Practice Essentials
In 1930, Wolff, Parkinson, and White described a series of young patients who experienced paroxysms of tachycardia and had characteristic abnormalities on electrocardiography (ECG). [1] Currently, Wolff-Parkinson-White (WPW) syndrome is defined as a congenital condition involving abnormal conductive cardiac tissue between the atria and the ventricles that provides a pathway for a reentrant tachycardia circuit, in association with supraventricular tachycardia (SVT). See the image below for a typical "preexcited" ECG.
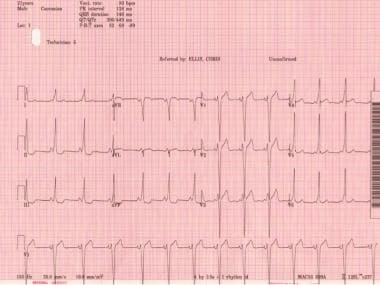 Wolff-Parkinson-White Syndrome. Classic Wolff-Parkinson-White electrocardiogram with short PR, QRS >120 ms, and delta wave.
Wolff-Parkinson-White Syndrome. Classic Wolff-Parkinson-White electrocardiogram with short PR, QRS >120 ms, and delta wave.
Signs and symptoms
The clinical manifestations of WPW syndrome reflect the associated tachyarrhythmia episodes—rather than the anomalous ventricular excitation per se. They may have their onset at any time from childhood to middle age, and they can vary in severity from mild chest discomfort or palpitations with or without syncope to severe cardiopulmonary compromise and cardiac arrest. Thus, presentation varies by patient age.
Infants may present with the following:
-
Tachypnea
-
Irritability
-
Pallor
-
Intolerance of feedings
-
Evidence of congestive heart failure if the episode has been untreated for several hours
-
A history of not behaving as usual for 1-2 days
-
An intercurrent febrile illness may be present
A verbal child with WPW syndrome usually reports the following:
-
Chest pain
-
Palpitations
-
Breathing difficulty
Older patients can usually describe the following:
-
Sudden onset of a pounding heartbeat
-
Pulse that is regular and “too rapid to count”
-
Typically, a concomitant reduction in their tolerance for activity
Physical findings include the following:
-
Normal cardiac examination findings in the vast majority of cases
-
During tachycardic episodes, the patient may be cool, diaphoretic, and hypotensive
-
Crackles in the lungs from pulmonary vascular congestion (during or following an SVT episode)
-
Many young patients may present with resting tachycardia on examination, with only minimal symptoms (eg, palpitations, weakness, mild dizziness) despite exceedingly fast heart rates
Clinical features of associated cardiac defects may be present, such as the following:
-
Cardiomyopathy
-
Ebstein anomaly
-
Hypertrophic cardiomyopathy ( AMPK mutation) [2]
See Clinical Presentation for more detail.
Diagnosis
Routine blood studies may be needed to help rule out noncardiac conditions triggering tachycardia. These may include the following:
-
Complete blood count
-
Chemistry panel, with renal function studies and electrolytes
-
Liver function tests
-
Thyroid panel
-
Drug screening
The diagnosis of WPW syndrome is typically made with a 12-lead electrocardiogram (ECG) and sometimes with ambulatory monitoring (eg, telemetry, Holter monitoring). SVT is best diagnosed by documenting a 12-lead ECG during tachycardia, although it is often diagnosed with a monitoring strip or even recorder. The index of suspicion is based on the history, and rarely, physical examination (Ebstein anomaly or hypertrophic cardiomyopathy [HOCM]). Although the ECG morphology varies widely, the classic ECG features are as follows:
-
A shortened PR interval (typically < 120 ms in a teenager or adult)
-
A slurring and slow rise of the initial upstroke of the QRS complex (delta wave)
-
A widened QRS complex (total duration >0.12 seconds)
-
ST segment–T wave (repolarization) changes, generally directed opposite the major delta wave and QRS complex, reflecting altered depolarization
Echocardiography is needed for the following:
-
Evaluation of left ventricular (LV) function, septal thickness, and wall motion abnormalities
-
Excluding cardiomyopathy and an associated congenital heart defect (eg, HOCM, Ebstein anomaly, L-transposition of the great vessels)
Stress testing is ancillary and may be used for the following:
-
To reproduce a transient paroxysmal SVT, which is triggered by exercise
-
To document the relationship of exercise to the onset of tachycardia
-
To evaluate the efficacy of antiarrhythmic drug therapy (class Ic antiarrhythmic medications and effects on antegrade preexcitation)
-
To determine whether consistent or intermittent preexcitation is present at different sinus (heart) rates
Electrophysiologic studies (EPS) can be used in patients with WPW syndrome to determine the following:
-
The mechanism of the clinical tachycardia
-
The electrophysiologic properties (eg, conduction capability, refractory periods) of the accessory pathway and the normal atrioventricular (AV) nodal and His Purkinje conduction system
-
The number and locations of accessory pathways (necessary for catheter ablation)
-
The response to pharmacologic or ablation therapy
See Workup for more detail.
