Practice Essentials
Bladder cancer is a common urologic cancer that has the highest recurrence rate of any malignancy. The most common type is urothelial carcinoma (UC). Other types include squamous cell carcinoma (see the image below) and adenocarcinomas.
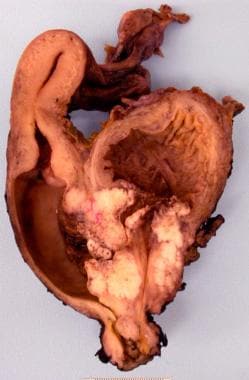 Bladder cancer. Cross-section through the bladder, uterus, and vagina with squamous cell carcinoma of the bladder infiltrating through the bladder wall into the vaginal wall.
Bladder cancer. Cross-section through the bladder, uterus, and vagina with squamous cell carcinoma of the bladder infiltrating through the bladder wall into the vaginal wall.
Signs and symptoms
Clinical manifestations of bladder cancer are as follows:
-
Painless gross hematuria - Approximately 80-90% of patients; classic presentation
-
Irritative bladder symptoms (eg, dysuria, urgency, frequency of urination) - 20-30% of patients
-
Pelvic or bony pain, lower-extremity edema, or flank pain - In patients with advanced disease
-
Palpable mass on physical examination - Rare in superficial bladder cancer
See Presentation for more detail.
Diagnosis
Urine studies include the following:
-
Urinalysis with microscopy
-
Urine culture to rule out infection, if suspected
-
Voided urinary cytology
-
Urinary tumor marker testing
Urinary cytology:
-
Standard noninvasive diagnostic method
-
Low sensitivity for low-grade and early stage cancers
-
Fluorescence in situ hybridization (FISH) may improve the accuracy of cytology
Cystoscopy:
-
The primary modality for the diagnosis of bladder carcinoma
-
Permits biopsy and resection of papillary tumors
Upper urinary tract imaging:
-
Usually necessary for the hematuria workup, but may be omitted on the basis of risk stratification
-
American Urologic Association Best Practice Policy recommends computed tomography (CT) scanning of the abdomen and pelvis with contrast, with preinfusion and postinfusion phases
-
Imaging is ideally performed with CT urography, using multidetector CT
-
Ultrasonography is commonly used, but it may miss urothelial tumors of the upper tract and small stones
The diagnostic strategy for patients with negative cystoscopy is as follows:
-
Negative urine cytology and FISH - Routine follow-up
-
Negative urine cytology, positive FISH - Increased frequency of surveillance
-
Positive urine cytology, positive or negative FISH - Cancer until proven otherwise
No blood tests are specific for bladder cancer, but a general evaluation is necessary prior to initiating therapy with intravesical bacillus Calmette-Guérin (BCG). Laboratory tests include the following:
-
Complete blood count (CBC)
-
Liver function tests
-
Bony fraction of alkaline phosphatase assay (if bone metastasis suspected)
-
Kidney function studies
See Workup for more detail.
Management
The treatment of non–muscle-invasive bladder cancer (Ta, T1, carcinoma in situ [CIS]) begins with transurethral resection of bladder tumor (TURBT). Subsequent treatment is as follows:
-
Small-volume, low-grade Ta bladder cancer - An immediate single, postoperative dose of intravesical chemotherapy
-
Intermediate-risk bladder cancer (recurrent low grade, high volume low grade, high-grade Ta) – Intravesical chemotherapy or BCG
-
High-risk Ta, T1, and CIS urothelial carcinoma - Intravesical BCG
-
Persistent or recurrent high-risk disease - Repeat resection prior to additional intravesical therapy; consider cystectomy for high-risk disease
The treatment of muscle-invasive bladder cancer is as follows:
-
Radical cystoprostatectomy in men
-
Radical cystectomy with anterior pelvic exenteration in women
-
Bilateral pelvic lymphadenectomy (PLND), standard or extended
-
Creation of a urinary diversion (eg, ileal conduit, Indiana pouch, orthotopic bladder substitution)
-
Neoadjuvant chemotherapy - May improve cancer-specific survival
Alternatively, a bladder-sparing approach of TURBT followed by concurrent radiation therapy and systemic chemotherapy (trimodality therapy) may be used.
