Background
Definitions
By definition, an aneurysm is a localized or diffuse dilation of an artery with a diameter at least 50% greater than the normal size of the artery. Aneurysmal degeneration can occur anywhere in the human aorta; most aortic aneurysms (AAs) occur in the abdominal aorta and thus are termed abdominal aortic aneurysms (AAAs). Although most AAAs are asymptomatic at the time of diagnosis, the most common complication remains life-threatening rupture with hemorrhage.
Aneurysmal degeneration that occurs in the thoracic aorta is termed a thoracic aortic aneurysm (TAA). Aneurysms that coexist in both segments of the aorta (thoracic and abdominal) are termed thoracoabdominal aneurysms (TAAAs). TAAs and TAAAs are also at risk for rupture. One population-based study suggests an increasing prevalence of TAAs.
TAAs are subdivided into the following three groups depending on location:
-
Ascending aortic aneurysms
-
Aortic arch aneurysms
-
Descending thoracic aneurysms or thoracoabdominal aneurysms
Aneurysms that involve the ascending aorta may extend as far proximally as the aortic annulus and as far distally as the innominate artery, whereas descending thoracic aneurysms begin beyond the left subclavian artery. Arch aneurysms are as the name implies.
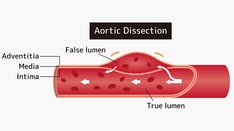
Dissection is another condition that may affect the thoracic aorta. An intimal tear causes separation of the walls of the aorta. A false passage for blood develops between the layers of the aorta. This false lumen may extend into branches of the aorta in the chest or abdomen, causing malperfusion, ischemia, or occlusion with resultant complications. The dissection can also progress proximally, to involve the aortic sinus, aortic valve, and coronary arteries.
Dissection can lead to aneurysmal change and early or late rupture. A chronic dissection is one that is diagnosed more than 2 weeks after the onset of symptoms. Dissection should not be termed dissecting aneurysm, because it can occur with or without aneurysmal enlargement of the aorta.
The shape of an aortic aneurysm is either saccular or fusiform. A fusiform (or true) aneurysm has a uniform shape with a symmetrical dilatation that involves the entire circumference of the aortic wall. A saccular aneurysm is a localized outpouching of the aortic wall, and it is the shape of a pseudoaneurysm.
For patient education resources, see Aortic Aneurysm.
Treatment
Treatment of AAAs, TAAAs, and TAAs involves surgical repair in good-risk patients with aneurysms that have reached a size sufficient to warrant repair. Surgical repair may involve endovascular stent grafting (in suitable candidates) [1] or traditional open surgical repair. [2]
The development of treatment modalities for TAAs followed successful treatment of AAAs. Estes' 1950 report revealed that the 3-year survival rate for patients with untreated AAAs was only 50%, with two thirds of deaths resulting from aneurysmal rupture. [3] From that tiem forward, increased attempts were made to devise methods of durable repair.
Most of these initial successful repairs involved the use of preserved aortic allografts, thus triggering the establishment of numerous aortic allograft banks. Simultaneously, Gross et al successfully used allografts to treat complex thoracic aortic coarctations, including those with aneurysmal involvement. [4]
In 1951, Lam and Aram reported the resection of a descending thoracic aneurysm with allograft replacement. [5] Ascending aortic replacement required the development of cardiopulmonary bypass and was first performed in 1956 by Cooley and DeBakey. [6] They successfully replaced the ascending aorta with an aortic allograft. Successful replacement of the aortic arch, with its inherent risk of cerebral ischemia, was understandably more challenging and was not reported until 1957 by DeBakey et al. [7]
Although the use of aortic allografts as aortic replacement was widely accepted in the early 1950s, the search for synthetic substitutes was well under way. Dacron was introduced by DeBakey. By 1955, Deterling and Bhonslay believed that Dacron was the best material for aortic substitution. [8] Numerous types of intricately woven hemostatic grafts have since been developed and are now used much more extensively than their allograft counterparts. Such Dacron grafts are used to replace ascending, arch, thoracic, and thoracoabdominal aortic segments.
