Practice Essentials
The clinical definition of cardiogenic shock is decreased cardiac output and evidence of tissue hypoxia in the presence of adequate intravascular volume. [1] Cardiogenic shock is the leading cause of death in acute myocardial infarction (MI), with mortality rates as high as 70-90% in the absence of aggressive, highly experienced technical care. See the image below.
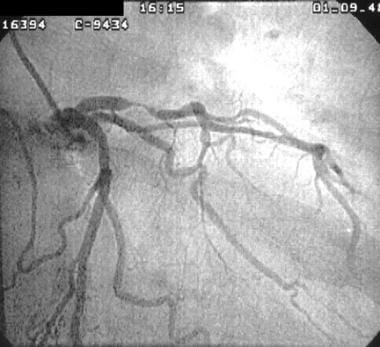 Cardiogenic shock. This image was obtained from a patient with an acute anterolateral myocardial infarction who developed cardiogenic shock. Coronary angiography images showed severe stenosis of the left anterior descending coronary artery, which was dilated by percutaneous transluminal coronary angioplasty.
Cardiogenic shock. This image was obtained from a patient with an acute anterolateral myocardial infarction who developed cardiogenic shock. Coronary angiography images showed severe stenosis of the left anterior descending coronary artery, which was dilated by percutaneous transluminal coronary angioplasty.
Signs and symptoms
The diagnosis of cardiogenic shock can sometimes be made at the bedside by observing the following:
-
Hypotension
-
Absence of hypovolemia
-
Clinical signs of poor tissue perfusion (ie, oliguria, cyanosis, cool extremities, altered mentation)
Findings on physical examination include the following:
-
Skin is usually ashen or cyanotic and cool; extremities are mottled
-
Peripheral pulses are rapid and faint and may be irregular if arrhythmias are present
-
Jugular venous distention and crackles in the lungs are usually (but not always) present; peripheral edema also may be present
-
Heart sounds are usually distant, and third and fourth heart sounds may be present
-
The pulse pressure may be low, and patients are usually tachycardic
-
Patients show signs of hypoperfusion, such as altered mental status and decreased urine output
-
Ultimately, patients develop systemic hypotension (ie, systolic blood pressure below 90 mm Hg or a decrease in mean blood pressure by 30 mm Hg)
See Presentation for more detail.
Diagnosis
Laboratory studies
-
Biochemical profile
-
CBC
-
Cardiac enzymes (eg, creatine kinase and CK-MB, troponins, myoglobin, LDH)
-
Arterial blood gases
-
Lactate
-
Brain natriuretic peptide
Imaging studies
-
Echocardiography should be performed early to establish the cause of cardiogenic shock
-
Chest radiographic findings are useful for excluding other causes of shock or chest pain (eg, aortic dissection, tension pneumothorax, pneumomediastinum)
-
Ultrasonography can be used to guide fluid management
-
Coronary angiography is urgently indicated in patients with myocardial ischemia or MI who also develop cardiogenic shock
Electrocardiography
-
Perform electrocardiography immediately to help diagnose MI and/or myocardial ischemia
-
A normal ECG, however, does not rule out the possibility of acute MI
Invasive hemodynamic monitoring
-
Swan-Ganz catheterization is very useful for helping exclude other causes and types of shock (eg, volume depletion, obstructive shock, and shock)
-
The hemodynamic measurements of cardiogenic shock are a pulmonary capillary wedge pressure (PCWP) greater than 15 mm Hg and a cardiac index less than 2.2 L/min/m 2
-
The presence of large V waves on the PCWP tracing suggests severe mitral regurgitation
-
A step-up in oxygen saturation between the right atrium and the right ventricle is diagnostic of ventricular septal rupture
-
High right-sided filling pressures in the absence of an elevated PCWP, when accompanied by ECG criteria, indicate right ventricular infarction
See Workup for more detail.