Management
Treatment of WPW associated arrhythmias comprises the following:
-
Radiofrequency ablation of the accessory pathway [3]
-
Antiarrhythmic drugs to slow accessory pathway conduction
-
AV nodal blocking medications in adult patients to slow AV nodal conduction in certain situations (ie, Mahaim or atriofascicular pathway-mediated SVT; typically, AV node-conduction blocking medications are avoided in the acute setting of WPW)
-
For adult WPW patients, address the triggers that perpetuate the dysrhythmia, which include coronary heart disease (CAD), ischemia, cardiomyopathy, pericarditis, electrolyte disturbances, thyroid disease, and anemia
Termination of acute episodes
Narrow-complex AV reentrant tachycardia (AVRT) and AV nodal reentrant tachycardia (AVNRT) are treated by blocking AV node conduction with the following:
-
Vagal maneuvers (eg, Valsalva maneuver, carotid sinus massage, splashing cold water or ice water on the face)
-
Adults: IV adenosine 6-12 mg via a large-bore line (the drug has a very short half-life)
-
Adults: IV verapamil 5-10 mg or diltiazem 10 mg
-
Pediatric patients: Adenosine and verapamil or diltiazem are dosed on the basis of weight.
Atrial flutter/fibrillation or wide-complex tachycardia is treated as follows:
-
IV procainamide or amiodarone if wide-complex tachycardia is present, ventricular tachycardia (VT) cannot be excluded, and the patient is stable hemodynamically
-
Ibutilide
The initial treatment of choice for hemodynamically unstable tachycardia is direct-current synchronized electrical cardioversion, biphasic, as follows:
-
A level of 100 J (monophasic or lower biphasic) initially
-
If necessary, a second shock with higher energy (200 J or 360 J)
Radiofrequency ablation
Radiofrequency ablation is indicated in the following patients:
-
Patients with symptomatic AVRT
-
Patients with AF or other atrial tachyarrhythmias that have rapid ventricular response via an accessory pathway (preexcited AF)
-
Patients with AVRT or AF with rapid ventricular rates found incidentally during EPS for unrelated dysrhythmia, if the shortest preexcited RR interval during AF is less than 250 ms [4]
-
Asymptomatic patients with ventricular preexcitation whose livelihood, profession, insurability, or mental well-being may be influenced by unpredictable tachyarrhythmias or in whom such tachyarrhythmias would endanger the public safety [5]
-
Patients with WPW and a family history of sudden cardiac death
Surgical treatment
Radiofrequency catheter ablation has virtually eliminated surgical open heart treatments in the vast majority of WPW patients, with the following exceptions:
-
Patients in whom RF catheter ablation (with repeated attempts) fails
-
Patients undergoing concomitant cardiac surgery (possible exception)
-
Patients with other tachycardias with multiple foci who require surgical intervention (very rare)
Long-term antiarrhythmic therapy
Oral medication is the mainstay of therapy in patients not undergoing radiofrequency ablation, although the response to long-term antiarrhythmic therapy for the prevention of further episodes of tachycardia in patients with WPW syndrome remains quite variable and unpredictable. Choices include the following:
-
Class Ic drugs (eg, flecainide, propafenone), typically used with an AV nodal blocking agent in low doses to avoid atrial flutter with a 1:1 conduction
-
Class III drugs (eg, amiodarone, sotalol), although these are less effective for altering accessory pathway conduction properties
-
In pregnancy, sotalol (class B) or flecainide (class C)
See Treatment and Medication for more detail.
Background
In 1930, Wolff, Parkinson, and White described a series of young patients who had a bundle branch block pattern on electrocardiography (ECG) findings, a short PR interval, and paroxysms of tachycardia. [1] Case reports began appearing in the literature in the late 1930s and early 1940s, and the term Wolff-Parkinson-White (WPW) syndrome was coined in 1940.
