Practice Essentials
Pancreas transplantation is principally performed to ameliorate type 1 diabetes mellitus and produce complete independence from injected insulin. In addition, pancreas transplantation in patients with type 2 diabetes has increased steadily in recent years. [1] The pancreas is usually procured from a deceased organ donor, although select cases of living-donor pancreas transplantations have been performed.
The number of pancreas transplants in the United States decreased from 2004 (when approximately 1500 were performed) to 2015. Subsequently, pancreas transplants have risen, mainly because of increased simultaneous pancreas-kidney (SPK) transplants, while pancreas transplant alone (PTA) continued on a downward trend. [1] In 2020, 135 PTAs were performed in the US, compared with 827 SPK transplantations. In the US, SPK transplantations are the most common multi-organ transplant; over 26,000 SPK transplantations were performed from 1988 through 2021. [1, 2] See the image below.
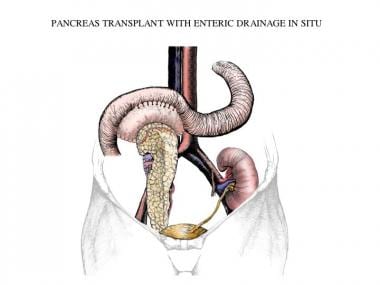 Simultaneous pancreas-kidney transplantation with enteric drainage. Illustrated by Simon Kimm, MD. Image courtesy of Landes Bioscience.
Simultaneous pancreas-kidney transplantation with enteric drainage. Illustrated by Simon Kimm, MD. Image courtesy of Landes Bioscience.
Pancreas transplantations are also performed after successful kidney transplantation (ie, pancreas-after-kidney [PAK] transplantation). PAK represented less than 10% of pancreas transplants in 2019. [1] The remaining cases are performed as PTA in nonuremic patients with labile and problematic diabetes.
An alternative therapy that may also ameliorate diabetes is islet cell transplantation. Pancreas and islet cell transplantation can be considered complementary transplant options and undergoing one or the other is not mutually exclusive. In an analysis of 40 pancreas transplantations (50% PTA, 27.5% SPK, 22.5% PAK) after islet cell transplantation graft failure, overall survival rates (97% at 1 year and 83% at 5 years) were not adversely affected. [3]
Background
Experiments in pancreas transplantation began long before the discovery of insulin. In 1891, pieces of dog pancreas were autotransplanted beneath the skin and were shown to prevent diabetes after removal of the intra-abdominal pancreas. Subsequent experimentation with intrasplenic transplantation did not succeed because of graft necrosis. In 1916, a sliced human pancreas was transplanted into two patients, but the grafts were wholly absorbed. The first pancreatic xenotransplantation was performed in 1893 in London; a 15-year-old boy underwent subcutaneous implantation of a pancreas.
Despite extensive animal experimentation, pancreatic transplantation did not become a reality until 1966, when W.D. Kelly performed the first human, whole-organ pancreatic transplantation to treat type 1 diabetes mellitus. Because of poor outcomes, few procedures were conducted until 1978. Much of the early work was performed by Sutherland and colleagues at the University of Minnesota. With improved immunosuppressive regimens and newer surgical techniques, the 1980s ushered in a new era in pancreas transplantation. [4] According to the International Pancreas Transplant Registry, nearly 10,000 pancreatic transplantations were recorded by 1998.
Most pancreatic transplantations are performed in patients with type 1 diabetes mellitus, who lack of insulin production. [5, 6] The most common indication is kidney failure; therefore, pancreas transplantation is typically performed simultaneously with kidney transplantation. [7, 8, 9, 10] In some patients with hypoglycemic unawareness or other diabetic complications, isolated pancreas transplantation has been performed. However, the results have been somewhat inferior to those of the combined procedure.
In patients undergoing pancreas transplantation, various technical concerns must be considered, including whether or not the venous drainage should be into the systemic circulation or into the portal vein. [4] Another controversial topic is whether the exocrine secretions should be drained enterically or into the bladder as initially described. The complications of graft pancreatitis and bladder leakage that plagued early experiences with pancreas transplantation have largely been resolved as a result of both better technical expertise and fewer rejection- and immunosuppression-related complications.
Pathophysiology
Type 1 diabetes mellitus is an autoimmune disease in which the insulin-producing pancreatic beta cells are destroyed selectively. Even with the increasing utilization of continuous glucose monitors (CGM) and insulin pumps, no practical mechanical insulin-delivery method exists that replaces pancreatic insulin secretion well enough to produce a near-constant euglycemic state without risk of hypoglycemia. Therefore, individuals with type I diabetes must manually regulate blood glucose levels by subcutaneous insulin injection or infusion and, as a consequence, typically exhibit wide deviations in plasma glucose levels from hour to hour and from day to day.
Hyperglycemia is the most important factor in the development and progression of the secondary complications of diabetes. These observations, and the fact that conventional exogenous insulin therapy cannot prevent the development of secondary complications of type I diabetes, have led to a search for alternative treatment methods.
