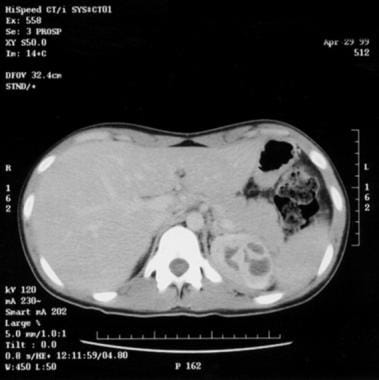
Practice Essentials
Acute pyelonephritis is a bacterial infection of the kidney parenchyma that can be organ- and/or life-threatening and that often leads to scarring of the kidney. The bacteria in these cases have usually ascended from the lower urinary tract, but may also reach the kidney via the bloodstream. Timely diagnosis and management of acute pyelonephritis has a significant impact on patient outcomes. [1, 2] See the image below.
Signs and symptoms
The classic presentation in patients with acute pyelonephritis is as follows:
-
Fever - This is not always present, but when it is, the temperature often exceeds 103°F (39.4°C)
-
Costovertebral angle pain - Pain may be mild, moderate, or severe; flank or costovertebral angle tenderness is most commonly unilateral over the involved kidney, although bilateral discomfort may be present
-
Nausea and/or vomiting - These vary in frequency and intensity, from absent to severe; anorexia is common in patients with acute pyelonephritis
Gross hematuria (hemorrhagic cystitis), unusual in males with pyelonephritis, occurs in 30-40% of females, most often young women, with the disorder.
Symptoms usually develop over hours or over the course of a day but may not occur at the same time. If the patient is male, elderly, or a child or has had symptoms for more than 7 days, the infection should be considered complicated until proven otherwise.
The classic manifestations of acute pyelonephritis observed in adults are often absent in children, particularly neonates and infants. In children aged 2 years or younger, the most common signs and symptoms of urinary tract infection (UTI) are as follows:
-
Failure to thrive
-
Feeding difficulty
-
Fever
-
Vomiting
Elderly patients may present with typical manifestations of pyelonephritis, or they may experience the following:
-
Fever
-
Mental status change
-
Decompensation in another organ system
-
Generalized deterioration
See Presentation for more detail.
Diagnosis
In the outpatient setting, pyelonephritis is usually suggested by a patient’s history and physical examination and supported by urinalysis results. Urine specimens can be collected through the following methods:
-
Clean catch
-
Urethral catheterization
-
Suprapubic needle aspiration
Urinalysis can include the following:
-
Dipstick leukocyte esterase test (LET) - Helps to screen for pyuria
-
Nitrite production test (NPT) - To screen for bacteriuria
-
Examination for hematuria (gross and microscopic) and proteinuria
Urine culture is indicated in any patient with pyelonephritis, whether treated in an inpatient or outpatient setting, because of the possibility of antibiotic resistance.
Imaging studies that may be used in assessing acute pyelonephritis include the following:
-
Computed tomography (CT) scanning - To identify alterations in renal parenchymal perfusion; alterations in contrast excretion, perinephric fluid, and nonrenal disease; gas-forming infections; hemorrhage; inflammatory masses; and obstruction
-
Magnetic resonance imaging (MRI) – To detect kidney infection or masses and urinary obstruction, as well as to evaluate renal vasculature
-
Ultrasonography - To screen for urinary obstruction in children admitted for febrile illnesses and to examine patients for kidney abscesses, acute focal bacterial nephritis, and stones (in xanthogranulomatous pyelonephritis)
-
Scintigraphy - To detect focal renal abnormalities
-
CT and MR urography - Used in the evaluation of hematuria
See Workup for more detail.
Management
Antibiotic therapy is essential in the treatment of acute pyelonephritis and prevents progression of the infection. Urine culture and sensitivity testing should always be performed, and empirical therapy should be tailored to the infecting uropathogen. [3]
Patients presenting with complicated pyelonephritis should be managed as inpatients and treated empirically with broad-spectrum parenteral antibiotics.
