Practice Essentials
Peritonitis is an inflammation of the peritoneum. It can result from any rupture (perforation) in the abdomen or occur as a complication of other medical conditions. Peritonitis may be primary (ie, occurring spontaneously and not as the result of some other medical problem) or secondary (ie, resulting from some other condition). It is most often due to infection by bacteria but may also be due to a chemical irritant. Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection. [1]
Given the scarcity of systemic therapies, [2] surgery remains a cornerstone of peritonitis treatment. Any operation should address the first two principles of the treatment of intraperitoneal infections:
-
Early and definitive source control
-
Elimination of bacteria and toxins from the abdominal cavity
The timing and adequacy of surgical source control are paramount concerns, in that an improper, untimely, or incorrect operation may have an overwhelmingly negative effect on outcome, compared with medical therapy.
The operative approach is directed by the underlying disease process and the type and severity of the intra-abdominal infection. [3, 4, 5] In many cases, the indication for operative intervention will be clear, as in cases of peritonitis caused by ischemic colitis, a ruptured appendix, or colonic diverticula. The surgeon should always strive to arrive at a specific diagnosis and delineate the intra-abdominal anatomy as accurately as possible before the operation.
In severe abdominal sepsis, however, delays in operative management may lead to a significantly higher need for reoperation and to worse outcomes overall [6] ; early exploration (ie, before completion of diagnostic studies) may be indicated. Surgical intervention may include resection of a perforated viscus with reanastomosis or the creation of a fistula. To reduce the bacterial load, lavage of the abdominal cavity may be performed, with particular attention paid to areas prone to abscess formation (eg, paracolic gutters and the subphrenic area). [7]
Laparoscopy is gaining wider acceptance in the diagnosis and treatment of abdominal infections. As with all indications for laparoscopic surgery, outcomes vary, depending on the skill and experience of the laparoscopic surgeon.
Initial laparoscopic examination of the abdomen can assist in determination of the etiology of peritonitis (eg, right-lower-quadrant [RLQ] pathology in female patients).
For additional information on this topic, see Peritonitis and Abdominal Sepsis.
Preoperative Preparation
Volume resuscitation and the prevention of secondary organ system dysfunction are of the utmost importance in the treatment of patients with intra-abdominal infections. Depending on the severity of the disease, placement of Foley catheters may be indicated to monitor urine output. Invasive hemodynamic monitoring is warranted in severely ill patients to guide volume resuscitation and inotropic support. Any existing serum electrolyte disturbances and coagulation abnormalities should be corrected to the extent possible before any intervention.
Empiric broad-spectrum systemic antibiotic therapy should be initiated as soon as the diagnosis of intra-abdominal infection is suspected, and therapy should subsequently be tailored according to the underlying disease process and the culture results.
Because patients with peritonitis often have severe abdominal pain, adequate analgesia with parenteral narcotic agents should be provided as soon as possible.
In the setting of significant nausea, vomiting, or abdominal distention caused by obstruction or ileus, nasogastric decompression should be instituted as soon as possible.
In patients with evidence of septic shock or altered mental status, intubation and ventilator support should be considered at an early stage to prevent further decompensation.
Even if patients do not appear critically ill initially, arranging for postoperative intensive care support before surgery is often wise, particularly in patients of advanced age and those with significant comorbidities.
In patients with severe infections and certain disease processes (eg, necrotizing pancreatitis and bowel ischemia), informed consent should include the potential need for several reoperations and enteric diversion. The involved physicians and surgeon should not downplay the significant morbidities associated with abdominal sepsis when discussing these issues with the patient or the family.
Considerations for Surgical Management
General principles of operative intervention
Discussion of the specific details of the operative treatment of all the potential etiologies of intraperitoneal infections is beyond the scope of this article. Certain principles always apply to the performance of celiotomies in patients with peritonitis.
Operative treatment of peritonitis has three main goals:
-
To eliminate the source of contamination
-
To reduce the bacterial inoculum
-
To prevent recurrent or persistent sepsis
A vertical midline incision is the incision of choice in most patients with generalized peritonitis because it allows access to the entire peritoneal cavity. In patients with localized peritonitis (eg, from acute appendicitis or cholecystitis), an incision directly over the site of the pathologic condition (eg, RLQ or right subcostal incision) is usually adequate. In cases where the etiology of the peritonitis is unclear, initial diagnostic laparoscopy may be useful.