Although "preexcitation" was first coined by Ohnell in a landmark publication in 1944, the term as defined by Durrer et al in 1970 provided a better description in the literature of what an accessory pathway is (before the advent of invasive electrophysiologic studies and ablation provided a clearer understanding): "Preexcitation exists, if in relation to atrial events, the whole or some part of the ventricular muscle is activated earlier by the impulse originating from the atrium than would be expected if the impulse reached the ventricles by way of the normal specific conduction system only." [6]
WPW syndrome is currently defined as a congenital abnormality involving the presence of abnormal conductive cardiac tissue between the atria and the ventricles in association with supraventricular tachycardia (SVT). It involves preexcitation, which occurs because of conduction of an atrial impulse not by means of the normal conduction system, but via an extra atrioventricular (AV) muscular connection, termed an accessory pathway (AP), that bypasses the AV node. [4, 7]
Classic ECG findings that are associated with WPW syndrome include the following:
-
Presence of a short PR interval (< 120 ms)
-
A wide QRS complex longer than 120 ms with a slurred onset of the QRS waveform, termed a delta wave, in the early part of QRS
-
Secondary ST-T wave changes (see the image below)
 Wolff-Parkinson-White Syndrome. Classic Wolff-Parkinson-White electrocardiogram with short PR, QRS >120 ms, and delta wave.
Wolff-Parkinson-White Syndrome. Classic Wolff-Parkinson-White electrocardiogram with short PR, QRS >120 ms, and delta wave.
Patients with WPW syndrome are potentially at an increased risk of dangerous ventricular arrhythmias as a consequence of conduction across the bypass tract resulting in a very rapid and chaotic depolarization of the ventricle if they develop atrial flutter or atrial fibrillation (AF).
Some patients have a concealed bypass tract. Although they have an accessory AV connection, it lacks antegrade conduction; accordingly, these patients do not have the classic abnormalities of the surface ECG. Most commonly, this is established by electrophysiologic study performed for the evaluation or treatment of SVT.
Only a small percentage of patients with WPW syndrome (< 1%) are at risk for sudden cardiac death (SCD). In patients who present with preexcited AF, cardiac electrophysiologic studies and radiofrequency (RF) catheter ablation may be curative. Other presentations include symptomatic SVT, which can also be cured by catheter ablation. Asymptomatic patients need periodic observation. The onset of cardiac arrhythmias, and possibly the sudden death risk, may be eliminated by prophylactic catheter ablation as well. [8]
This review discusses the pathogenesis, clinical presentation, evaluation, and treatment of patients with WPW syndrome.
Pathophysiology
Accessory pathways or connections between the atrium and ventricle are the result of anomalous embryonic development of myocardial tissue bridging the fibrous tissues that separate the two chambers. This allows electrical conduction between the atria and ventricles at sites other than the AV node. Passage through APs circumvents the usual conduction delay between the atria and ventricles, which normally occurs at the AV node, and predisposes the patient to develop tachydysrhythmias.
Although dozens of locations for bypass tracts can exist in preexcitation, including atriofascicular, fasciculoventricular, nodofascicular, or nodoventricular, the most common bypass tract is an accessory AV pathway otherwise known as a Kent bundle. This is the anomaly seen in WPW syndrome. The primary feature that differentiates WPW syndrome from other AP-mediated supraventricular tachycardias (SVTs) is the ability of the AP to conduct in either an antegrade (ie, from atrium to ventricles) or a retrograde manner.
The presence of an AP sets up the potential for reentrant tachycardia circuits to be established or for preexcited tachycardia in the setting of atrial fibrillation, atrial flutter, or SVT with a bystander accessory pathway. This reentrant mechanism is the typical cause of the SVT of which patients with preexcitation are at risk. The genesis of reentrant SVT involves the presence of dual conducting pathways between the atria and the ventricles [9] :
-
The natural AV nodal His-Purkinje tract
-
One or more AV accessory tract(s) (ie, AV connection or AP, Kent fibers, Mahaim fibers)
These pathways usually exhibit different conduction properties and refractory periods that facilitate reentry. The effective refractory period (ERP, the time necessary for the electrical recovery needed to conduct the next impulse) of the accessory tract is often longer than that of the normal AV nodal His-Purkinje tract and requires time for conduction to recover before allowing reentry.