Outpatient care
Outpatient treatment is appropriate for patients who have an uncomplicated infection that does not warrant hospitalization. Treatment can be with a single dose of a parenteral antibiotic followed by oral therapy, provided that the patient is monitored within the first 48 hours.
Inpatient care
Inpatient care includes the following:
-
Supportive care
-
Monitoring of urine and blood culture results
-
Monitoring of comorbid conditions for deterioration
-
Maintenance of hydration status with IV fluids until hydration can be maintained with oral intake
-
IV antibiotics until defervescence and significant symptomatic improvement occur; convert to an oral regimen tailored to urine or blood culture results
Surgery
In addition to antibiotics, surgery may be necessary to treat the following manifestations of acute pyelonephritis:
-
Renal cortical abscess (renal carbuncle): Surgical drainage (if patients do not respond to antibiotic therapy); other surgical options are enucleation of the carbuncle and nephrectomy
-
Renal corticomedullary abscess: Incision and drainage, nephrectomy
-
Perinephric abscess: Drainage, nephrectomy
-
Calculi-related urinary tract infection (UTI): Extracorporeal shockwave lithotripsy (ESWL) or endoscopic, percutaneous, or open surgery
-
Renal papillary necrosis: CT scan ̶ guided drainage or surgical drainage with debridement
-
Xanthogranulomatous pyelonephritis: Nephrectomy
See Treatment and Medication for more detail.
Background
Acute pyelonephritis is a potentially organ- and/or life-threatening infection that characteristically causes scarring of the kidney. [4] An episode of acute pyelonephritis may lead to significant renal damage; acute kidney injury; abscess formation (eg, nephric, perinephric); sepsis; or sepsis syndrome, septic shock, and multiorgan system failure.
A population-based study of acute pyelonephritis in the United States found overall annual rates of 15–17 cases per 10,000 females and 3–4 cases per 10,000 males. [5]
Diagnosing and managing acute pyelonephritis is not always straightforward. Wide variation exists in the clinical presentation, severity, options, and disposition of the disease.
Patients in the age range of 5-65 years typically present with lower urinary tract infection (UTI) symptoms (eg, dysuria, frequency, urgency, gross hematuria, suprapubic pain) and classic upper UTI symptoms (eg, flank pain, back pain), with or without systemic signs and symptoms (eg, fever, chills, abdominal pain, nausea, vomiting, costovertebral angle tenderness) and with or without leukocytosis. However, acute pyelonephritis can present as nonspecific symptoms.
A number of studies using immunochemical markers have shown that many women who initially present with lower urinary tract symptoms actually have pyelonephritis. Their pyelonephritis is often identified when short-course therapy for uncomplicated cystitis fails.
With patients at the extremes of age, the presentation may be so atypical that pyelonephritis is not in the differential diagnosis. Infants may present with feeding difficulty or fever, and the elderly may have mental status change or fever.
Acute pyelonephritis is complex, and there is no consistent set of signs and symptoms that is both sensitive and specific for the diagnosis. Therefore, clinicians must maintain a high index of suspicion.
In contrast to the plethora of data available for the treatment of cystitis, less substantial data are available regarding the appropriate antibiotic choice or duration of therapy for acute pyelonephritis. An additional cause for concern is the growing resistance of uropathogens to standard agents. Nevertheless, useful recommendations can be made.
The current emphasis on cost-effectiveness and the advent of newer antibiotics have led clinicians to reevaluate the benefit of hospitalization in patients with acute pyelonephritis. Most cases of uncomplicated pyelonephritis in young women can be managed successfully on an outpatient basis. However, outpatient management of acute pyelonephritis requires close follow-up care. The first follow-up visit should occur in 1-2 days, depending on the clinician's estimation of the severity of the infection. Any deterioration or unsatisfactory improvement warrants admission for intravenous antibiotics and evaluation for any complications. (See Treatment.)