Management
Cardiogenic shock is an emergency requiring the following:
-
Fluid resuscitation to correct hypovolemia and hypotension, unless pulmonary edema is present
-
Prompt initiation of pharmacologic therapy to maintain blood pressure and cardiac output
-
Admission to an intensive care setting (eg, cardiac catheterization suite or ICU or critical care transport to a tertiary care center)
-
Early and definitive restoration of coronary blood flow; at present, this represents standard therapy for patients with cardiogenic shock due to myocardial ischemia
-
Correction of electrolyte and acid-base abnormalities (eg, hypokalemia, hypomagnesemia, acidosis)
Invasive procedures include the following:
-
Placement of a central line may facilitate volume resuscitation, provide vascular access for multiple infusions, and allow invasive monitoring of central venous pressure
-
An arterial line may be placed to provide continuous blood pressure monitoring
-
An intra-aortic balloon pump may be placed as a bridge to percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG)
Pharmacologic therapy
-
Patients with MI or acute coronary syndrome are given aspirin and heparin
-
Inotropic and/or vasopressor drug therapy may be necessary in patients with inadequate tissue perfusion and adequate intravascular volume, so as to maintain mean arterial pressure (MAP) of 60 or 65 mm Hg
-
Diuretics are used to decrease plasma volume and peripheral edema
Features of dopamine are as follows:
-
Dopamine is the drug of choice to improve cardiac contractility in patients with hypotension
-
Dopamine may increase myocardial oxygen demand
-
Dopamine is usually initiated at a rate of 5-10 mcg/kg/min IV
-
The infusion rate is adjusted according to the blood pressure and other hemodynamic parameters
-
Often, patients may require doses as high as 20 mcg/kg/min
Features of dobutamine are as follows:
-
Dobutamine may be preferable to dopamine if the systolic blood pressure is higher than 80 mm Hg
-
Compared with dopamine, dobutamine has less effect on myocardial oxygen demand
-
Tachycardia from dobutamine may preclude its use in some patients
If the patient remains hypotensive despite moderate doses of dopamine, a direct vasoconstrictor may be administered, as follows:
-
Norepinephrine is started at a dose of 0.5 mcg/kg/min and titrated to maintain an MAP of 60 mm Hg
-
The dose of norepinephrine may vary from 0.2-1.5 mcg/kg/min
-
Doses as high as 3.3 mcg/kg/min have been used
Phosphodiesterase inhibitors (eg, inamrinone [formerly amrinone], milrinone) are inotropic agents with vasodilating properties and long half-lives that are beneficial in patients with cardiac pump failure, but they may require concomitant vasopressor administration
PCI and CABG
-
Either PCI or CABG is the treatment of choice for cardiogenic shock
-
PCI should be initiated within 90 minutes after presentation
-
PCI remains helpful, as an acute intervention, within 12 hours after presentation
-
Thrombolytic therapy is second best but should be considered if PCI and CABG are not immediately available
See Treatment and Medication for more detail.
Background
Cardiogenic shock is a physiologic state in which inadequate tissue perfusion results from cardiac dysfunction, most often systolic. It is a major, and frequently fatal, complication of a variety of acute and chronic disorders, occurring most commonly following acute myocardial infarction (MI). (See Pathophysiology, Etiology, and Prognosis.)
Although ST-segment elevation MI (STEMI, previously termed Q-wave MI) is encountered in most patients, cardiogenic shock may also develop in patients with non ̶ ST-segment elevation acute coronary syndrome (NSTEMI, NSTACS, or unstable angina). (See the image below.)
 Cardiogenic shock. This image was obtained from a patient with an acute anterolateral myocardial infarction who developed cardiogenic shock. Coronary angiography images showed severe stenosis of the left anterior descending coronary artery, which was dilated by percutaneous transluminal coronary angioplasty.
Cardiogenic shock. This image was obtained from a patient with an acute anterolateral myocardial infarction who developed cardiogenic shock. Coronary angiography images showed severe stenosis of the left anterior descending coronary artery, which was dilated by percutaneous transluminal coronary angioplasty.