The intra-abdominal anatomy may be significantly distorted by the presence of inflammatory masses and adhesions. Normal tissue planes and boundaries may be obliterated. The inflamed organs are often very friable, and the surgeon must exercise great caution when exploring the patient with peritoneal infection.
Hemodynamic instability may occur at any time during treatment as a consequence of bacteremia and cytokine release. Patients often demonstrate significant fluid shifts with third-spacing. Swelling of the bowel, retroperitoneum, and abdominal wall may preclude safe abdominal closure after prolonged cases in patients who are severely ill.
Inflammation causes regional hyperemia, and sepsis may cause coagulation deficits and platelet dysfunction, leading to increased bleeding. Careful dissection and meticulous hemostasis are of the utmost importance.
When faced with extensive abdominal inflammatory disease and septic shock, the surgeon may be better advised to drain the infection temporarily, control the visceral leak quickly (eg, with oversewing or enteric diversion), and defer any definitive repair until after the patient has recovered from the initial insult (ie, a damage-control operation).
Open abdomen vs closed abdomen
One of the critical decisions in the surgical treatment of patients with severe peritonitis concerns whether to use an open-abdomen or a closed-abdomen technique.
The goal of the open-abdomen technique is to provide easy, direct access to the affected area. Source control is achieved through repeated reoperations or through open packing of the abdomen. This technique may be well suited for initial damage control in extensive peritonitis. [8, 9] (See Temporary Abdominal Closure Techniques.) It may be considered for elderly patients as well as for younger ones. [10]
The open-abdomen technique should also be considered in patients who are at high risk for the development of abdominal compartment syndrome (ACS)—such as patients with intestinal distention or extensive edema of the abdominal wall or intra-abdominal organs—because attempts to perform primary fascial closure under significant tension in these circumstances are associated with an increased incidence of multiple organ (eg, renal and respiratory) failure, necrotizing abdominal-wall infections, and mortality.
The goal of the closed-abdomen technique is to provide definitive surgical treatment at the initial operation. Primary fascial closure is employed, and repeat laparotomy is performed only when clinically indicated.
Pancreatitis-associated peritonitis
Among the causes of peritonitis, pancreatitis is unique in several ways. Patients may present with significant abdominal symptoms and a severe systemic inflammatory response, yet they may have no clear organ-specific indications for emergency exploration. Not all cases of severe (ie, necrotizing) pancreatitis and peripancreatic fluid collection are associated with a superinfection.
Patients with pancreatitis-associated peritonitis may be best served by a period of 12-24 hours of observation and intensive medical support. Deterioration of the patient's clinical status or the development of organ-specific indications (eg, intra-abdominal bleeding or gas-forming infection of the pancreas) should lead to prompt operation.
Percutaneous treatment is reserved for the management of defined peripancreatic fluid collections in stable patients.
Pancreatic abscess or infected pancreatic necrosis generally should be treated with surgical debridement and repeated exploration.
Dehiscence
If an anastomotic dehiscence is suspected, percutaneous drainage is of limited value, and the patient should be treated surgically. The image below demonstrates the results of anastomotic dehiscence following colon cancer surgery.
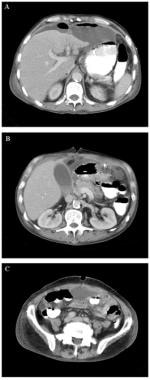 A 48-year-old man underwent suprapubic laparotomy, right hemicolectomy, and gastroduodenal resection for right colon cancer invading the first portion of the duodenum. He developed abdominal pain and distension. Computed tomography (CT) scanning was used to confirm an anastomotic dehiscence. Figure A shows a contrast-enhanced scan of the abdomen and pelvis that reveals multiple fluid collections, perihepatic ascites, and mild periportal edema. A collection of fluid containing an air-fluid level is visible anterior to the left lobe of the liver. A second collection is anterior to the splenic flexure of the colon. In figure B, a third fluid collection is present in the inferior aspect of the lesser space and in the transverse mesocolon. Figure C shows the pelvis with a collection of free fluid in the rectovesical pouch.