Chemotherapeutic regimens for locally advanced or metastatic bladder cancer include the following [1] :
-
Gemcitabine and cisplatin (GC) with avelumab maintenance
-
Dose-dense methotrexate, vinblastine, doxorubicin (Adriamycin), and cisplatin (DDMVAC) with avelumab maintenance
Inhibitors of programmed cell death 1 (PD-1) protein and its ligands PD-L1 and PD-L2, are first-line agents in patients with metastatic urothelial carcinoma who are not candidates for platinum-containing chemotherapy, and second-line agents for those with disease progression despite cisplatin-based chemotherapy. Agents in this category include the following [1] :
-
Pembrolizumab
-
Atezolizumab
-
Nivolumab
-
Avelumab
Erdafitinib, a fibroblast growth factor receptor inhibitor, is approved for locally advanced or metastatic urothelial carcinoma that has FGFR2 or FGFR3 genetic alterations and has progressed during or following at least 1 line of prior platinum-containing chemotherapy.
See Treatment and Medication for more detail.
See Bladder Cancer Treatment Protocols for more information on this topic. Go to Oncology Decision Point for expert commentary on bladder cancer treatment decisions and related guidelines. To view a multidisciplinary tumor board case discussion, see Memorial Sloan Kettering e-Tumor Boards: Muscle Invasive Bladder Cancer.
For patient education information, see Bladder Cancer.
Background
Bladder cancer is a common urologic cancer. Almost all bladder cancers originate in the urothelium, which is a 3- to 7-cell mucosal layer within the muscular bladder.
Urothelial carcinoma
In North America, South America, Europe, and Asia, the most common type of urothelial tumor is urothelial carcinoma (UC); it constitutes more than 90% of bladder cancers in those regions. UC can arise anywhere in the urinary tract, including the renal pelvis, ureter, bladder, and urethra, but it is usually found in the urinary bladder. Carcinoma in situ (CIS) is frequently found in association with high-grade or extensive UC. (See the image below.)
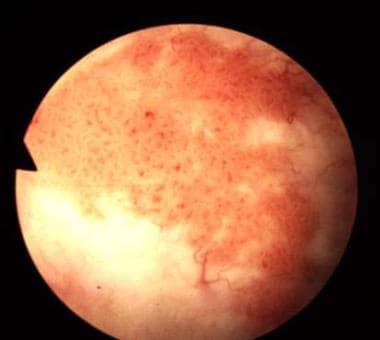 Bladder cancer. The classic appearance of carcinoma in situ is as a flat, velvety patch. However, using special staining techniques such as 5-aminolevulinic acid, it has been shown that significant areas of carcinoma in situ are easily overlooked by conventional cystoscopy. Courtesy of Abbott and Vysis Inc.
Bladder cancer. The classic appearance of carcinoma in situ is as a flat, velvety patch. However, using special staining techniques such as 5-aminolevulinic acid, it has been shown that significant areas of carcinoma in situ are easily overlooked by conventional cystoscopy. Courtesy of Abbott and Vysis Inc.
Squamous cell carcinoma
Squamous cell carcinoma (SCC) is the second most common cell type associated with bladder cancer in industrialized countries. In the United States, around 5% of bladder cancers are SCCs. [2] Worldwide, however, SCC is the most common form of bladder cancer, accounting for 75% of cases in developing nations (see Epidemiology).
In the United States, the development of SCC is associated with persistent inflammation from long-term indwelling Foley catheters and bladder stones, as well as, possibly, infections. In developing nations, SCC is often associated with bladder infection by Schistosoma haematobium (see Etiology).