The clinical definition of cardiogenic shock is decreased cardiac output and evidence of tissue hypoxia in the presence of adequate intravascular volume. Hemodynamic criteria for cardiogenic shock are sustained hypotension (systolic blood pressure < 90 mm Hg for ≥30 min) and a reduced cardiac index (< 2.2 L/min/m2) in the presence of normal or elevated pulmonary capillary wedge pressure (>15 mm Hg) or right ventricular end-diastolic pressure (RVEDP) (>10 mm Hg). (See DDx, Workup.)
Cardiogenic shock continues to be a difficult clinical problem; the management of this condition requires a rapid and well-organized approach. (See Prognosis, Treatment, and Medication.)
The diagnosis of cardiogenic shock may be made at the bedside by observing hypotension, absence of hypovolemia, and clinical signs of poor tissue perfusion, which include oliguria, cyanosis, cool extremities, and altered mentation. These signs usually persist after attempts have been made to correct hypovolemia, arrhythmia, hypoxia, and acidosis. (See Presentation, DDx.)
Types of circulatory shock
Shock is identified in most patients on the basis of findings of hypotension and inadequate organ perfusion, which may be caused by either low cardiac output or low systemic vascular resistance (SVR). Circulatory shock can be subdivided into four distinct classes according to the underlying mechanism and characteristic hemodynamic findings. In all patients, before a definite diagnosis of septic shock is established, the following four classes of shock should be considered and systematically differentiated. (See Pathophysiology, Etiology, Presentation, and Workup.)
Cardiogenic shock
Cardiogenic shock characterized by primary myocardial dysfunction renders the heart to be unable to maintain adequate cardiac output. These patients demonstrate clinical signs of low cardiac output, with adequate intravascular volume. The patients have cool and clammy extremities, poor capillary refill, tachycardia, narrow pulse pressure, and low urine output.
Hypovolemic shock
Hypovolemic shock results from loss of blood volume, the possible reasons for which include gastrointestinal bleeding, extravasation of plasma, major surgery, trauma, and severe burns.
Obstructive shock
Obstructive shock results from impedance of circulation by an intrinsic or extrinsic obstruction. Pulmonary embolism, dissecting aneurysm, and pericardial tamponade all result in obstructive shock.
Distributive shock
Distributive shock is caused by conditions producing direct arteriovenous shunting and is characterized by decreased SVR or increased venous capacitance because of the vasomotor dysfunction. These patients have high cardiac output, hypotension, high pulse pressure, low diastolic pressure, and warm extremities with good capillary refill. Such findings upon physical examination strongly suggest a working diagnosis of septic shock.
Pathophysiology
Cardiogenic shock is recognized as a low-cardiac-output state secondary to extensive left ventricular (LV) infarction, development of a mechanical defect (eg, ventricular septal defect or papillary muscle rupture), or right ventricular (RV) infarction.
Autopsy studies show that cardiogenic shock is generally associated with the loss of more than 40% of the LV myocardial muscle. [2] The pathophysiology of cardiogenic shock in the setting of coronary artery disease, is described below.
Myocardial pathology
Cardiogenic shock is characterized by systolic and diastolic dysfunction leading to end organ hypoperfusion. The interruption of blood flow in an epicardial coronary artery causes the zone of myocardium supplied by that vessel to lose the ability to shorten and perform contractile work. If a sufficient area of myocardium undergoes ischemic injury, LV pump function become depressed and systemic hypotension develops.
Patients who develop cardiogenic shock from acute MI consistently have evidence of progressive myocardial necrosis with infarct extension. Decreased coronary perfusion pressure and cardiac output as well as increased myocardial oxygen demand play a role in the vicious cycle that leads to cardiogenic shock and potentially death. [3]
Patients suffering from cardiogenic shock often have multivessel coronary artery disease with limited coronary blood flow reserve. Ischemia remote from the infarcted zone is an important contributor to shock. Myocardial diastolic function is also impaired, because ischemia decreases myocardial compliance and impairs filling, thereby increasing LV filling pressure and leading to pulmonary edema and hypoxemia.