The degree of preexcitation on a surface ECG in a person with WPW pattern can be estimated by the width of the QRS and the length of the PR interval. A wider or more preexcited QRS with a short PR interval with absent or nearly absent isoelectric component reveals that most (or all) of the ventricular depolarization initiates through the AP insertion rather than through the AV node/His Purkinje system. This would be typical with right free wall pathways where the atrial insertion is close to the sinoatrial (SA) node.
However, the QRS width may vary, becoming narrower during more rapid heart rates. This is possible because catecholamines permit the AV node to contribute more (or entirely) to ventricular depolarization by enhancing AV node conduction; the AV node connects to the entire and usual His-Purkinje system, resulting in the narrow QRS complex.
Types of SVT include orthodromic tachycardia (down the AV nodal His-Purkinje system and retrograde conduction up an AP), orthodromic tachycardia with a concealed AP (retrograde conduction only), and antidromic tachycardia (down the AP and retrograde conduction up the His-Purkinje system and AV node). In patients with WPW in which the AP participates in the reentrant circuit, 95% of SVT is due to orthodromic tachycardia and 5% is due to antidromic tachycardia.
Orthodromic tachycardia
When a premature ectopic atrial impulse advances towards the ventricle, it has the potential to block at the AP but conduct down the normal AVN/His Purkinje pathway. The impulse then reenters the AP in a retrograde fashion to perpetuate a circus movement of the impulse. Such reentrant tachycardia is described as orthodromic. Premature ventricular contractions (PVCs) can also initiate orthodromic tachycardia.
In orthodromic tachycardia, the normal pathway is used for ventricular depolarization, and the AP is used for the retrograde conduction essential for reentry. On ECG findings, the delta wave is absent, the QRS complex is normal, and P waves are typically inverted in the inferior and lateral leads.
Orthodromic tachycardia with concealed accessory pathway
Some APs are unable to conduct in an antegrade fashion. These are called concealed APs, because "manifest" preexcitation is a delta wave that is visible on a surface 12-lead ECG. (Technically, concealed pathways should not be classified as a WPW syndrome, because there is no delta wave.) They account for about 30% of all SVTs induced on EPS.
Although no evidence of the pathway is present during sinus rhythm (ie, no preexcitation on ECG), orthodromic tachycardias can occur. Orthodromic tachycardia may also occur when there are two or more accessory connections, and in that case, the retrograde conduction may occur through the AV node, through one of the accessory connections, or through both.
This type of SVT may be difficult to distinguish from the usual AV nodal reentrant tachycardia (AVNRT) on a standard surface ECG. In adults, if the heart rate is higher than 200 bpm or a retrograde P wave is visible in the ST segment (long R-P tachycardia), a concealed AP-mediated orthodromic reentrant tachycardia (ORT) may be the diagnosis. However, this determination is most accurately made with electrophysiologic studies (EPS), or if SVT terminates with a single PVC. Other differentiating factors include the following [10] :
-
Anterior AVNRT: Presence of pseudo r' wave in lead V1, or a pseudo S wave in leads II, III, and aVF
-
Posterior AVNRT: Presence of a more than 20-ms difference in R-P interval between leads I and III
-
AVNRT: Presence of an AV block or AV dissociation (uncommon and of short duration) excludes the presence of an AP; development of a bundle branch block with an unchanged atrial-atrial (AA) or His-His (HH) interval
-
Orthodromic AVRT: Development of a bundle branch block in the presence of a significant change in the ventriculoatrial (VA) interval (diagnostic), with localization of the AP to the same side as the block.
Antidromic tachycardia
Less commonly, a shorter refractory period in the AP may cause blockade of an ectopic atrial impulse in the normal pathway, with antegrade conduction down the AP and then retrograde reentry of the normal AV nodal pathway. This type of tachycardia is called antidromic tachycardia.
On ECG, the QRS is wide, reflecting an exaggeration of the delta wave during sinus rhythm (ie, wide-QRS tachycardia). Such tachycardias are difficult to differentiate from ventricular tachycardias and often have a slurred R wave upstroke with QRS duration longer than 160 ms.