A 48-year-old man underwent suprapubic laparotomy, right hemicolectomy, and gastroduodenal resection for right colon cancer invading the first portion of the duodenum. He developed abdominal pain and distension. Computed tomography (CT) scanning was used to confirm an anastomotic dehiscence. Figure A shows a contrast-enhanced scan of the abdomen and pelvis that reveals multiple fluid collections, perihepatic ascites, and mild periportal edema. A collection of fluid containing an air-fluid level is visible anterior to the left lobe of the liver. A second collection is anterior to the splenic flexure of the colon. In figure B, a third fluid collection is present in the inferior aspect of the lesser space and in the transverse mesocolon. Figure C shows the pelvis with a collection of free fluid in the rectovesical pouch.
Open-Abdomen Approach
Second-look surgery
In certain situations, staging the operative approach to intraperitoneal infections is appropriate. Staging may be performed as a scheduled second-look operation or through open management, with or without temporary closure (eg, with mesh or vacuum-assisted closure [VAC]). [11, 12]
Second-look operations may be employed in a damage-control fashion. In these cases, the patient at initial operation is severely ill and unstable from septic shock or coagulopathy (eg, mediator liberation or disseminated intravascular coagulation [DIC]). The goal of the initial operation is to provide preliminary drainage and to remove obviously necrotic tissue. The patient is then resuscitated and stabilized in an intensive care unit (ICU) setting for 24-36 hours and returned to the operating room for more definitive drainage and source control.
In conditions related to bowel ischemia, the initial operation aims to remove all frankly devitalized bowel. The second-look operation serves to reevaluate for further demarcation and decision-making regarding reanastomosis or diversion.
Closure of abdomen
Temporary closure of the abdomen to prevent herniation of the abdominal contents and contamination of the abdominal cavity from the outside can be achieved by using gauze and large, impermeable, self-adhesive membrane dressings; mesh (eg, Vicryl, Dexon); nonabsorbable mesh (eg, GORE-TEX, polypropylene), with or without zipper or Velcrolike closure devices; and VAC devices (see Table 1 below). [13] The advantages of this management strategy include avoidance of ACS and provision of easy access for reexploration. The disadvantages include significant disruption of respiratory mechanics and potential contamination of the abdomen with nosocomial pathogens.
Table 1. Options for Temporary and Permanent Closure After Celiotomy (Open Table in a new window)
Closure Technique |
Description |
Advantages |
Disadvantages |
Self-adhesive impermeable membranes |
Abdominal dressing with gauze and coverage of the entire wound with impermeable membrane with and without placement of drains between the layers |
Inexpensive Easy application |
Difficult to maintain seal Potentially large volume losses Fistula formation |
Vicryl or Dexon mesh |
Suturing of the mesh to the fascial edges; different options for dressing |
Can be applied directly over bowel Allows for drainage of peritoneal fluid |
Rapid loss of tensile strength (in the setting of infection) Potentially large volume losses Higher incidence of later ventral hernia development No reopen-and-close option Fistula formation |
Polypropylene mesh |
Suturing of the mesh to the fascial edges; different options for dressing |
Good tensile strength Allows for drainage of peritoneal fluid |
Risk of intestinal erosion when applied directly over bowel Potentially large volume losses High risk of mesh infection Fistula formation |
GORE-TEX mesh |
Suturing of the mesh to the fascial edges; different options for dressing |
Good tensile strength Reopen and close option |
Potential fluid accumulation underneath the mesh Limited tissue integration and granulation tissue formation over the mesh Risk of mesh infection Fistula formation |
Human acellular dermis |
Suturing of the mesh to the fascial edges |
Good tensile strength |
Expensive Needs 10 minutes of rehydration |
Vacuum-assisted closure device |
Sponges applied over mesh and attached to controlled, low-level suction |
Controlled drainage of secretions Accelerated granulation tissue formation Wound debridement Can remain in place for longer than 48 hours |
Cost Risk of intestinal erosion when applied directly over bowel Fistula formation |
Wittmann patch |
Suturing of artificial burr (ie, Velcro) to fascia, staged abdominal closure by application of controlled tension |
Good tensile strength Allows for easy reexploration and eventual primary fascial closure |
Fistula formation |
For delayed primary closure (permanent), our experience with the use of human acellular dermis (commercially known as AlloDerm) has been satisfactory, though this option has the disadvantage of being more expensive than others.