One such treatment, pancreas transplantation, has the potential to achieve better glycemic control and alter the progression of long-term complications. Successful pancreas transplantation accomplishes the following:
-
Produces a normoglycemic and exogenous insulin–independent state
-
Reverses some of the diabetic changes in the native kidneys of patients with very early diabetic nephropathy
-
Prevents recurrent diabetic nephropathy, in patients undergoing SPK transplantation
-
Stabilizes and potentially partially reverses peripheral sensory neuropathy
-
Stabilizes advanced diabetic retinopathy
-
Significantly improves patients' quality and quantity of life
The insulin released by the endocrine pancreas graft is secreted into the bloodstream. The exocrine pancreas produces about 800-1000 mL of fluid per day, which must be diverted into the bladder or the bowel. This pancreatic fluid is rich in bicarbonate, so if the pancreas graft is attached to the bladder, the fluid loss may produce relative acidosis. This usually is treated by bicarbonate supplementation.
Because the pancreas graft comes from another individual, the recipient's immune system can mount a rejection reaction and destroy the graft. To prevent rejection, patients must take immunosuppressive medications daily for the rest of their lives. Long-term immunosuppression elevates the risk of viral and fungal infections and some types of malignancy.
Epidemiology
An estimated 34.2 million people in the United States have diabetes and over 58,000 individuals annually develop end-stage kidney disease with diabetes as the primary cause. [11] Although almost 2400 candidates were on the waitlist for pancreas transplants (13.4% PTA, 76.6% SPK, 10% PAK) at the end of 2019, only about 1000 pancreas transplantations were performed. [1] The number of transplants is limited in part by the number of high-quality donor organs available for transplantation. See Table 1 below for a breakdown of patient characteristics.
Table 1. Characteristics of adult recipients of pancreas transplantation, United States, 2019 [1] (Open Table in a new window)
Patient Characteristic |
All Candidates |
PAK | PTA | SPK | ||||
| Number | PCT | Number | PCT | Number | PCT | Number | PCT | |
Age 18-34 y |
226 |
22.7% |
4 | 9.1% | 20 | 24.7% | 202 | 23.2% |
Age 35-49 y |
520 |
52.3% |
30 | 68.2% | 34 | 42.0% | 520 | 52.3% |
Age 50-60 y |
213 |
21.4% |
9 | 20.5% | 20 | 24.7% | 213 | 21.4% |
Age > 60 y |
35 |
3.5% |
1 | 2.3% | 7 | 8.6% | 27 | 3.1% |
Male |
606 |
61.0% |
26 | 59.1% | 37 | 45.7% | 543 | 62.5% |
Female |
388 |
39.0% |
18 | 40.9% | 44 | 54.3% | 326 | 37.5% |
White |
519 |
52.2% |
27 | 61.4% | 66 | 81.5% | 426 | 49.0% |
Black |
271 |
27.3% |
8 | 18.2% | 9 | 11.1% | 254 | 29.2% |
Hispanic |
157 |
15.8% |
7 | 15.9% | 6 | 7.4% | 144 | 16.6% |
Asian |
43 | 4.3% |
2 | 4.5% | 0 | 0% | 41 | 4.7% |
| Other | 4 | 0.4% | 0 | 0% | 0 | 0% | 4 | 0.5% |
| All recipients | 1015 | 100% | 44 | 4% | 99 | 10% | 872 | 86% |
Prognosis
Assessment of pancreas graft outcome rates has been hampered by lack of uniformity in the criteria for graft failure. Some programs do not report a failed graft if C peptide production continues, whereas others report a graft failure if the recipient is no longer insulin independent. To resolve this problem, the Organ Procurement and Transplantation Network/United Network for Organ Sharing (OPTN/UNOS) established a uniform definition for pancreas graft failure, which includes any of the following criteria:
-
Removal of a transplanted pancreas
-
Re-registration for a pancreas transplant
-
Registration for an islet transplant after undergoing pancreas transplant
-
Death
Total insulin use of 0.5 units/kg/day or more for 90 consecutive days can also be used to define pancreas graft failure. However, this may be problematic if the recipient's starting insulin dose was less than 0.5 units/kg/day.
The new pancreas graft failure definition was implemented in February 2018. It was used in the first full year of pancreas graft survival data in the OPTN/Scientific Registry of Transplant Recipients 2020 Annual Data Report. [1]
Nevertheless, the number of recipients alive with a functioning pancreas allograft has continued to rise over the past decade and exceeded 19,000 in June 2019. Mortality has decreased consistently among all pancreas transplant groups as a result of safer and more effective immunosuppressive regimens. Five-year survival rates for patients transplanted in 2011-2012 were 87.8% for PAK, 79.5% for PTA, and 91.7% for SPK.