Complicated pyelonephritis results from structural and functional abnormalities, urologic manipulations, or underlying disease; it includes pyelonephritis in men and in the elderly. Any patient with complicated pyelonephritis should be referred, if possible, to a physician trained in the management of these cases (eg, a nephrologist, urologist, or infectious disease specialist). The patient will require ongoing management, including repeat cultures, metabolic studies, and appropriate imaging studies.
Pathophysiology
Acute pyelonephritis results from bacterial invasion of the renal parenchyma. Bacteria usually reach the kidney by ascending from the lower urinary tract. [6] In all age groups, episodes of bacteriuria occur commonly, but most are asymptomatic and do not lead to infection. The development of infection is influenced by bacterial factors and host factors. [7]
Bacteria may also reach the kidney via the bloodstream. Hematogenous sources of gram-positive organisms, such as Staphylococcus, are intravenous drug abuse and endocarditis. Experimental evidence suggests that hematogenous spread of gram-negative organisms to the kidney is less likely unless an underlying problem exists, such as an obstruction. Little or no evidence supports lymphatic spread of uropathogens to the kidney.
Most bacterial data are derived from research with Escherichia coli, which accounts for 70-90% of uncomplicated UTIs and 21-54% of complicated UTIs (ie, UTIs that are secondary to anatomic or functional abnormalities that impair urinary tract drainage; are associated with metabolic disorders; or involve unusual pathogens). A subset of E coli, the uropathogenic E coli (UPEC), also termed extraintestinal pathogenic E coli (ExPEC), accounts for most clinical isolates from UTIs.
UPEC derives commonly from the phylogenetic groups B2 and D, which express distinctive O, K, and H antigens. UPEC genes encode several postulated virulence factors (VFs), including adhesins, siderophores, protectins, and toxins, as well as having the metabolic advantage of synthesizing essential substances.
Virulence factors
Adhesins have specific regions that attach to cell receptor epitopes in a lock-and-key fashion. Mannose-sensitive adhesins (usually type 1 fimbriae) are present on essentially all E coli. They contribute to colonization (eg, bladder, gut, mouth, vagina) and possibly pathogenesis of infection; however, they also attach to polymorphonuclear neutrophils (PMNs), leading to bacterial clearance.
Mannose-resistant adhesins permit the bacteria to attach to epithelial cells, thereby resisting the cleansing action of urine flow and bladder emptying. They also allow the bacteria to remain in close proximity to the epithelial cell, enhancing the activity of other VFs.
The P fimbriae family of adhesins is epidemiologically associated with prostatitis, pyelonephritis (70-90% of strains), and sepsis. This family of adhesins is associated with less than 20% of asymptomatic bacteriuria (ABU) strains. The AFA/Dr family is associated with diarrhea, UTI, and particularly pyelonephritis in pregnancy. The S/F1C family is associated with neonatal meningitis and UTI.
Siderophores are involved in iron uptake, an essential element for bacteria, and possibly adhesion. Protectins and their contributions to virulence include the following:
-
Lipopolysaccharide (LPS) coatings: resist phagocytosis
-
Tra T and Iss: resist action of complement
-
Omp T: cleave host defense proteins (eg, immunoglobulins)
Toxins, which affect various host cell functions, include the following:
-
Alpha-hemolysin
-
Cytotoxic necrotizing factor–1
-
Cytolethal distending toxin
-
Secreted autotransporter toxin
No single VF is sufficient or necessary to promote pathogenesis. Apparently, multiple VFs are necessary to ensure pathogenesis, although adhesins play an important role.
Asymptomatic bacteriuria strains
Bacterial strains that produce ABU may in some instances provide a measure of protection against symptomatic infections from UPEC and other organisms. On the other hand, ABU may also cause increased morbidity and mortality. Once bacteriuria is established, these strains appear to stop producing adhesins, allowing them to survive and persist without producing an inflammatory reaction.
