Practice Essentials
Testicular torsion is defined as a twisting of the spermatic cord structures (see the image below), followed by venous congestion, loss of arterial inflow, and subsequent ischemia of the ipsilateral testis. This is considered a urologic emergency; it necessitates expeditious diagnosis and treatment to preserve testicular vitality. The testicular salvage rate decreases significantly if treatment is delayed by more than six hours from symptom onset. [1]
The acute scrotum includes a variety of disease processes and can present in any age group. Testicular torsion has a bimodal age distribution, primarily affecting neonates and adolescents. However, it has been noted in adult males and should therefore be included in the differential workup of testicular pain, regardless of patient age. [1]
Diagnosis of torsion is clinical, and when there is a high level of suspicion, no adjunctive test should preclude definitive treatment. If the diagnosis is in question, testicular ultrasound can serve as a valuable and often prompt diagnostic tool. Various scoring methods have been used to clinically identify patients with high probability for torsion (see Workup).
Prompt surgical treatment is crucial to preventing ischemic injury to the testis and possible testicular loss. Manual detorsion may be attempted during the initial examination, but even if manual detorsion is successful, surgical exploration and testicular fixation should be performed prior to the patient being discharged from the hospital.
Testicular torsion remains a frequent cause for malpractice litigation. Most cases being litigated had an atypical presentation; the most common misdiagnosis was epididymitis, and most patients were noted to be older (mean patient age, 24.3 years). [2]
For patient education information, see the Testicular Torsion.
For additional information, see Testicular Torsion in Emergency Medicine and Pediatric Testicular Torsion .
Background
Historical perspective
Torsion was first described in 1810 by Hunter, the first case report was in 1840 by Delasiauve, [3] and the first recorded detorsion and fixation was by Curling in 1857. [4] Several institutions have formulated a streamlined and standardized approach to assessment of the acute scrotum. [5]
Orchiopexy, the surgical procedure in which the testis is anchored to the scrotal wall, was initially developed as treatment for cryptorchidism, with the first successful orchiopexy performed in the 1870s by Annandale. Subsequently, the main technical improvements have been in how the testis is anchored to the scrotal wall. Anchoring mechanisms have ranged from using an external cage to fastening the testis to the fascia lata of the thigh or the contralateral testis for lengthening of the spermatic cord. The current method of attaching the testis to the scrotal pouch was initially described the 1930s. [6]
Anatomy
The testis is a paired organ within the scrotum that functions as a male reproductive and endocrine organ. It is an ovoid, longitudinally oriented structure that is on average 15 to 25 mL in volume. The testis parenchyma is surrounded by a three-layer capsule that from external to internal consists of the tunica vaginalis, tunica albuginea, and tunica vasculosa.
The epididymis is located along the posterolateral surface of the testis and is encapsulated by the tunica vaginalis. It functions as an organ of transport, storage, and maturation for sperm, and receives vascular supply in conjunction with the testis. In normal anatomy, the posterolateral attachments are a point of fixation that prevents the testis from twisting.
The spermatic cord follows the route of testicular abdominal descent and contains the ductus deferens and associated vasculature, testicular artery, pampiniform plexus, and genital branch of the genitofemoral nerve. It is a tubular structure that is comprised of layers of spermatic fascia, which are extensions of the abdominal wall. The external oblique forms the external spermatic fascia, which attaches to border the external inguinal ring. The internal oblique forms the cremasteric muscles and fascia and attaches to the inguinal ligament, iliopsoas, and pubic tubercle. The transversalis fascia forms the internal spermatic fascia. [7]
Testicular development commences in the gonadal ridge located near the fetal mesonephric kidney. Testicular descent into the scrotum is essential for normal sperm maturation and function.