Cellular pathology
Tissue hypoperfusion, with consequent cellular hypoxia, causes anaerobic glycolysis, the accumulation of lactic acid, and intracellular acidosis. Also, myocyte membrane transport pumps fail, which decreases transmembrane potential and causes intracellular accumulation of sodium and calcium, resulting in myocyte swelling.
If ischemia is severe and prolonged, myocardial cellular injury becomes irreversible and leads to myonecrosis, which includes mitochondrial swelling, the accumulation of denatured proteins and chromatin, and lysosomal breakdown. These events induce fracture of the mitochondria, nuclear envelopes, and plasma membranes.
Additionally, apoptosis (programmed cell death) may occur in peri-infarcted areas and may contribute to myocyte loss. Activation of inflammatory cascades, oxidative stress, and stretching of the myocytes produces mediators that overpower inhibitors of apoptosis, thus activating the apoptosis.
Reversible myocardial dysfunction
Large areas of myocardium that are dysfunctional but still viable can contribute to the development of cardiogenic shock in patients with MI. This potentially reversible dysfunction is often described as myocardial stunning or as hibernating myocardium. Although hibernation is considered a different physiologic process than myocardial stunning, the conditions are difficult to distinguish in the clinical setting and they often coexist.
Myocardial stunning represents postischemic dysfunction that persists despite restoration of normal blood flow. By definition, myocardial dysfunction from stunning eventually resolves completely. The mechanism of myocardial stunning involves a combination of oxidative stress, abnormalities of calcium homeostasis, and circulating myocardial depressant substances.
Hibernating myocardium is a state of persistently impaired myocardial function at rest, which occurs because of the severely reduced coronary blood flow. Hibernation appears to be an adaptive response to hypoperfusion that may minimize the potential for further ischemia or necrosis. Revascularization of the hibernating (and/or stunned) myocardium generally leads to improved myocardial function.
Consideration of the presence of myocardial stunning and hibernation is vital in patients with cardiogenic shock because of the therapeutic implications of these conditions. Hibernating myocardium improves with revascularization, whereas the stunned myocardium retains inotropic reserve and can respond to inotropic stimulation.
Cardiovascular mechanics of cardiogenic shock
Cardiogenic shock is the most severe clinical expression of LV failure. The primary mechanical defect in cardiogenic shock is a shift to the right for the LV end-systolic pressure-volume curve, because of a marked reduction in contractility. As a result, at a similar or even lower systolic pressure, the ventricle is able to eject less blood volume per beat. Therefore, the end-systolic volume is usually greatly increased in persons with cardiogenic shock. The degree to which LV end systolic volume increases is a powerful hemodynamic predictor of mortality following STEMI. [4]
To compensate for the diminished stroke volume, the curvilinear diastolic pressure-volume curve also shifts to the right, with a decrease in diastolic compliance. This leads to increased diastolic filling and increased LV end-diastolic pressure. The attempt to enhance cardiac output by this mechanism comes at the cost of having a higher LV diastolic filling pressure, which ultimately increases myocardial oxygen demand and can lead to pulmonary edema.
As a result of decreased contractility, the patient develops elevated LV and RV filling pressures and low cardiac output. Mixed venous oxygen saturation falls because of the increased tissue oxygen extraction, which is due to the low cardiac output. This, combined with the intrapulmonary shunting that is often present, contributes to substantial arterial oxygen desaturation.
Systemic effects
When a critical mass of LV myocardium becomes ischemic and fails to pump effectively, stroke volume and cardiac output are curtailed. The LV pump function becomes depressed; cardiac output, stroke volume, and blood pressure decline while end-systolic volume increases. [5] Myocardial ischemia is further exacerbated by impaired myocardial perfusion due to hypotension and tachycardia.