Pathogens
As noted above, UPEC account for most uncomplicated pyelonephritis cases and a significant portion of complicated pyelonephritis cases. The following microorganisms are also commonly isolated:
-
Staphylococcus saprophyticus
-
Klebsiella pneumoniae
-
Proteus mirabilis
-
Enterococci
-
S aureus
-
Pseudomonas aeruginosa
-
Enterobacter species
This is the same spectrum of organisms cultured in cystitis. In 10-15% of symptomatic cystitis cases, cultures using routine methods remain negative, although the symptoms typically respond to antibiotic therapy. In some cases, cultures using selective media have grown Gardnerella vaginalis, Mycoplasma hominis, and Ureaplasma urealyticum. These data cannot be extended to acute pyelonephritis, but they do illustrate the difficulties in isolating the causative organism.
Epithelial attachment and inflammatory response
Evidence suggests that the pathogenesis of pyelonephritis takes a 2-step path. First, UPEC attaches to the epithelium and triggers an inflammatory response involving at least 2 receptors, glycosphingolipid (GSL) and Toll-like receptor 4 (TLR4). In the mouse model, GSL is the primary receptor and TLR4 is recruited and is an important receptor for the release of chemokines. When TLR4 is genetically absent, an asymptomatic carrier state develops in the infected mice.
Second, as a result of the inflammatory response, chemokines (eg, interleukin-8 [IL-8], which is chemotactic for PMNs) are released and attach to the neutrophil-activating chemokine receptor 1 (CXCR1), allowing PMNs to cross the epithelial barrier into the urine. In children prone to pyelonephritis, for example, CXCR1 expression has been shown to be significantly lower than in control subjects.
Several other host factors militate against symptomatic UTI. Phagocytosis of bacteria in urine is maximized at pH 6.5-7.5 and osmolality of 485 mOsm; values deviating from these ranges lead to significantly reduced or absent phagocytosis. Other important factors are the flushing action of urine flow in the ureter and bladder, the inhibition of attachment of type 1 fimbriae E coli to uroepithelial cells by tubular cell–secreted Tamm-Horsfall protein, and the inhibition of attachment by some surface mucopolysaccharides on the uroepithelial cells.
Complicated infection
Complicated UTI is an infection of the urinary tract in which the efficacy of antibiotics is reduced because of the presence of one or more of the following:
-
Structural abnormalities of the urinary tract
-
Functional abnormalities of the urinary tract
-
Metabolic abnormalities predisposing to UTIs
-
Unusual pathogens
-
Recent antibiotic use
-
Recent urinary tract instrumentation
For more information on this topic, see Pathophysiology of Complicated Urinary Tract Infections.
Obstruction
Obstruction is the most important factor. It negates the flushing effect of urine flow; allows urine to pool (urinary stasis), providing bacteria a medium in which to multiply; and changes intrarenal blood flow, affecting neutrophil delivery. Obstruction may be extrinsic or intrinsic. Extrinsic obstruction occurs with chronic constipation (particularly in children), prostatic swelling/mass (eg, hypertrophy, infection, cancer), and retroperitoneal mass.
Intrinsic obstruction occurs with bladder outlet obstruction, cystocele, fungus ball, papillary necrosis, stricture, and urinary stones. With increasing size of stone, the probability of stone passage decreases while the probability of obstruction increases. Nonetheless, stones as small as 2 mm have resulted in obstruction, while 8-mm stones have occasionally passed spontaneously.
Infectious stones, urease stones, or triple-phosphate stones composed of magnesium ammonium phosphate or struvite and apatite account for 10-15% of all urinary stones. They develop secondary to the action of urea-splitting organisms and can grow rapidly and branch out (ie, staghorn calculi).
If left untreated, staghorn calculi will destroy the kidney and may cause the death of the patient. Complications include azotemia, hydropyonephrosis, perinephric abscess, pyelonephritis (severe or end-stage), sepsis, and xanthogranulomatous pyelonephritis.
Incomplete bladder emptying may be related to medication (eg, anticholinergics). The spermicide nonoxynol-9 inhibits the growth of lactobacilli. Lactobacilli produce hydrogen peroxide, which protects the vaginal ecosystem against pathogens. Frequent sexual intercourse causes local mechanical trauma to the urethra in both partners.