Other types of bladder cancer
Approximately 2% of bladder cancers are adenocarcinomas. Nonurothelial primary bladder tumors are extremely rare and may include small cell carcinoma, carcinosarcoma, primary lymphoma, and sarcoma (see Pathophysiology). Small cell carcinoma of the urinary bladder accounts for only 0.3-0.7% of all bladder tumors. High-grade urothelial carcinomas can also show divergent histologic differentiation, such as squamous, glandular, neuroendocrine, and sarcomatous features.
Phenotypes
Clinical and pathologic data indicate that at least 3 different phenotypes, as follows, exist in urothelial carcinoma [2, 3] :
-
Low-grade proliferative lesions that develop into non–muscle-invasive tumors; these account for approximately 80% of bladder cancers
-
Highly proliferative invasive tumors with a propensity to metastasize
-
CIS, which can penetrate the lamina propria and eventually progress
Clinical course
The clinical course of bladder cancer is marked by a broad spectrum of aggressiveness and risk. Low-grade, superficial bladder cancers have minimal risk of progression to death; however, high-grade non–muscle-invasive cancers frequently progress and muscle-invasive cancers are often lethal (see Prognosis).
The classic presentation of bladder cancer is painless gross hematuria, which is seen in approximately 80-90% of patients. Physical examination results are often unremarkable (see Presentation). Cystoscopy, cytology, and biopsy when necessary are the principal diagnostic tests (see Workup).
Upon presentation, 55-60% of patients have low-grade, noninvasive disease, which is usually treated conservatively with transurethral resection of bladder tumor (TURBT) and periodic cystoscopy. Intravesical agents may also be given selectively to decrease the frequency of recurrences. The remaining patients have high-grade disease, of which 50% is muscle invasive and is typically treated with radical cystectomy or with trimodality therapy (ie, TURBT followed by concurrent radiation therapy and systemic chemotherapy; see Treatment).
Carcinoma in situ (CIS) is managed by TURBT and instillation of chemotherapeutic or immunotherapeutic agents—most commonly, immunotherapy with bacillus Calmette-Guérin (BCG)—into the bladder via catheter. These intravesical treatments are not effective patients in whom cancer has invaded the bladder wall muscle; those cases require cystectomy or a combination of radiation therapy and chemotherapy (see Treatment).
Bladder cancer has the highest recurrence rate of any malignancy. Although most patients with bladder cancer can be treated with organ-sparing therapy, most experience either recurrence or progression, creating a great need for accurate and diligent surveillance (see Treatment).
For more information on bladder cancer, see the following:
Anatomy
The bladder is an extraperitoneal muscular urine reservoir that lies behind the pubis symphysis in the pelvis. At the dome of the bladder lies the median umbilical ligament, a fibrous cord that is anchored to the umbilicus and that represents the obliterated urachus (allantois). The ureters, which transport urine from kidney to bladder, approach the bladder obliquely and posterosuperiorly, entering at the trigone (the area between the interureteric ridge and the bladder neck). The intravesical ureteral orifices are roughly 2-3 cm apart and form the superolateral borders of the trigone.
In males, the seminal vesicles, vas deferens, ureters, and rectum border the inferoposterior aspect of the bladder. Anterior to the bladder is the space of Retzius, which is composed of fibroadipose tissue and the prevesical fascia. The dome and posterior surface of the bladder are covered by parietal peritoneum, which reflects superiorly to the seminal vesicles and is continuous with the anterior rectal peritoneum. In females, the posterior peritoneal reflection is continuous with the uterus and vagina.
The vascular supply to the bladder arrives primarily via the internal iliac (hypogastric) arteries, branching into the superior, middle, and inferior vesical arteries, which are often recognizable as lateral and posterior pedicles. The arterial supply also arrives via the obturator and inferior gluteal artery and, in females, via the uterine and vaginal arteries. Bladder venous drainage is a rich network that often parallels the named arterial vessels, most of which ultimately drain into the internal iliac vein.
Initial lymphatic drainage from the bladder is primarily into the external iliac, obturator, internal iliac (hypogastric), and common iliac nodes. Following the drainage to these sentinel pelvic regions, spread may continue to the presacral, paracaval, interaortocaval, and para-aortic lymph node chains.