Testicular descent can be broken down into two separate phases. The transabdominal phase, occurring at 8-15 weeks' gestation, involves swelling of the gubernaculum (stimulated by insulin-like hormone 3) and regression of the cranial suspensory ligament (stimulated by androgens). The inguinoscrotal phase, occurring between 25 and 35 weeks' gestation, involves migration of the gubernaculum into the scrotum, formation of the processus vaginalis, and finally descent of the testis into the scrotum. The role of the gubernaculum has been postulated to be dilation of the inguinal canal to facilitate transinguinal testicular descent. This process is regulated by calcitonin gene-related peptide (CGRP), produced by the genitofemoral nerve in response to androgen stimulation. [8]
Prenatal vascular injury has been postulated to account for so-called "vanishing" testis. This term describes a situation in which an infant presents with a solitary hypertrophic testis, and blind-ending contralateral spermatic vessels are noted on laparoscopic evaluation. This condition is considered to occur in the third trimester, given the location of the blind-ending vessels and testis nubbins that are found postnatally. When vanished testis is noted on later evaluation for undescended testis, contralateral fixation is not indicated. [9]
Pathophysiology
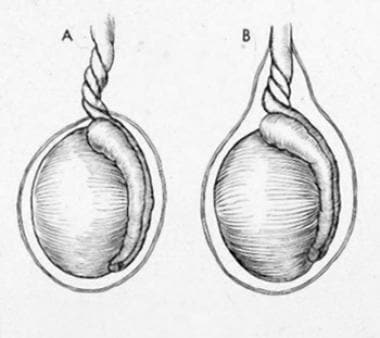
Testicular torsion can be distinguished as two separate mechanisms, extravaginal and intravaginal, depending on whether the torsion includes the tunica vaginalis or occurs within the tunica vaginalis. Extravaginal torsion is also known as perinatal testicular torsion, as it occurs exclusively within this stage of life. In extravaginal torsion, the entire cord including the tunica vaginalis becomes twisted, as it has not yet become fixed within the scrotum. [5] Intravaginal torsion occurs when there is an abnormally high fixation of the tunica vaginalis to the spermatic cord (bell clapper deformity), allowing the spermatic cord and testis to twist within the tunica vaginalis.
The significant consequence of torsion of the spermatic cord is testicular ischemia. The magnitude of ischemia is determined by both the degree of rotation and the duration of the torsion.
Depending on the severity of ischemia, the morphologic changes range from vascular congestion to hemorrhagic necrosis of the testis. Animal studies have demonstrated a loss of all spermatogenic and Sertoli cells after six hours, and loss of Leydig cells after 10 hours of complete absence of vascular flow. Clinically, most patients will experience testicular atrophy after 10 hours of torsion greater than 360 degrees. After the torsion has been reduced, reperfusion injury can further damage cellular DNA via reactive oxygen species causing lipid peroxidation, which leads to cellular and mitochondrial membrane disruption. [10]
Under normal physiologic conditions, the testis is isolated by a blood-testis barrier. When this barrier is disrupted, the immune system can develop antibodies against sperm antigens. Agglutinating antisperm antibodies are found in 20% of patients after torsion. However, no correlation with infertility has been demonstrated. A proposed hypothesis for post-torsion reduction of contralateral testicular vitality is that torsion may result in reflex vasoconstriction involving the contralateral testis, which may contribute to contralateral testicular injury. [1] Retention of the torsed testis in the body for a prolonged time can lead to reduction in spermatogonia and spermatids in the contralateral testis. [11]
Etiology
The exact precipitating event for spermatic cord torsion is unknown. The only consistently reported anatomic factor noted has been the lack of appropriate testicular fixation: the bell clapper deformity in intravaginal torsion, or the lack of fixation of the tunica in extravaginal torsion. Understanding of how the bell clapper deformity forms remains speculative. Suggestions have included a congenital anomaly of formation of the embryonic scrotum, spermatic cord, and testicle.