However, some patients required replacement of the aortic root, as well. Subsequently, combined operations that replaced the ascending aneurysm in conjunction with replacement of the aortic valve and reimplantation of the coronary arteries were performed by Bentall and De Bono in 1968, using a mechanical valve with a Dacron conduit. [9] Ross, in 1962, and Barratt-Boyes, in 1964, successfully implanted the aortic homograft in the orthotopic position. [10, 11] In 1985, Sievers reported the use of stentless porcine aortic roots. [12]
Subsequently, less invasive therapies for descending TAA were developed. Dake et al reported the first endovascular thoracic aortic repair in 1994. [13] In March 2005, the US Food and Drug Administration (FDA) approved the first thoracic aortic stent graft, the GORE TAG graft (W.L. Gore and Associates; Flagstaff, AZ). Since 2005, two other devices have gained FDA approval: the Talent Thoracic endograft (Medtronic; Santa Rosa, CA) and the Cook TX2 endograft (Cook; Bloomington, IN). Several successive next-generation reiterations of all of these devices have also gained approval.
Given the relative acceptance of the indications for thoracic endografts as an alternative to open procedures in the treatment of uncomplicated diseases of the descending thoracic aorta, experienced users of the devices began to use them "off label" in increasingly more complex indications, including use via "hybrid procedures" in the ascending aorta and aortic arch. However, little long-term data are available at this time to support use in this fashion.
Anatomy
A blood vessel has the following three layers:
-
Intima (inner layer made of endothelial cells)
-
Media (containing muscular elastic fibers)
-
Adventitia (outer connective tissue)
Aneurysms are either true or false. The wall of a true aneurysm involves all three layers, and the aneurysm is contained inside the endothelium. The wall of a false or pseudoaneurysm only involves the outer layer and is contained by the adventitia. An aortic dissection is formed by an intimal tear and is contained by the media; hence, it has a true lumen and a false lumen.
Ascending aortic aneurysms occur as proximally as the aortic annulus and as distally as the innominate artery. They may compress or erode into the sternum and ribs, causing pain or fistula. They also may compress the superior vena cava or airway. When symptomatic by rupture or dissection, they may involve the pericardium, aortic valve, or coronary arteries. They may rupture into the pericardium, causing tamponade. They may dissect into the aortic valve, causing aortic insufficiency, or into the coronary arteries, causing myocardial infarction.
Aortic arch aneurysms involve the aorta where the innominate artery, left carotid, and left subclavian originate. They may compress the innominate vein or airway. They may stretch the left recurrent laryngeal nerve, causing hoarseness.
Descending thoracic aneurysms originate beyond the left subclavian artery and may extend into the abdomen. Thoracoabdominal aneurysms are stratified according to the Crawford classification, as follows:
-
Type I involves the descending thoracic aorta from the left subclavian artery down to the abdominal aorta above the renal arteries
-
Type II extends from the left subclavian artery to the renal arteries and may continue distally to the aortic bifurcation
-
Type III begins at the mid-to-distal descending thoracic aorta and involves most of the abdominal aorta as far distal as the aortic bifurcation
-
Type IV extends from the upper abdominal aorta and all or none of the infrarenal aorta
Descending thoracic aneurysms and thoracoabdominal aneurysms may compress or erode into surrounding structures, including the trachea, bronchus, esophagus, vertebral body, and spinal column.
Pathophysiology
Aneurysms are usually defined as a localized dilation of an arterial segment greater that 50% its normal diameter. Most aortic aneurysms occur in the infrarenal segment (95%). The average size for an infrarenal aorta is 2 cm; therefore, AAAs are usually defined by diameters greater than 3 cm. The normal size for the thoracic and thoracoabdominal aorta is larger than that of the infrarenal aorta, and aneurysmal degeneration in these areas is defined accordingly. The average diameter of the mid-descending thoracic aorta is 26-28 mm, compared with 20-23 mm at the level of the celiac axis.