A study by Mutafchiyski et al included 108 patients with diffuse peritonitis and open abdomen who were treated either with mesh-foil laparostomy without negative pressure or with VAC. [14] The investigators found VAC to be associated with higher overall and late primary fascial closure rates, a lower incidence of necrotizing fasciitis, fewer intra-abdominal abscesses and enteroatmospheric fistulas, reduced overall mortality, and shorter ICU and hospital stays.
In a study that involved 53 patients with peritonitis who underwent open-abdomen management, Willms et al found that regardless of the process underlying the peritonitis, the use of a combination of VAC and mesh-mediated fascial traction (MMFT) was able to achieve high rates of fascial closure. [15]
In a study that involved 152 patients who underwent open-abdomen therapy at a single center, more than half of whom had sepsis (33.3%) or peritonitis (24.2%) as the indication, Berrevoet et al found that patients who started open-abdomen management with MMFT and negative-pressure wound therapy (NPWT) from the initial surgery had a significantly better fascial closure rate than those who started 3 or more days later. [16] The use of VAC in conjunction with MMFT yielded high rates of fascial closure. Absence of initial intraperitoneal NPWT and delayed initiation of MMFT were risk factors for nonfascial closure.
Laparoscopic Approach
Laparoscopic surgery is commonly used in the treatment of uncomplicated appendicitis, though there is evidence to indicate that it can yield positive outcomes for complicated appendicitis as well. [17, 18]
For both complicated and uncomplicated appendicitis, the laparoscopic approach is associated with a shorter length of hospital stay and fewer wound infections than is the open approach. However, laparoscopic surgery may be associated with a higher rate of intra-abdominal abscess.
Laparoscopic diagnosis and peritoneal lavage in patients with peritonitis secondary to diverticulitis has been shown to be safe and has helped to avoid the need for colostomy in many patients in small clinical trials. [19]
In a prospective study comparing laparoscopic peritoneal lavage with an open Hartmann procedure for perforated diverticulitis with generalized peritonitis, peritoneal lavage without operative intervention was found to be feasible, with a comparable mortality and a low risk of short-term recurrence.
A retrospective cohort study by Campana et al found that diverticulitis recurrence was high after successful laparoscopic peritoneal lavage in Hinchey III peritonitis but that to recurrence rate decreased after the first year. [20]
A study by Illuminati et al found laparoscopic lavage/drainage (LALA) to be a potentially effective as a bridge treatment before endovascular aneurysm exclusion and elective colon resection in patients presenting with perforated diverticulitis with purulent peritonitis associated with an uncomplicated abdominal aortic aneurysm. [21]
Successful laparoscopic treatment of gastroduodenal as well as colorectal perforation has been reported. [22]
No definitive guidelines have been established regarding the optimal selection of patients for successful laparoscopic repair. Studies have investigated scoring systems (eg, APACHE II, Boey score) for patient risk stratification, in order to allow better selection of patients for laparoscopic repair.
The treatment of perihepatic infections via the laparoscopic approach has been well established in acute cholecystitis, where laparoscopic cholecystectomy has become the mainstay of therapy. Primary treatment of subphrenic abscesses and laparoscopic ultrasonography (US)-assisted drainage of pyogenic liver abscesses have also been performed successfully.
Individual reports also describe successful drainage of peripancreatic fluid collections and complicated intra-abdominal abscesses that are not amenable to percutaneous drainage guided by either computed tomography (CT) or US.
As minimally invasive procedures continue to advance technologically, use of these approaches is likely to increase, reducing the need for the open surgical approach for peritoneal abscess drainage.
Multiple Reexplorations
In severe peritonitis, particularly when it includes extensive retroperitoneal involvement (eg, necrotizing pancreatitis), open treatment with repeat reexploration, debridement, and intraperitoneal lavage has been shown to be effective.
The decision to perform a series of reexplorations may be made during the initial surgery if additional debridement and lavage are needed beyond what can be achieved in the first procedure. Indications for planned repeat laparotomy may include failure to achieve adequate source control, diffuse fecal peritonitis, hemodynamic instability, and intra-abdominal hypertension.
Multiple reoperations may be associated with significant risks, including those from a substantial inflammatory response, fluid and electrolyte shifts, and hypotension; however, these must be balanced against the risks of persistent necrotic or infectious abdominal foci.