The LV pump failure increases ventricular diastolic pressures concomitantly, causing additional wall stress and thereby elevating myocardial oxygen requirements. Systemic perfusion is compromised by decreased cardiac output, with tissue hypoperfusion intensifying anaerobic metabolism and instigating the formation of lactic acid (lactic acidosis), which further deteriorates the systolic performance of the myocardium.
Depressed myocardial function also leads to the activation of several physiologic compensatory mechanisms. These include sympathetic stimulation, which increases the heart rate and cardiac contractility [6] and causes renal salt and fluid retention, hence augmenting the LV preload. The elevated heart rate and contractility increases myocardial oxygen demand, further worsening myocardial ischemia.
Fluid retention and impaired LV diastolic filling triggered by tachycardia and ischemia worsen pulmonary venous congestion and hypoxemia. Sympathetically mediated vasoconstriction to maintain systemic blood pressure amplifies myocardial afterload, which additionally impairs cardiac performance.
Finally, excessive myocardial oxygen demand with simultaneous inadequate myocardial perfusion worsens myocardial ischemia, initiating a vicious cycle that ultimately ends in death, if uninterrupted. [3]
Usually, a combination of systolic and diastolic myocardial dysfunction is present in patients with cardiogenic shock. Metabolic derangements that impair myocardial contractility further compromise systolic ventricular function. Myocardial ischemia decreases myocardial compliance, thereby elevating LV filling pressure at a given end-diastolic volume (diastolic dysfunction), which leads to pulmonary congestion and congestive heart failure.
Shock state
Shock state, irrespective of the etiology, is described as a syndrome initiated by acute systemic hypoperfusion that leads to tissue hypoxia and vital organ dysfunction. All forms of shock are characterized by inadequate perfusion to meet the metabolic demands of the tissues. A maldistribution of blood flow to end organs begets cellular hypoxia and end organ damage, the well-described multisystem organ dysfunction syndrome. The organs of vital importance are the brain, heart, and kidneys.
A decline in higher cortical function may indicate diminished perfusion of the brain, which leads to an altered mental status ranging from confusion and agitation to flaccid coma. The heart plays a central role in propagating shock. Depressed coronary perfusion leads to worsening cardiac dysfunction and a cycle of self-perpetuating progression of global hypoperfusion. Renal compensation for reduced perfusion results in diminished glomerular filtration, causing oliguria and subsequent renal failure.
Etiology
Cardiogenic shock can result from the following types of cardiac dysfunction:
-
Systolic dysfunction
-
Diastolic dysfunction
-
Valvular dysfunction
-
Cardiac arrhythmias
-
Coronary artery disease
-
Mechanical complications
The vast majority of cases of cardiogenic shock in adults are due to acute myocardial ischemia. Indeed, cardiogenic shock is generally associated with the loss of more than 40% of the LV myocardium, although in patients with previously compromised LV function, even a small infarction may precipitate shock. Cardiogenic shock is more likely to develop in people who are elderly or diabetic or in persons who have had a previous inferior MI.
Complications of acute MI, such as acute mitral regurgitation, large RV infarction, rupture of the interventricular septum or LV free wall, and tamponade can result in cardiogenic shock. Conduction abnormalities (eg, atrioventricular blocks, sinus bradycardia) are also risk factors.
Many cases of cardiogenic shock occurring after acute coronary syndromes may be due to medication administration. The use of beta blockers and angiotensin-converting enzyme (ACE) inhibitors in acute coronary syndromes must be carefully timed and monitored. [3, 7, 8]
In children, preceding viral infection may cause myocarditis. In addition, children and infants may have unrecognized congenital structural heart defects that are well compensated until there is a stressor. These etiologies plus toxic ingestions make up the three primary causes of cardiogenic shock in children.