Precipitating factors for the development of torsion include exercise and injury. Cold temperatures have been hypothesized to trigger torsion by causing asymmetric contraction of the cremaster muscle in susceptible individuals; however, a review of 2413 pediatric cases in the United States found that episodes of testicular torsion were evenly distributed across all seasons, even when analysis was limited to states with cold winters. [12]
Cryptorchid testes are at increased risk of torsion and are difficult to assess due to their extrascrotal location. [5] These patients typically present with a painful inguinal mass and an empty ipsilateral scrotum. In one series of 22 patients with cryptorchid testicular torsion, the testicular salvage rate was significantly lower in prepubertal patients than in postpubertal ones (16.7% versus 60.0%, respectively). [13]
There may be a familial predisposition to torsion, but the genetic transmission is unknown. [5] Evaluation of a familial basis for testis torsion has also been evaluated in animal models. Although candidate genes INSL3 and RXFP2 were identified in a mouse model of testis torsion, no functionally significant mutations were found. [14]
Testicular tumors were noted in 6% of patients presenting with testicular torsion, in one series. [15] Although malignancy remains an uncommon cause of torsion, it should be considered in postpubertal males presenting with acute scrotal pain.
Possible risk factors for extravaginal torsion include high birth weight, prolonged labor, pre-eclampsia, and gestational diabetes. However, those predispositions have yet to be confirmed in controlled studies.
Epidemiology
Extravaginal testicular torsion typically affects newborn boys, with an incidence of 1 in 7500. It constitutes 12% of all testicular torsions. Most of the torsions in this group (72%) occur prior to delivery. Postnatal torsion (normal testis at birth) within 30 days of birth comprises 28% of this cohort. It is typically unilateral and affects left and right testes equally, but bilateral torsion has been noted in 11 – 21% of newborns and is synchronous in 80%.
The annual incidence of intravaginal testicular torsion has been noted to be 4.5 per 100,000 males. Approximately 86% of cases occur in males older than 10 years (median age, 15 years). However, torsion has been reported in men up to the age of 78 years. [1]
Prognosis
The likelihood that a torsed testis will remain viable is inversely related to the time elapsed between symptom onset and detorsion. A published series including 1140 patients found the risk of undergoing orchiectomy to be 5%, 20%, 40% 16%, and 90% at 0-6, 7-12, 13-18, 19-24, longer than 24, and longer than 48 hours after symptom onset, respectively. [5]
Several risk factors are associated with needing orchiectomy rather than orchiopexy. In one retrospective review of data collected from the Pediatric Health Information System database, risk factors for undergoing orchiectomy included age 1 to 9 years, Black race, and lack of private insurance. [16] A more recent study found that younger age at presentation and longer duration from symptom onset to treatment were significant risk factors for orchiectomy, but that ethnicity, referral pattern, insurance, and timing of seasonal or daily timing of presentation were not significant risk factors. [17]
Regardless of whether orchiectomy or orchiopexy is performed, fertility after torsion remains poorly understood. In some studies, semen analysis shows subtle qualitative abnormalities. Semen density is often normal and is associated with a shorter period of torsion and lessened testicular atrophy. Studies have shown conflicting results on the effect of testicular torsion on fertility.
Endocrine function has been compared among men who presented with testis torsion and had orchiopexy or orchiectomy. Compared with controls, no difference was found between serum follicle-stimulating hormone (FSH), luteinizing hormone (LH), and testosterone levels. Serum inhibin B was diminished in both sets of torsion patients compared to the controls. [5]
-
Testicular torsion: (A) extravaginal; (B) intravaginal.
-
A 17-year-old adolescent boy with a 72-hour history of scrotal pain.
-
Intraoperative findings in testicular torsion.
-
Transverse power Doppler image of both testes illustrates an enlarged, avascular left testicle.
-
Testicular torsion. Transverse color Doppler image of the left groin illustrates an undescended testicle without flow.
-
Example of scrotal appearance in testicular torsion.
-
Extravaginal torsion in a newborn.
-
Intravaginal torsion in a child.