The occurrence and expansion of an aneurysm in a given segment of the arterial tree probably involves local hemodynamic factors and factors intrinsic to the arterial segment itself.
The medial layer of the aorta is responsible for much of its tensile strength and elasticity. Multiple structural proteins make up the normal medial layer of the human aorta. Of these, collagen and elastin are probably the most important. The elastin content of the ascending aorta is high and diminishes progressively in the descending thoracic and abdominal aorta. The infrarenal aorta has a relative paucity of elastin fibers in relation to collagen and compared with the thoracic aorta, possibly accounting for the increased frequency of aneurysms in this area.
In addition, the activity and amount of specific enzymes is increased, which leads to the degradation of these structural proteins. Elastic fiber fragmentation and loss with degeneration of the media result in weakening of the aortic wall, loss of elasticity, and consequent dilation.
Hemodynamic factors probably play a role in the formation of aortic aneurysms. The human aorta is a relatively low-resistance circuit for circulating blood. The lower extremities have higher arterial resistance, and the repeated trauma of a reflected arterial wave on the distal aorta may injure a weakened aortic wall and contribute to aneurysmal degeneration. Systemic hypertension compounds the injury, accelerates the expansion of known aneurysms, and may contribute to their formation.
Hemodynamically, the coupling of aneurysmal dilation and increased wall stress is defined by the law of Laplace. Specifically, the law of Laplace states that the (arterial) wall tension is proportional to the pressure times the radius of the arterial conduit (T = P × R). As diameter increases, wall tension increases, which contributes to increasing diameter. As tension increases, risk of rupture increases. Increased pressure (systemic hypertension) and increased aneurysm size aggravate wall tension and therefore increase the risk of rupture.
Aneurysm formation is probably the result of multiple factors affecting that arterial segment and its local environment.
Etiology
Aneurysmal degeneration occurs more commonly in the aging population. Aging results in changes in collagen and elastin, which lead to weakening of the aortic wall and aneurysmal dilation. According to the law of Laplace, luminal dilation results in increased wall tension and the vicious cycle of progressive dilation and greater wall stress. Pathologic sequelae of the aging aorta include elastic fiber fragmentation and cystic medial necrosis. Arteriosclerotic (degenerative) disease is the most common cause of thoracic aneurysms.
A previous aortic dissection with a persistent false channel may produce aneurysmal dilation; such aneurysms are the second most common type. False aneurysms are more common in the descending aorta and arise from the extravasation of blood into a tenuous pocket contained by the aortic adventitia. Because of increasing wall stress, false aneurysms tend to enlarge over time.
Authorities strongly agree that genetics play a role in the formation of aortic aneurysms. [14] Of first-degree relatives of patients with aortic aneurysms, 15% have an aneurysm. This appears especially true in first-degree relatives of female patients with aortic aneurysms. Thus, inherited disorders of connective tissue appear to contribute to the formation of aortic aneurysms.
Marfan syndrome is a potentially lethal connective-tissue disease characterized by skeletal, heart valve, and ocular abnormalities. Individuals with this disease are at risk for aneurysmal degeneration, especially in the thoracic aorta. Marfan syndrome is an autosomal dominant genetic condition that results in abnormal fibrillin, a structural protein found in the human aorta. Patients with Marfan syndrome may develop annuloaortic ectasia of the sinuses of Valsalva, commonly associated with aortic valvular insufficiency and aneurysmal dilation of the ascending aorta.
Type IV Ehlers-Danlos syndrome results in a deficiency in the production of type III collagen, and individuals with this disease may develop aneurysms in any portion of the aorta. Imbalances in the synthesis and degradation of structural proteins of the aorta have also been discovered, which may be inherited or spontaneous mutations.