A systemic inflammatory response syndrome–type mechanism has also been implicated in the etiology of cardiogenic shock. Elevated levels of white blood cells, body temperature, complement, interleukins, and C-reactive protein are often seen in large myocardial infarctions. Similarly, inflammatory nitric oxide synthetase (iNOS) is also released in high levels during myocardial stress. Nitric oxide production induced by iNOS may uncouple calcium metabolism in the myocardium resulting in a stunned myocardium. Additionally, iNOS leads to the expression of interleukins, which may themselves cause hypotension.
Left ventricular failure
Systolic dysfunction
The primary abnormality in systolic dysfunction is abated myocardial contractility. Acute MI or ischemia is the most common cause; cardiogenic shock is more likely to be associated with anterior MI. The causes of systolic dysfunction leading to cardiogenic shock can be summarized as follows:
-
Ischemia/MI
-
Global hypoxemia
-
Valvular disease
-
Myocardial depressant drugs (eg, beta blockers, calcium-channel blockers, and antiarrhythmics)
-
Myocardial contusion
-
Respiratory acidosis
-
Metabolic derangements (eg, acidosis, hypophosphatemia, and hypocalcemia)
-
Severe myocarditis
-
End-stage cardiomyopathy (including valvular causes)
-
Prolonged cardiopulmonary bypass.
-
Cardiotoxic drugs (eg, doxorubicin [Adriamycin])
Diastolic dysfunction
Increased LV diastolic chamber stiffness contributes to cardiogenic shock during cardiac ischemia, as well as in the late stages of hypovolemic shock and septic shock. Increased diastolic dysfunction is particularly detrimental when systolic contractility is also depressed. The causes of cardiogenic shock due primarily to diastolic dysfunction can be summarized as follows:
-
Ischemia
-
Ventricular hypertrophy
-
Restrictive cardiomyopathy
-
Prolonged hypovolemic or septic shock
-
Ventricular interdependence
-
External compression by pericardial tamponade
Greatly increased afterload
Increased afterload, which can impair cardiac function, can be caused by the following:
-
Aortic stenosis
-
Hypertrophic cardiomyopathy
-
Dynamic aortic outflow tract obstruction
-
Coarctation of the aorta
-
Malignant hypertension
Valvular and structural abnormality
Valvular dysfunction may immediately lead to cardiogenic shock, or it may aggravate other etiologies of shock. Acute mitral regurgitation secondary to papillary muscle rupture or dysfunction is caused by ischemic injury. Rarely, acute obstruction of the mitral valve by a left atrial thrombus may result in cardiogenic shock by means of severely decreased cardiac output. Aortic and mitral regurgitation reduce forward flow, raise end-diastolic pressure, and aggravate shock associated with other etiologies.
Valvular and structural abnormalities associated with cardiogenic shock include the following:
-
Mitral stenosis
-
Endocarditis
-
Mitral aortic regurgitation
-
Obstruction due to atrial myxoma or thrombus
-
Papillary muscle dysfunction or rupture
-
Ruptured septum or free wall arrhythmias
-
Tamponade
Decreased contractility
Reduced myocardial contractility can result from the following:
-
RV infarction
-
Ischemia
-
Hypoxia
-
Acidosis
Right ventricular failure
Greatly increased afterload
Afterload increase associated with RV failure can result from the following:
-
Pulmonary embolism (PE)
-
Pulmonary vascular disease (eg, pulmonary arterial hypertension and veno-occlusive disease)
-
Hypoxic pulmonary vasoconstriction
-
Peak end-expiratory pressure (PEEP)
-
High alveolar pressure
-
Acute respiratory distress syndrome (ARDS)
-
Pulmonary fibrosis
-
Sleep-disordered breathing
-
Chronic obstructive pulmonary disease (COPD)
Arrhythmias
Ventricular tachyarrhythmias are often associated with cardiogenic shock. Furthermore, bradyarrhythmias may cause or aggravate shock due to another etiology. Sinus tachycardia and atrial tachyarrhythmias contribute to hypoperfusion and aggravate shock.