Atrophic vaginal mucosa in postmenopausal women predisposes to the colonization of urinary tract pathogens and UTIs because of the higher pH (5.5 vs 3.8) and the absence of lactobacilli. Bacterial prostatitis (acute or chronic) produces bacteriuria, whereas nonbacterial prostatitis and pelviperineal pain syndrome (prostadynia) do not.
Unusual organisms include Mycoplasma, Pseudomonas, and urea-splitting organisms. Pseudomonas aeruginosa has several mechanisms that promote adherence, including alginate, other membrane proteins, pili, and surface-associated exoenzyme S.
Urea-splitting organisms produce urease, which hydrolyzes urea, yielding ammonia, bicarbonate, and carbonate; this leads to a more alkaline urine and allows crystal formation (staghorn calculus) from the supersaturation of carbonate apatite and struvite. Staghorn calculi continue to grow in size, leading to infection, obstruction, or both.
Complications of obstruction with superimposed infection include hydronephrosis, pyonephrosis, urosepsis, and xanthogranulomatous pyelonephritis. Additionally, the pathogens can sequester in the struvite stones, protected from the host’s immune system. Proteus species are the most common urea-splitting organisms. E coli, Klebsiella, Pseudomonas, and Staphylococcus can also produce urease, however, and are sometimes involved in staghorn calculus formation.
Pregnancy
Pregnancy produces hormonal and mechanical changes that predispose the woman to upper urinary traction infections. Hydroureter of pregnancy, secondary to both hormonal and mechanical factors, manifests as dilatation of the renal pelvis and ureters (greater on the left than on the right), with the ureters containing up to 200 mL of urine. Progesterone decreases ureteral peristalsis and increases bladder capacity. The enlarging uterus displaces the bladder, contributing to urinary stasis.
Diabetes
Diabetes mellitus produces autonomic bladder neuropathy, glucosuria, leukocyte dysfunction, microangiopathy, and nephrosclerosis; additionally, it leads to recurrent bladder instrumentation secondary to the neuropathy. Complicated UTIs in patients who have diabetes mellitus include the following:
-
Renal and perirenal abscess
-
Emphysematous cystitis
-
Fungal infections
-
Papillary necrosis
Etiology
Uropathogens that cause the majority of acute pyelonephritis cases and the frequency with which they are cultured in uncomplicated versus complicated cases, are listed in Table 1. Unusual pathogens include mycobacteria, yeasts and fungi, and opportunistic pathogens such as Corynebacterium urealyticum.
Table 1. Bacterial Etiology of Urinary Tract Infections (Open Table in a new window)
Bacteria |
% Uncomplicated |
% Complicated |
Gram negative |
|
|
Escherichia coli |
70-95 |
21-54 |
Proteus mirabilis |
1-2 |
1-10 |
Klebsiella spp |
1-2 |
2-17 |
Citrobacter spp |
< 1 |
5 |
Enterobacter spp |
< 1 |
2-10 |
Pseudomonas aeruginosa |
< 1 |
2-19 |
Other |
< 1 |
6-20 |
Gram positive |
|
|
Coagulase-negative staphylococci |
5-10* |
1-4 |
Enterococci |
1-2 |
1-23 |
Group B streptococci |
< 1 |
1-4 |
Staphylococcus aureus |
< 1 |
1-23 |
Other |
< 1 |
2 |
Adapted from Hooton TM. The current management strategies for community-acquired urinary tract infection. Infect Dis Clin North Am. Jun 2003;17(2):303-32. [QxMD MEDLINE Link]. * S saprophyticus |
||
Epidemiology
Epidemiologic data on the incidence of pyelonephritis are limited. A population-based study of acute pyelonephritis in the United States found overall annual rates of 15-17 cases per 10,000 females and 3-4 cases per 10,000 males. [5] In women aged 18−49 years, the estimated incidence is 28 cases per 10,000. [8] At least 250,000 cases of pyelonephritis are diagnosed annually in the United States. The cost of treating acute pyelonephritis has been estimated to be $2.14 billion per year. [5]
Acute pyelonephritis develops in 20-30% of pregnant women with untreated asymptomatic bacteriuria (ABU) (2-9.5%), most often during the late second and early third trimesters. The incidence of pyelonephritis in infants and children is difficult to ascertain because of the infrequency of typical symptoms. Up to 25% of children with UTI and no signs or symptoms of pyelonephritis do have bacteria demonstrable in the upper urinary tract.