Atherosclerosis may play a role. Whether atherosclerosis contributes to the formation of an aneurysm or whether they occur concomitantly is not established. Other causes of aortic aneurysms are infection (ie, bacterial [mycotic or syphilitic]), arteritis (ie, giant cell, Takayasu, Kawasaki, Behçet), and trauma. Aortitis due to granulomatous disease is rare, but it can lead to the formation of aortic and, on occasion, pulmonary artery aneurysms. Aortitis caused by syphilis may cause destruction of the aortic media followed by aneurysmal dilation.
Traumatic dissection is a result of shearing from deceleration injury due to a high-speed motor vehicle accident (MVA) or a fall from heights. The dissection occurs at a point of fixation, usually at the aortic isthmus (ie, at the ligamentum arteriosum, distal to the origin of the left subclavian artery), the ascending aorta, the aortic root, and the diaphragmatic hiatus.
The true etiology of aortic aneurysms is probably multifactorial, and the condition occurs in individuals with multiple risk factors. Risk factors include smoking, chronic obstructive pulmonary disease (COPD), hypertension, atherosclerosis, male sex, older age, high body mass index (BMI), bicuspid or unicuspid aortic valves, genetic disorders, and family history.
In late 2018, the FDA issued a warning that fluoroquinolone use can increase the risk of AA and urged healthcare providers to avoid prescribing these antibiotics to patients with or at risk for an AA, such as those with peripheral atherosclerotic vascular disease, hypertension, or certain genetic conditions (eg, Marfan syndrome and Ehlers-Danlos syndrome), as well as the elderly. [15]
Epidemiology
Although findings from autopsy series vary widely, the prevalence of aortic aneurysms probably exceeds 3-4% in individuals older than 65 years. Aortic aneurysms are more common in men than in women and are more common in persons with COPD than in those without lung disease.
The incidence of TAAs has been estimated to be about 6 cases per 100,000 person-years. In a systematic review and meta-analysis of the incidence and prevalence of TAAs in 22 population-based studies, Melo et al found a pooled incidence of 5.3 per 100,000 individuals per year and a prevalence of 0.16%. [16]
Death from aneurysmal rupture is one of the 15 leading causes of death in most series. In addition, the overall prevalence of aortic aneurysms has increased significantly in the past 30 years. This is partly due to an increase in diagnosis based on the widespread use of imaging techniques. However, the prevalence of fatal and nonfatal rupture has also increased, suggesting a true increase in prevalence.
Population-based studies have suggested an incidence of acute aortic dissection of 3.5 per 100,000 persons; an incidence of thoracic aortic rupture of 3.5 per 100,000 persons; and an incidence of abdominal aortic rupture of 9 per 100,000 persons. An aging population probably plays a significant role.
Prognosis
According to Culliford et al from 1982, [17] Cabrol et al from 1988, [18] and Donaldson and Ross from 1982, [19] the early hospital mortality following repair of ascending aneurysms is 4-10%. Contemporary surgical series demonstrated a continued wide range in operative mortality (2-17%). Stroke occurs in 2-5% of patients.
As would be expected, the early mortality after repair of arch aneurysms is considerably higher, approaching 25% in series by Crawford and Saleh from 1981, [20] by Crawford et al from 1979, [21] by Columbi et al from 1983, [22] by Ergin et al from 1982, [23] and by Galloway et al from 1989. [24] More contemporary results from Coselli and Ueda demonstrate an operative mortality of 6-12%. Stroke rate varied from 3-22%. Renal failure that necessitated dialysis occurred in 7% of patients.
The mortality after repair of descending thoracic aneurysms is lower, approximately 5-15% according to Crawford et al from 1981, [20] to Donahoo et al from 1977, [25] to Livesay et al from 1985, [26] and to Pressler and McNamara from 1985. [4] Contemporary results are unchanged, with 12-15% mortality.