Epidemiology
United States statistics
The incidence rate of cardiogenic shock ranges from 5% to 10% in patients with acute MI. [9] In the Worcester Heart Attack Study, a community-wide analysis, the reported incidence rate was 7.5%. [10] The literature contains few data on cardiogenic shock in patients without ischemia.
A 2014 review of the 2003-2010 Nationwide Inpatient Sample (NIS) databases revealed a 7.9% incidence in patients with STEMI. [9] Overall, of cases with cardiogenic shock and STEMI, 42.3% were located in the anterior wall, 38.6% in the inferior wall, and 19.1% at other sites. [9]
As many as 3% of patients with NSTACS develop cardiogenic shock. [11]
International statistics
Several multicenter thrombolytic trials in Europe reported a prevalence rate of cardiogenic shock following MI of approximately 7%.
Race-, sex-, and age-related demographics
Asian/Pacific Islanders have a higher incidence of cardiogenic shock (11.4%) than white (8%), black (6.9%), and Hispanic (8.6%) patients. [9]
Although the overall incidence of cardiogenic shock has traditionally been higher in men than in women, a difference resulting from the increased prevalence of coronary artery disease in males, the 2003-2010 NIS data revealed women had a higher overall incidence of cardiogenic shock (8.5%) than men (76%) during this period. [9] Moreover, a higher percentage of female patients with MI developed cardiogenic shock than did males with MI. [9]
Median age for cardiogenic shock mirrors the bimodal distribution of disease. For adults, the median age ranges from 65-66 years. For children, cardiogenic shock presents as a consequence of fulminant myocarditis or congenital heart disease.
Overall, the 2003-2010 NIS data revealed patients aged 75 years and older suffered cardiogenic shock more often than those younger than 75 years. [9]
Prognosis
Cardiogenic shock is the leading cause of death in acute MI, [12] with a 50% mortality in this setting. [13] In the absence of aggressive, highly experienced technical care, mortality rates among patients with cardiogenic shock are exceedingly high (up to 70-90%). [1, 24] The key to achieving a good outcome is rapid diagnosis, prompt supportive therapy, and expeditious coronary artery revascularization in patients with myocardial ischemia and infarction. [14, 15, 16] Thus, with the implementation of prompt revascularization, improved interventional procedures, and better medical therapies and mechanical support devices, the mortality rates from cardiogenic shock may continue to decline.
The overall in-hospital mortality rate for patients with cardiogenic shock is 39%, [9] with a range of 27% to 51%. [9]
Race-stratified inpatient mortality rates from cardiogenic shock are as follows (race-based mortality differences persisted even after adjustment for early mechanical revascularization status) [9] :
-
Hispanic patients - 40.6%
-
Black patients - 39.9%
-
White patients - 38.9%
-
Asians/others - 37.6%
Mortality rates are similar for patients with cardiogenic shock secondary to STEMI or NSTACS. [17, 18]
Evidence of RV dilatation on an echocardiogram may indicate a worse outcome in patients with cardiogenic shock, as may RV infarction on a right-side electrocardiogram. [19] The prognosis for patients who survive cardiogenic shock is not well studied but may be favorable if the underlying cause of shock is expeditiously corrected.
Age and cardiac index predict survival and ability to wean from short-term mechanical circulatory support device following acute MI that is complicated by cardiogenic shock. [20] Although results of angiography and the cardiac index predict ventricular recovery, half of patients who are optimally revascularized still require heart replacement therapy.