No racial predilection of pyelonephritis has been demonstrated. Pyelonephritis is significantly more common in females than in males, although this difference narrows considerably with increasing age, especially in patients aged 65 years and older. In females, pyelonephritis shows a trimodal distribution, with an elevated incidence in girls aged 0-4 years, a peak in women 15-35 years of age, and a gradual increase after age 50 years to another peak at 80 years of age. [5]
In males, the age distribution of pyelonephritis is bimodal. Males also demonstrate a peak incidence of pyelonephritis at 0-4 years of age. Rates gradually increase after 35 years of age and peak at 85 years of age. [5]
Acute pyelonephritis shows a seasonal variation. In Washington state, cases occurred most frequently during the months of July and August among females and during August and September in the male population. [5]
Prognosis
Timely diagnosis and management of acute pyelonephritis has a significant impact on the outcome. Any patient with acute pyelonephritis who deteriorates suddenly or does not respond to conventional therapy may have a complication, resistant organism, or unrecognized comorbidity.
Pyelonephritis causes considerable morbidity. Data can be extrapolated from the morbidity data for acute lower urinary tract infections: specifically, acute cystitis in women produces approximately 6.1 days with symptoms, 2.4 days of restricted activity, 1.2 days that the patient is unable to work or attend class, and 0.4 days bedridden. In women aged 18−49 years, 7% of pyelonephritis cases require hospital admission. [8]
For patients with pyelonephritis who have an organ-threatening infection, the follow-up examination is important to be sure that the patient is progressing satisfactorily and that recovery is complete. Failure to diagnose these complications in a timely fashion could predispose the patient to a poor outcome. Pregnant patients with pyelonephritis are at significant risk for premature labor.
Kofteridis et al found that acute pyelonephritis is linked to bacteremia, longer hospital stays, and higher mortality in patients with diabetes mellitus. [9] In a retrospective study of 206 elderly patients, 88 of whom had diabetes, bacteremia occurred in 30.7% of patients with diabetes but in only 11% of the control patients. Moreover, compared with the control patients, patients with diabetes had longer-lasting fevers (median, 4.5 vs 2.5 days), longer hospital stays (median, 10 vs 7 days), and higher mortality (12.5% vs 2.5%).
Mortality is higher in patients older than 65 years; it is also higher with septic shock, bedridden status, and immunosuppression. In men, mortality is also increased with use of antibiotics during the previous month. Morbidity (prolonged hospital stay) in both men and women is increased with a change in initial treatment, diabetes mellitus, and long-term indwelling catheter.
Uncomplicated pyelonephritis
In healthy, nonpregnant women with uncomplicated disease, the prognosis is excellent for full recovery and minimal damage to the kidney. In healthy men without any known complicating conditions, the prognosis is good for full recovery; however, urologic evaluation is recommended to rule out an underlying complicating condition. In infants and children, the prognosis is good. Importantly, children should undergo a urologic evaluation after the first episode to rule out structural abnormalities.
Uncomplicated pyelonephritis is not a fatal disease in the antibiotic era. Pyelonephritis becomes a potentially fatal disease when secondary conditions develop, such as emphysematous pyelonephritis (20-80% mortality rate), perinephric abscess (20-50% mortality rate), or one of the sepsis syndromes (>25% overall mortality rate).
Emphysematous pyelonephritis
In emphysematous pyelonephritis, the mortality rate is 60% in cases in which the gas is localized to the renal parenchyma, regardless of treatment. The mortality is 80% if the gas has spread in the perinephric space and the patient is treated with antibiotics alone. In emphysematous pyelitis, the mortality rate is 20%.