For SPK, the 5-year survival rates were similiar in patients with type 1 and type 2 diabetes (91.1% and 93.1% respectively), despite the older age and comorbidity associated with type 2 diabetes. This is likely due to selecting candidates with type 2 diabetes whose cardiovascular status can tolerate the high operative risks. [1]
In one published retrospective study, differences in mortality were examined in consecutive patients with diabetes who were older than 50 years compared with well-matched recipients younger than 50 years undergoing pancreas transplantation (the majority were simultaneous kidney-pancreas transplants) at a high-volume European center. Despite US data suggesting an increased risk of mortality in recipients older than 45 years compared with patients younger than 45 years, it is becoming clear that carefully selected patients with diabetes who are older than 50 years can undergo successful pancreas transplantation with similar patient and allograft survival outcomes. [12]
This trend toward considering pancreas transplantation in older recipients appears to have begun earlier in the United States and is now gaining momentum in Europe. It must be emphasized that careful cardiac evaluation is essential to this patient selection process.
Effect of pancreas transplantation on secondary complications of diabetes
Recipients of successful pancreas transplantation maintain normal plasma glucose levels without exogenous insulin therapy. This results in the normalization of glycosylated hemoglobin levels and has a beneficial effect on many secondary complications of diabetes. The durability of the transplanted endocrine pancreas has been established with the demonstration that normalization of glycosylated hemoglobin is maintained as long as the allograft functions. The potential lifespan of the transplanted pancreas is not known precisely because, at present, survivors with functioning pancreas transplantations still are doing well more than 16 years after transplantation. The implications of prolonged normalization of glycemia and glycosylated hemoglobin levels are significant with respect to patients' quality of life, kidney structure, and motor-sensory and nerve function.
One long-term follow-up study of 15 years showed that pancreas transplantation in patients with type 1 diabetes mellitus and end-stage kidney failure has long-term functional viability. However, some deterioration in pancreas function should be expected, as shown in oral glucose tolerance test results. [13]
The quality of life of pancreas transplantation recipients has been well studied. Patients with a functioning pancreas graft describe their quality of life and rate their health significantly more favorably than those with nonfunctioning pancreas grafts. Satisfaction encompasses not only the physical capacities but also relates to psychosocial and vocational aspects. The functioning pancreas graft leads to even better quality of life when compared with recipients of kidney transplantation alone. [14, 15] Virtually all patients with a successful pancreas transplantation report that managing their life, even with the added need for immunosuppression, has become much easier. Successful pancreas transplantation will not elevate all patients with diabetes to the general population's level of health and functioning. Still, transplant recipients consistently report significantly better quality of life than patients who remain diabetic.
The development of diabetic nephropathy in transplanted kidneys residing in patients with type I diabetes has been well established. Marked variability is observed in the rate of kidney pathology, including mesangial expansion and widening of the glomerular basement membrane, in patients with type 1 diabetes and kidney transplantation alone. The onset of pathological lesions can be detected within a few years of kidney transplantation. Clinical deterioration of kidney allograft function can lead to loss 10-15 years after transplantation.
Successful pancreas transplantation prevents glomerular structure changes of kidney allografts in patients with type I diabetes. This has been observed in transplanted kidneys of patients undergoing SPK transplantation and in kidneys of recipients undergoing pancreas after kidney transplantation. These studies provide evidence of the efficacy of normalizing blood glucose and glycosylated hemoglobin levels to prevent the progression of diabetic glomerulopathy in renal allografts.
Furthermore, successful pancreas transplantation will halt or reverse the pathology in the native kidneys of patients with type I diabetes and very early proteinuria. Pancreas transplantation recipients all had persistently normal glycosylated hemoglobin values for 5-10 years after transplantation. The thickness of the glomerular and tubular basement membranes and mesangial volume steadily decrease over a 10-year interval. These early studies have important implications for the role of pancreas transplantation alone in patients with type I diabetes and very early changes in native kidney function.
Successful pancreas transplantation has been shown to halt, and in many cases, reverse motor-sensory and autonomic neuropathy 12-24 months after transplantation. This has been studied most extensively in recipients of SPK transplantations. This raises the possibility that improvement of diabetic neuropathy occurs partly because of the reduction in uremic neuropathy. However, pancreas transplantation alone in preuremic patients also has also been shown to improve diabetic neuropathy. Many patients report subjective improvements in peripheral sensation 6-12 months after pancreas transplantation. Interestingly, the reversal of autonomic neuropathy in patients with type 1 diabetes with pancreas transplantation has been associated with better patient survival rates than in patients with failed or no transplantation. [16]
Pancreas transplantation does not have an immediate dramatic beneficial effect on established diabetic retinopathy. Retinopathy appears to progress for at least 2 years following transplantation of the pancreas, but it begins to stabilize in 3-4 years compared with diabetic recipients of kidney transplantation only. [17] Longer-term studies of 5-10 years have not been reported.
-
Simultaneous pancreas-kidney transplantation with enteric drainage. Illustrated by Simon Kimm, MD. Image courtesy of Landes Bioscience.
-
Solitary pancreas transplantation with enteric drainage. Illustrated by Simon Kimm, MD. Image courtesy of Landes Bioscience.
-
Solitary pancreas transplantation with bladder drainage. Illustrated by Simon Kimm, MD. Image courtesy of Landes Bioscience.