Almost all bladder cancers originate in the urothelium, which is a 3- to 7-cell mucosal layer within the muscular bladder. Squamous cell carcinoma of the bladder can involve multiple sites; however, the lateral wall and trigone are more commonly involved by this tumor. All small cell carcinomas of the urinary system identified so far have been located in the urinary bladder, most commonly in the dome and vesical lateral wall. [4]
See Bladder Anatomy.
Pathophysiology
Bladder cancer is often described as a polyclonal field change defect with frequent recurrences due to a heightened potential for malignant transformation. However, bladder cancer has also been described as resulting from implantation of malignant cells that have migrated from a previously affected site. The latter occurs less often and may account for only a small percentage of cases.
Use of the common term superficial bladder cancer should be discouraged. The term implies a harmless nature, which is misleading in many instances. Because it was used to describe the disparate disorders of low-grade papillary bladder cancer and the markedly more aggressive form, carcinoma in situ (CIS), the World Health Organization (WHO) has recommended it be abandoned.
In its place, the term non–muscle-invasive bladder cancer should be used and qualified with the appropriate American Joint Committee on Cancer stage (ie, Ta, T1, Tis). Stage T1 cancer invades lamina propria but not the muscle of the bladder. High-grade T1 tumor associated with CIS carries a relatively high risk for disease recurrence and progression (approximately 60%).
The current WHO/International Society of Urological Pathology (ISUP) system classifies bladder cancers as low grade or high grade. [5] Tumors are also classified by growth patterns: papillary (70%), sessile or mixed (20%), and nodular (10%). (See the images below.)
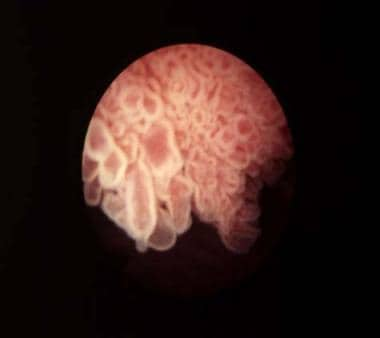 Bladder cancer. Papillary bladder tumors such as this one are typically of low stage and grade (Ta-G1). Courtesy of Abbott and Vysis Inc.
Bladder cancer. Papillary bladder tumors such as this one are typically of low stage and grade (Ta-G1). Courtesy of Abbott and Vysis Inc.
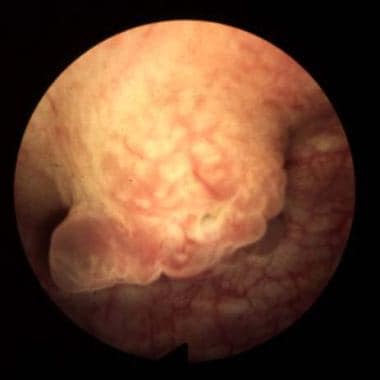 Bladder cancer. Sessile lesions as shown usually invade muscle, although occasionally a tumor is detected at the T1-G3 stage prior to muscle invasion. Courtesy of Abbott and Vysis Inc.
Bladder cancer. Sessile lesions as shown usually invade muscle, although occasionally a tumor is detected at the T1-G3 stage prior to muscle invasion. Courtesy of Abbott and Vysis Inc.
Urothelial carcinoma
Urothelial carcinoma (UC) arises from stem cells that are adjacent to the basement membrane of the epithelial surface. Depending on the genetic alterations that occur, these cells may follow different pathways in the expression of their phenotype.
The most common molecular biologic pathway for UCs involves the development of a papillary tumor that projects into the bladder lumen and, if untreated, eventually penetrates the basement membrane, invades the lamina propria, and then continues into the bladder muscle, where it can metastasize.
This progression occurs with high-grade cancers only. Low-grade cancers rarely, if ever, progress and are thought to have a distinct molecular pathway, different from the high-grade cancers and CIS.
