epidural and facial spaces for years following a myelogram. Chronic inflammatory arachnoiditis has been associated with a combination of trauma (bleeding) with administration of Pantopaque.
-
Localization of thoracic lesion prior to surgical correction. A needle/wire localization technique is used to ensure the correct surgical level. Such preoperative localizations save time in the operating suite while reducing the need for intraoperative radiology.
-
Sagittal 3-dimensional CT reconstruction of the lumbar spine in a patient with multiple myeloma. The central portions of the vertebral bodies (yellow arrows) have been replaced by the nonossified tumor.
-
Biopsy (yellow arrow) of a multiple myeloma mass (black arrow) that has replaced the lumbar spinal canal (blue arrow) completely.
-
Multiple myeloma. Photomicrograph of an aspiration biopsy specimen.
-
Three-dimensional surface CT image of the lumbar spine following transpedicular screw placement across the L4-L5 interspace. Note how the tips of the screws project beyond the anterior margins of the L5 vertebral body.
-
Axial CT image taken through L5 in a patient in whom transpedicular screws have been placed. Note that the screws (black arrows) are too far lateral and anterior. The iliac veins lie just anterior to tips of the screws (white arrows). Both the angle of screw placement and the length of the screws must be tailored to the individual patient.
-
Spinal stenosis. Sagittal multiplanar reconstruction (MPR) image from a CT scan of the lumbar spine following posterior decompression and fusion of the L4-L5 interspace. The interposition graft (white arrow) is posterior to the desired position. The patient remained asymptomatic. Follow-up imaging should focus upon the stability of the posterior fusion, the position of the pedicle screws, and the position of the interposition graft.
-
Sagittal reformatted image from a CT of the cervical spine following anterior spinal decompression and fusion. Surgical treatment of spinal canal stenosis often involves anterior vertebrectomy and bone graft interposition. The goal in such cases is to restore cervical spinal alignment (white line) while securing anterior stability. In this patient, the bone graft (double black arrows) has migrated forward (double yellow arrows).