Only about 5% of the tachycardias in patients who have WPW syndrome are antidromic tachycardias; the remaining 95% are orthodromic. Even when the AP conducts solely in a retrograde fashion, it can still participate in the reentrant circuit and produce an orthodromic AV reciprocating tachycardia with a narrow QRS morphology. The presence of an antidromic tachycardia should prompt a careful search for a second bypass tract. [10, 11] About 10-15% of patients with WPW have a second pathway. [11]
Etiology
APs are considered congenital phenomena that are related to a failure of insulating tissue maturation within the AV ring—even though their manifestations are often detected in later years, making them appear to be "acquired." On rare occasions, acquired WPW syndrome has occurred in patients who have undergone congenital heart surgery, which may be owing to an acquired functional epicardial AV connection. [12]
Family studies, as well as molecular genetic investigations, indicate that WPW syndrome, along with associated preexcitation disorders, may have a genetic component. It may be inherited as a familial trait, with or without associated congenital heart defects (CHDs) [13] ; 3.4% of those with WPW syndrome have first-degree relatives with preexcitation.
The familial form is usually inherited as a mendelian autosomal dominant trait. Although rare, mitochondrial inheritance has also been described. The syndrome may also be inherited with other cardiac and noncardiac disorders, such as familial atrial septal defects, familial hypokalemic periodic paralysis, and tuberous sclerosis.
Clinicians have long recognized the association of WPW syndrome with autosomal dominant familial hypertrophic cardiomyopathy. However, only comparatively recently was a genetic substrate linking hypertrophic cardiomyopathy to WPW syndrome and skeletal myopathy described. [2]
Patients with mutations in the gamma 2 subunit of adenosine monophosphate (AMP)-activated protein kinase (PRKAG2) develop cardiomyopathy characterized by ventricular hypertrophy, WPW syndrome, AV block, and progressive degenerative conduction system disease. The mutation is believed to produce disruption of the annulus fibrosus by accumulation of glycogen within myocytes, which causes preexcitation. This is thought to be the case in Pompe disease, Danon disease, and other glycogen-storage diseases.
Infantile Pompe disease or glycogen-storage disease type II is a fatal genetic muscle disorder that is caused by deficiency of acid alpha-glucosidase (GAA). These patients have a shortened PR interval, large left ventricular (LV) voltages, and an increased QT dispersion (QTd).
More recently, investigators appeared to have identified a novel locus in a family with WPW, MYH6 p.E1885K. [14] All of the family members with WPW but none of the unaffected relatives demonstrated this variant. MYH6 variants have been associated with atrial septal defects, cardiomyopathies, and sick sinus syndrome. [14]
Mutations in the lysosome-associated membrane protein 2 (LAMP2), which cause accumulation of cardiac glycogen, are thought to be the etiology of a significant number of hypertrophic cardiomyopathies in children, especially when skeletal myopathy, WPW syndrome, or both are present.
For example, Danon disease is an X-linked lysosomal cardioskeletal myopathy; males are more often and more severely affected than females. It is caused by mutations in the LAMP2 that produce proximal muscle weakness and mild atrophy, left ventricle hypertrophy, WPW syndrome, and intellectual disability.
Patients with the Ebstein anomaly may develop WPW syndrome. They frequently have multiple accessory bypass tracts, mostly on the right, in the posterior part of the septum or the posterolateral wall of the right ventricle. The orthodromic reciprocating tachycardia in such patients often exhibits right bundle-branch block (RBBB) and a long ventriculoatrial (VA) interval.
Preexcitation can be surgically created, as in certain types of Bjork modifications of the Fontan procedure, if atrial tissue is flapped onto and sutured to ventricular tissue. Certain tumors of the AV ring, such as rhabdomyomas, may also cause preexcitation.
Epidemiology
United States statistics
The prevalence of ventricular preexcitation is thought to be 0.1-0.3%, or 1-3 per 1000 people in the general population. Estimates of arrhythmia incidence in patients with preexcitation vary widely, ranging from 12% to 80% in several surveys.
The incidence of preexcitation and WPW syndrome ranges from 0.1 to 3 cases per 1000 population (average, 1.5 cases per 1000 population) in otherwise healthy persons. This includes only patients with manifest preexcitation (delta wave evident on surface 12-lead ECG). About 60-70% of these individuals have no other evidence of heart disease. Approximately four newly diagnosed cases of WPW syndrome per 100,000 population occur each year.
In a review of ECG findings from 22,500 healthy aviation personnel, 0.25% exhibited findings consistent with the WPW pattern, with a 1.8% reported incidence of tachycardia.