CIS, which constitutes 10% of UCs, follows a different molecular pathway. This is a flat, noninvasive, high-grade UC that spreads along the surface of the bladder, staying superficial to the basement membrane. Over time, this may progress to an invasive form of cancer that behaves the same as invasive UC.
Many urothelial tumors are primarily UC but contain areas of squamous differentiation, squamous cell carcinoma (SCC), or adenocarcinoma.
Squamous cell carcinoma
SCC of the urinary bladder is a malignant neoplasm that is derived from bladder urothelium and has a pure squamous phenotype. [6, 7, 8] SCC of the bladder is essentially similar to squamous cell tumors arising in other organs. Because many urothelial carcinomas contain a minor squamous cell component, a diagnosis of SCC of the bladder should be rendered only when the tumor is solely composed of squamous cell components, with no conventional urothelial carcinoma component.
Reportedly, SCC has less of a tendency for nodal and vascular distant metastases than does urothelial carcinoma. [9, 10]
Rare forms of bladder cancer
Adenocarcinomas account for less than 2% of primary bladder tumors. These lesions are observed most commonly in exstrophic bladders and are often associated with malignant degeneration of a persistent urachal remnant.
Other rare forms of bladder cancer include leiomyosarcoma, rhabdosarcoma, carcinosarcoma, lymphoma, and small cell carcinoma. Leiomyosarcoma is the most common sarcoma of the bladder. Rhabdomyosarcomas most commonly occur in children. Carcinosarcomas are highly malignant tumors that contain a combination of mesenchymal and epithelial elements. Primary bladder lymphomas arise in the submucosa of the bladder. Except for lymphomas, all these rare bladder cancers carry a poor prognosis.
Small cell carcinoma of the urinary bladder is a poorly differentiated, malignant neoplasm that originates from urothelial stem cells and has variable expression of neuroendocrine markers. Morphologically, it shares features of small cell carcinoma of other organs, including the lung.
Genetic factors in pathogenesis
Divergent, yet interconnected and overlapping, molecular pathways are likely responsible for the development of noninvasive and invasive bladder tumors. Somatic mutations in fibroblast growth receptor3 (FGFR-3) and tumor protein p53 (TP53) in tumor cells appear to be important early molecular events in the noninvasive and invasive pathways, respectively.
FGFR-3, Ras, and PIK3CA mutations occur with high frequency in noninvasive tumors, leading to upregulation of Akt and mitogen-activated protein kinase (MAPK). [11, 12] Loss of heterozygosity (LOH) on chromosome 9 is among the most frequent genetic alterations in bladder tumors and is considered an early event. [13]
Large numbers of genomic changes have been detected using karyotyping and comparative genomic hybridization (CGH) analysis in urothelial carcinoma. Numerically common are losses of 2q, 5q, 8p, 9p, 10q, 18q, and Y. Gains of 1q, 5p, 8q, and 17q are frequently present, and high-level amplifications can be found; however, the target genes in the regions of amplifications have not been conclusively identified. [14]
Alterations in the TP53 gene are noted in approximately 60% of invasive bladder cancers. Progression-free survival is significantly shorter in patients with TP53 mutations and is an independent predictor of death among patients with muscle-invasive bladder cancer. [15]
Additionally, alterations in retinoblastoma (Rb), PTEN, and p16 are common in high-grade invasive cancers. [16] Overexpression of JUN, MAP2K6, STAT3, and ICAM1 and molecules associated with survival (Bcl-2, caspase-3, p53, survivin), as well as insensitivity to antigrowth signals (p53, p21, p16, pRB), has been demonstrated. [17]
In advanced disease, multiple mechanisms may lead to tumor progression. These include those that promote proliferation, survival, invasion, and metastasis, as well as those that involve deficiencies in DNA damage repair and the finding of stemlike cells.