As a group, including all repairs, according to Crawford et al from 1978, [27] Crawford et al from 1981, [20] and Kitamura et al from 1983, [28] survival rates after surgery for chronic aortic aneurysms are approximately 60% at 5 years and 30-40% at 10 years.
The longest follow-up data for a multicenter trial comparing endovascular and open techniques for management of TAAs are the results of a phase II trial for the GORE-TAG thoracic endovascular stent. A 1.5% 30-day mortality for endovascular repairs was demonstrated, temporary or permanent spinal cord paraplegia occurred in 3% of patients and stroke in 4% of patients. [29] At 2 years, aneurysm survival was 97% and overall survival 75%. [29] For the Medtronic Talent device, the incidence of paraplegia in the stent group was 0-9%; stroke, 3.7-8.1%; 30-day mortality, 2.9-9.7%; and procedural success, more than 95%. [30]
When endovascular stent grafting was compared with open surgery for the GORE-TAG device, the rate of paraplegia was 3% in the stent group vs 14% in the open group [31] ; operative mortality was 1% vs 6%, and early death was 2% vs 10%. [32] The patients in the stent group had shorter ICU and hospital stays, a quicker recovery time, and a lower incidence of major adverse events (except for vascular complications). Complications at 2 years included 4% proximal stent migration, 6% migration of the graft components, and 15% of patients had an endoleak.
Overall, survival rates were equivalent between the endovascular and open groups at both 2-years and 5-years, 80% and 70% respectively, but aneurysm-related survival significantly favored endovascular repairs at 5 years (97% vs 88%). [33] However, more contemporary "real world" experienced application has not been as supportive of this discrepancy, as noted by Greenberg et al, who discerned no significant differences in mortality or paraplegia in their population at 30 days (5.7 vs 8.3%) or at 1 year (15.6% vs 15.9%). [34]
Midterm results comparing open descending thoracic aneurysm repair with endovascular stent grafting demonstrate less early operative mortality with endovascular repair (10% for stent grafting vs 15% for open repair) but similar late survival (actuarial survival rate at 48 months of 54% for stent grafting vs 64% for open repair).
Success with the results of endovascular repair of contained, degenerative TAAs of the descending aorta have created an environment to use endografts for treatment of arch aneurysms as well as acute catastrophes of both the arch and descending aortas.
Data from a multicenter, nonrandomized, prospective study of the use of endografts in emergency pathologies of the descending aorta were published by Cambria et al. [35] In situations that have reported mortality as high as 90%, the authors found that in the management of acute type B dissections, traumatic aortic tears, or ruptured aortic aneurysms, endovascular management compared to open resulted in a 14% vs 30% 30-day composite mortality/paraplegia rate.
Although freedom from aortic-related events was 84.5% at 1 year for the endovascular cohort, survival was only 66% with the subset of ruptured aneurysms have the worst survival (37%). Another multicenter trial evaluating use in ruptured aneurysms confirmed the perioperative mortality but also noted considerable neurologic complications (8%), procedure-related complications such as endoleak (18%), and ongoing aneurysm-related death (25% at 4 years). [36]
Others have used endografts for arch pathologies, which usually necessitates a "hybrid" approach, a combination of endovascular and open techniques. Small (N < 30) single-institution series with limited follow-up have reported perioperative mortality, stroke, and paraplegia rates of 0-25%, 0-25%, and 0-4%, respectively, questioning the durability and futility of the repairs. [37, 38] However, a series from a single tertiary care medical center highlighted the results of 400 consecutive patients, demonstrating a 6.5% and 53% 30-day and 4-year mortality, respectively, and a paraplegia and stroke rate of 4.5% and 3%, respectively. [39]
-
Chest radiograph showing widening of superior mediastinum.
-
Computed tomography (CT) scan depicting descending thoracic aortic aneurysm with mural thrombus at level of left atrium.
-
Ascending aortogram showing ascending aortic aneurysm. Patient also underwent computed tomography (CT).