The location of the accessory pathways (APs), in descending order of frequency, is (1) 53%, the left free wall, (2) 36%, posteroseptal, (3) 8%, right free wall, and (4) 3%, anteroseptal. The presence of concealed APs accounts for approximately 30% of patients with apparent SVT referred for electrophysiologic studies (EPS). These patients do not have "classic" WPW syndrome because no delta wave is present, but they do have the potential for orthodromic tachycardia.
Approximately 80% of patients with WPW syndrome have a reciprocating tachycardia, 15-30% will develop atrial fibrillation (AF), and 5% have atrial flutter. VT is uncommon. Patients with mitral valve prolapse have an association with WPW, but the mechanism is unclear.
International statistics
Worldwide, the incidence and prevalence of WPW syndrome parallel those seen in the United States.
Age-related demographics
WPW syndrome is found in persons of all ages. Most patients with WPW syndrome present during infancy. However, a second peak of presentation is noted in school-aged children and in adolescents. This interesting bimodal age distribution is due to permanent or transitory loss of preexcitation during infancy in some patients and during late adolescence in others.
The prevalence of WPW syndrome decreases with age as a consequence of apparent attenuation of conduction speed in the AP. About one fourth of patients lose preexcitation over a 10-year period, probably as a result of fibrotic changes at the site of insertion of the accessory bypass tract with loss of electrical conduction properties between cardiac chambers. Cases have been described in which ECG evidence of preexcitation disappears completely. One tenth of patients with concealed APs lose retrograde conduction over 10 years.
In asymptomatic patients, antegrade conduction across the AP may spontaneously disappear with advancing age (one fourth of patients lose antegrade bypass tract conduction over 10 years).
In patients with abnormal ECG findings indicative of WPW syndrome, the frequency of SVT paroxysms increases from 10% in people aged 20-39 years to 36% in people older than 60 years. [15] Overall, about 50% of patients with WPW develop tachyarrhythmias.
Sex-related demographics
WPW pattern appears to affect the two sexes equally; however, WPW syndrome has been found to be more frequent in males. One study documented a male-to-female ratio of approximately 2:1. Another reported 1.4 cases of WPW syndrome per 1000 men and 0.9 cases per 1000. A third study found a 3.5-fold higher prevalence of WPW syndrome in men.
Race-related demographics
No clear racial predilection appears to exist.
Prognosis
Once identified and appropriately treated, WPW syndrome is associated with an excellent prognosis, including the potential for permanent cure through radiofrequency (RF) catheter ablation.
Asymptomatic patients with only preexcitation on ECG generally have a very good prognosis. Many develop symptomatic arrhythmias over time, which can be prevented with prophylactic EPS and RF catheter ablation. [16] Patients with a family history of sudden cardiac death (SCD) or significant symptoms of tachyarrhythmias or cardiac arrest have worse prognoses. However, once definitive therapy is performed, including curative ablation, the prognosis is once again excellent.
Noninvasive risk stratification (eg, Holter monitoring, exercise stress test) [17] can be useful if abrupt and complete loss of preexcitation occurs with exercise or procainamide infusion. However, this is not an absolute predictor for the absence of arrhythmic episodes.
Mortality/morbidity
Mortality in WPW syndrome is rare and is related to SCD. The incidence of SCD in WPW syndrome is approximately 1 in 100 symptomatic cases when followed for up to 15 years. Although relatively uncommon, SCD may be the initial presentation in as many as 4.5% of cases.
Even in patients with asymptomatic WPW, the risk of SCD is increased above that of the general population. Medical therapy with agents such as digoxin may increase this risk if the patient has AF or atrial flutter by favoring atrial-to-ventricular conduction over the bypass tract rather than the AV node. The risk in asymptomatic patients is low and can be reduced further with prophylactic catheter ablation of the accessory pathway (EPS and RF ablation).
Other factors that appear to influence the risk of SCD are the presence of multiple bypass tracts, short AP refractory periods (< 240 ms), AF and atrial flutter, or a family history of premature sudden death. SCD is unusual without preceding symptoms.
The cause of SCD in WPW syndrome is rapid conduction of AF to the ventricles via the AP, resulting in ventricular fibrillation (VF). AF develops in one fifth to one third of patients with WPW syndrome; the reasons for this and the effects of AP ablation on its development are unclear.