The open-abdomen technique allows thorough drainage of the intra-abdominal infection, but the specific indications are not clearly defined. Many trials lack control groups or use historical controls; outcome variables (eg, mortality) are often not specific enough, and data on resource use are limited.
To date, no conclusive data suggest a clear advantage for the open approach over the closed approach in the treatment of severe abdominal sepsis; however, in the author's experience, bowel edema and subsequent inflammatory changes limit the use of the closed-abdomen technique. Secondary ACS may ensue if abdominal closure is performed before the inflammatory process has resolved.
In some cases, staged operative interventions will be planned. In other cases, patients may present with continued peritonitis or abscess formation requiring "on-demand" relaparotomy.
A 2004 study suggested that the mortality of on-demand laparotomy is higher for those patients receiving intervention more than 48 hours after their index operation.
Postoperative Care
Postoperatively, all patients should be closely monitored in the appropriate clinical setting for adequacy of volume resuscitation, resolution or persistence of sepsis, and the development of organ system failure. Appropriate systemic broad-spectrum antibiotic coverage must be continued without interruption for the appropriate amount of time.
The patient's overall condition should improve significantly and progressively within 24-72 hours of the initial treatment (as evidenced by resolution of the signs and symptoms of infection and mobilization of interstitial fluid). However, this time course may be prolonged in patients who are critically ill with significant multiple organ dysfunction syndrome (MODS).
A lack of improvement should prompt an aggressive search for a persistent or recurrent intraperitoneal or new extraperitoneal infectious focus.
All patients who are critically ill and patients who are receiving prolonged antibiotic therapy are at increased risk for the development of secondary, opportunistic infections (eg, Clostridioides (Clostridium) difficile colitis, fungal infections, central venous catheter infections, and ventilator-associated pneumonia). Accordingly, they should be closely monitored for signs and symptoms of these complications.
Patients with severe abdominal infections demonstrate higher incidences of fascial dehiscence and incisional hernia development necessitating later reoperation.
Surgical-Site Infection and Delayed Healing
Patients requiring surgical intervention for peritonitis demonstrate a significantly increased risk for surgical-site infection (SSI) and failed wound healing; they should therefore be closely monitored for these potential complications.
The incidence of SSI increases with the degree of contamination; therefore, SSI occurs at much higher rates after operations for peritonitis and peritoneal abscess (ie, 5-15%, compared with < 5% for elective abdominal operations for noninfectious etiologies).
SSI may be expected if the wound is closed in the setting of gross abdominal contamination (see Table 2 below). Perioperative systemic antibiotic therapy, wound-protector devices, and wound lavage at the end of therapy do not reliably prevent this complication. These wounds should be left open and should be treated with wet-to-dry dressing changes several times a day, or VAC dressing should be applied.
Table 2. Wound Classification and Risk of Surgical-Site Infection (SSI) (Open Table in a new window)
Classification |
Examples |
Incidence of SSI (%) |
Clean |
Elective surgery without violation of the gut or infected spaces |
< 2 |
Clean-contaminated |
Elective bowel surgery (prepared bowel, mechanical and antibiotic) |
5-15 |
Contaminated |
Emergency bowel surgery (unprepared bowel, minor spillage), drainage of infected spaces |
15-30 |
Dirty |
Grossly contaminated traumatic wounds, significant intestinal spillage, grossly infected and devitalized tissue (necrotizing infection) |
>30 |
The same factors that impair the clearance of the abdominal infection contribute to increased problems related to wound healing (eg, malnutrition, severe sepsis, MODS, advanced age, and immunosuppression) and should be addressed aggressively.
-
A 48-year-old man underwent suprapubic laparotomy, right hemicolectomy, and gastroduodenal resection for right colon cancer invading the first portion of the duodenum. He developed abdominal pain and distension. Computed tomography (CT) scanning was used to confirm an anastomotic dehiscence. Figure A shows a contrast-enhanced scan of the abdomen and pelvis that reveals multiple fluid collections, perihepatic ascites, and mild periportal edema. A collection of fluid containing an air-fluid level is visible anterior to the left lobe of the liver. A second collection is anterior to the splenic flexure of the colon. In figure B, a third fluid collection is present in the inferior aspect of the lesser space and in the transverse mesocolon. Figure C shows the pelvis with a collection of free fluid in the rectovesical pouch.