Morbidity and mortality
Complications of cardiogenic shock may include the following:
-
Cardiopulmonary arrest
-
Dysrhythmia
-
Renal failure
-
Multisystem organ failure
-
Ventricular aneurysm
-
Thromboembolic sequelae
-
Stroke
-
Death
The following predictors of mortality were identified from the Global Utilization of Streptokinase and Tissue-Plasminogen Activator for Occluded Coronary Arteries (GUSTO-I) trial [21] :
-
Increasing age
-
Prior MI
-
Altered sensorium
-
Cold, clammy skin
-
Oliguria
Echocardiographic findings such as LV ejection fraction (LVEF) and mitral regurgitation are independent predictors of mortality. An ejection fraction of less than 28% has been associated with a survival rate of 24% at 1 year, compared to a survival rate of 56% with a higher ejection fraction. [22] Moderate or severe mitral regurgitation was found to be associated with a 1-year survival rate of 31%, compared to a survival rate of 58% in patients with no regurgitation. [22] The time to reperfusion is an important predictor of mortality in acute MI complicated by cardiogenic shock. In patients with shock, the in-hospital mortality rate increased progressively with increasing time-to-reperfusion.
Outcomes in cardiogenic shock significantly improve only when rapid revascularization can be achieved. The SHOCK (Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock?) trial demonstrated that overall mortality when revascularization occurs is 38%. [23] When rapid revascularization is not attempted, mortality rates approach 70%. Rates vary depending on the procedure (eg, percutaneous transluminal coronary angioplasty, stent placement, thrombolytic therapy).
Patient Education
Patients should receive instruction regarding the early warning signs of acute MI and how to access the emergency medical system (eg, calling 911).
Instruct patients on cardiac risk factors, particularly those that are reversible and subject to change (eg, smoking, diet, exercise).
Discuss the benefits and limitations of palliative care. [1, 24]
For patient education information, see the First Aid & Emergencies Center, Healthy Living Center, and Heart Health Center, as well as Shock and Cardiopulmonary Resuscitation (CPR).
-
Cardiogenic shock. This image was obtained from a patient with an acute anterolateral myocardial infarction who developed cardiogenic shock. Coronary angiography images showed severe stenosis of the left anterior descending coronary artery, which was dilated by percutaneous transluminal coronary angioplasty.
-
Cardiogenic shock. This coronary angiogram from a patient with cardiogenic shock demonstrates severe stenosis of the right coronary artery.
-
Cardiogenic shock. This coronary angiogram from a patient with cardiogenic shock reveals severe stenosis of the right coronary artery. Following angioplasty of the critical stenosis, coronary flow was reestablished. The patient recovered from cardiogenic shock.
-
Cardiogenic shock. This electrocardiogram shows evidence of an extensive anterolateral myocardial infarction. The patient subsequently developed cardiogenic shock.
-
Cardiogenic shock. The electrocardiogram tracing shows further evolutionary changes in a patient with cardiogenic shock.
-
Cardiogenic shock. The electrocardiogram tracing was obtained from a patient who developed cardiogenic shock secondary to pericarditis and pericardial tamponade.
-
Cardiogenic shock. A 63-year-old man was admitted to the emergency department with clinical features of cardiogenic shock. The electrocardiogram revealed findings indicative of wide-complex tachycardia, likely ventricular tachycardia. Following cardioversion, his shock state improved. Myocardial ischemia was the cause of the ventricular tachycardia.
-
Cardiogenic shock. A short-axis view of the left ventricle demonstrates small pericardial effusion, low ejection fraction, and segmental wall motion abnormalities. Courtesy of Michael Stone, MD, RDMS.
-
Cardiogenic shock. Pleural sliding in an intercostal space demonstrates increased lung comet artifacts suggestive of pulmonary edema. Courtesy of Michael Stone, MD, RDMS.
-
Cardiogenic shock. HeartMate II Left Ventricular Assist Device. Reprinted with the permission of Thoratec Corporation.
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Treatment
- Approach Considerations
- Prehospital Care
- Resuscitation, Ventilation, and Pharmacologic Intervention
- Hemodynamic Support
- Thrombolytic Therapy
- Intra-Aortic Balloon Pump
- Ventricular Assist Devices
- Percutaneous Transluminal Coronary Angioplasty
- Coronary Artery Bypass Grafting
- Revascularization in the SHOCK Trial
- Patient Transfer
- Prevention
- Consultations
- Show All
- Guidelines
- Medication
- Questions & Answers
- Media Gallery
- References