However, a study hypothesized that two mechanisms are involved in the pathogenesis of AF in patients with WPW syndrome: one is related to the AP that predisposes the atria to fibrillation, and the other is independent from the AP and is related to increased atrial vulnerability present in these individuals. [18] Notably, AF may still occur and be symptomatic in some patients after successful ablation of the bypass tract, [11, 19] but AF does not then carry the same associated risk of SCD.
In a study that evaluated the long-term (median, 6.9 y) natural history of WPW in adult patients treated with (n = 872) and without catheter ablation (n = 1461) compared to a control group (n = 11,175), Bunch et al found similarly low death rates but higher incident AF risk in patients with WPW versus the control group. [20] The risk of long-term mortality was higher in those who did not undergo ablation compared to the group treated with ablation, whereas the risk of incident AF was higher in the ablation group. Thus, ablation did not reduce the risk of AF.
According to the literature, risk factors for the development of AF in the setting of WPW syndrome include advancing age (two peak ages for AF occurrence are recognized, one at 30 years and the other at 50 years), male sex, and prior history of syncope. [21]
Certain factors increase the likelihood of VF, including rapidly conducting APs and multiple pathways. [22] Cases have also been reported in association with esophageal studies, digoxin, and verapamil. A few reports document spontaneous VF in WPW syndrome, and SVT may degenerate into AF, thus leading to VF [23] ; however, both scenarios are rare in pediatric patients.
Morbidity may be related to rapid near syncope or syncopal arrhythmias. Even when syncope is absent, the arrhythmia episodes may be highly symptomatic. In most patients, the SVT is well tolerated and is not life threatening. However, the potential for syncope, hemodynamically compromising rhythms, or sudden death may prevent patients with WPW syndrome from participating in competitive sports or hazardous occupations until the substrate is definitively addressed and cured by a catheter ablation procedure.
Complications
Complications include the following:
-
Tachyarrhythmia
-
Palpitations
-
Dizziness or syncope
-
Sudden cardiac death
-
Complications of drug therapy (eg, proarrhythmia, organ toxicity)
-
Complications associated with invasive procedures and surgery
-
Recurrence
Patient Education
Patient education is of paramount importance in patients with WPW syndrome. This is especially true in asymptomatic young patients who have been told of their abnormal ECG results. Periodic follow-up care of such patients is necessary, along with thoughtful discussions of consideration for EPS and prophylactic catheter ablation.
Urge patients to carry a sample ECG in sinus rhythm and a medical identification bracelet in case of cardiac arrest.
Educate patients who are being treated with drug therapy thoroughly regarding the disease and the type of medications they are taking. Such patients must be taught the following:
-
How to recognize disease recurrence
-
How to perform vagal maneuvers, when needed
-
To keep their follow-up appointments
-
To identify the adverse effects of antiarrhythmic drugs
-
Typically, to avoid aggressive competitive sports
-
To learn about ablative options and the indications for ablation
Patients with WPW syndrome should also educate their family members, and their siblings should be screened for preexcitation with 12-lead ECG.
-
Wolff-Parkinson-White Syndrome. Classic Wolff-Parkinson-White electrocardiogram with short PR, QRS >120 ms, and delta wave.
-
Wolff-Parkinson-White Syndrome. Preexcited atrial fibrillation.
-
Wolff-Parkinson-White Syndrome. Variants of Wolff-Parkinson-White syndrome (unusual accessory pathways).
-
Wolff-Parkinson-White Syndrome. Accessory pathway potential and local AV fusion at successful RF ablation site with loss of preexcitation and return of normal HV interval.
-
Wolff-Parkinson-White Syndrome. Electrocardiogram of an asymptomatic 17-year-old male who was incidentally discovered to have Wolff-Parkinson-White pattern. It shows sinus rhythm with evident preexcitation. To locate the accessory pathway (AP), initial 40 ms of QRS (delta wave) is evaluated. Note that delta wave is positive in I and aVL, negative in III and aVF, isoelectric in V1, and positive in rest of precordial leads. Therefore, this is likely posteroseptal AP.
-
Wolff-Parkinson-White Syndrome. 12-lead electrocardiogram from an asymptomatic 7-year-old boy with Wolff-Parkinson-White pattern. Delta waves are positive in I and aVL; negative in II, III, and aVF; isoelectric in V1; and positive in the rest of the precordial leads. This predicts a posteroseptal location for an accessory pathway.
-
Wolff-Parkinson-White Syndrome. 12-lead electrocardiogram showing short PR interval and delta waves consistent with the presence of an accessory pathway.