Sepsis
The genitourinary system is the source of severe sepsis in 9.1% of all cases annually. The mortality for these GU-related cases is 16.1%. Overall mortality from severe sepsis significantly increases with chronic kidney disease (36.7%), acute renal dysfunction (38.2%), and age older than 64 years (25-42% with progressively increasing age to older than 85 years). In the age range of 0-4 years, the mortality is 5%; for ages 5-50 years, it is less than 3%.
Acute kidney injury
Rarely, acute pyelonephritis can cause acute kidney injury (AKI) in children, healthy adults, and pregnant women. When this occurs, characteristically, recovery proceeds more slowly than with AKI from other causes. In most instances, other factors are thought to contribute to the AKI (eg, medications, hypovolemia, obstruction, sepsis). In a retrospective review of 403 adult patients hospitalized for acute pyelonephritis, multivariate analysis showed that age over 65, complicated or bilateral pyelonephritis, and initial shock were independent risk factors for the occurrence of AKI. [10]
A Danish population-based cohort study of 8760 patients found that preadmission impaired kidney function is a strong risk factor for development of AKI in patients with pyelonephritis. The 30-day risk of AKI rose steadily from 16% in patients with a preadmission estimated glomerular filtration ration (eGFR) ≥90 mL/min/1.73 m2 to 47% for patients with preadmission eGFR of < 30. [11]
Kidney scarring
In children, kidney scarring can be detected in 6-15% after a febrile UTI. Of these patients, almost all males and some females have demonstrable kidney scarring and a globally small kidney with smooth renal outlines in infancy, usually associated with vesicoureteral reflux (VUR) and thought to be congenital. Most female infants do not have demonstrable scarring on initial imaging, but they subsequently develop it. Delay in treatment of cystitis or pyelonephritis, recurrent UTIs, urinary obstruction, and VUR increase the risk of scarring. In children, normal ultrasonogram findings alone cannot reliably rule out the presence of either reflux or kidney scarring. [12]
Acute pyelonephritis (single episode; first UTI ever in one half of cases) in an adult woman leads to kidney scarring in 46%, as demonstrated by scintigraphic scanning 10 years later. Subsequent UTIs do not appear to affect the risk of future scarring.
Kidney scarring has been demonstrated to be 4 times more likely after pyelonephritis in pregnant women than in nonpregnant women. In addition, acute pyelonephritis during pregnancy is associated with the following complications:
-
Acute kidney dysfunction (elevated creatinine) in 2% of cases (20-25% in the past)
-
Acute kidney injury in 0.03% of cases
-
Acute respiratory distress syndrome (bilateral chest radiograph infiltrates and hypoxemia without pulmonary hypertension) in 1-8% of cases
-
Low birth weight (< 2500 g) in 7% of cases
-
Preterm delivery (< 37 wk gestation) in 5% of cases (6-50% in the past)
-
Recurrence prior to delivery in 18-20% of cases
-
Sepsis (positive blood cultures) in 17% of cases
Kidney transplant pyelonephritis
Acute kidney transplant pyelonephritis occurring in the first 3 months after transplant has a significant association with graft loss (>40%) by 96 months, as compared with all kidney transplant cases with or without the occurrence of pyelonephritis at any time after the transplant up to 96 months (25-30%).
Patient Education
Patients must take antibiotics as directed and complete the course as prescribed. This minimizes the risk of recurrence and the development of resistant organisms.
Patients should be advised to drink plenty of fluids, as avoidance of dehydration is important for both patient well-being and kidney function. When under stress, men typically drink only enough liquid to replace two thirds of the loss. When ill, individuals drink less and predispose themselves to dehydration. Unavoidable daily water loss is 1.5 L, of which approximately 500 mL is replaced by the oxidation of carbohydrates. Because patients cannot measure urine specific gravity at home, they should drink enough water or other liquid to produce light-colored urine, almost like water.
For patient education information, see Kidney Infection.
-
Nonobstructing distal left ureteral calculus 2 X 1 X 2 cm.
-
Multiple abscesses, upper pole of left kidney.
-
Bilateral hydronephrosis.