In addition to tumor cell alterations, the microenvironment may promote tumor growth by paracrine influences, including vascular endothelial growth factor (VEGF) production and aberrant E-cadherin expression. Finally, a growing body of research over the last decade indicates that epigenetic alterations may silence tumor suppressor genes and that they represent important events in tumor progression. [18, 19, 20]
Etiology
Up to 80% of bladder cancer cases are associated with environmental exposure. Tobacco use is by far the most common cause of bladder cancer in the United States and is increasing in importance in some developing countries. Smoking duration and intensity are directly related to increased risk. [21, 22, 2]
The risk of developing bladder carcinoma is 2-6 times greater in smokers than in nonsmokers. This risk appears to be similar between men and women. [23] Nitrosamine, 2-naphthylamine, and 4-aminobiphenyl are possible carcinogenic agents found in cigarette smoke.
A number of occupations involve exposure to substances that may increase risk for bladder cancer. Of occupationally related bladder cancer cases, the incidence rate is highest in workers exposed to aromatic amines, while mortality is greatest in those exposed to polycyclic aromatic hydrocarbons and heavy metals. [24]
Numerous occupations associated with diesel exhaust, petroleum products, and solvents (eg, auto work, truck driving, plumbing, leather and apparel work, rubber and metal work) have also been associated with an increased risk of bladder cancer. In addition, increased bladder carcinoma risk has been reported in persons, including the following, who work with organic chemicals and dyes:
-
Beauticians
-
Dry cleaners
-
Painters
-
Paper production workers
-
Rope-and-twine industry workers
-
Dental workers
-
Physicians
-
Barbers
People living in urban areas are also more likely to develop bladder cancer. The etiology in these cases is thought to be multifactorial, potentially involving exposure to numerous carcinogens.
Arsenic exposure may be a factor in the development of bladder cancer. Results of a population-based case-control study in Maine, New Hampshire, and Vermont support an association between low-to-moderate levels of arsenic in drinking water and bladder cancer risk in those states, where incidence rates of bladder cancer have long been about 20% higher than in the United States overall. [25] A likely source of the arsenic is residue of arsenic-based pesticides, which were used extensively on crops such as blueberries, apples, and potatoes in that region from the 1920s through to the 1950s. [26]
Several medical risk factors are associated with an increased risk of bladder cancer, including the following:
-
Radiation treatment of the pelvis
-
Chemotherapy with cyclophosphamide - Increases the risk of bladder cancer via exposure to acrolein, a urinary metabolite of cyclophosphamide [27]
-
Spinal cord injuries requiring long-term indwelling catheters - A 16- to 20-fold increase in the risk of developing SCC of the bladder
Although certain common genetic polymorphisms appear to increase susceptibility in persons with occupational exposure associated with increased bladder cancer risk, [28] no convincing evidence exists for a hereditary factor in the development of bladder cancer. Nevertheless, familial clusters of bladder cancer have been reported.
Schistosomiasis
In many developing countries, particularly in the Middle East, Schistosoma haematobium infection causes most cases of bladder SCC. In a study from Egypt, 82% of patients with bladder carcinoma harbored S haematobium eggs in the bladder wall. In egg-positive patients, the tumor tended to develop at a younger age (with SCC predominant) than it did in egg-negative persons. A higher degree of adenocarcinoma has also been reported in schistosomal-associated bladder carcinomas. [29]
Along with S haematobium, the species S mansoni and S japonicum are responsible for schistosomiasis in humans. The eggs reside in the pelvic and mesenteric venous plexus. In the bladder, a severe inflammatory response and fibrosis secondary to the deposition of Schistosoma eggs is common. (See the image below.)
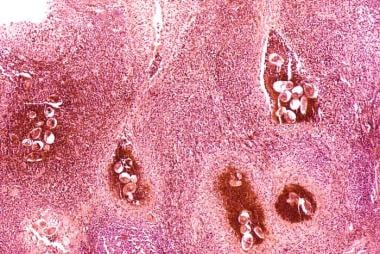 Bladder cancer. Histopathology of bladder shows eggs of Schistosoma haematobium surrounded by intense infiltrates of eosinophils and other inflammatory cells.
Bladder cancer. Histopathology of bladder shows eggs of Schistosoma haematobium surrounded by intense infiltrates of eosinophils and other inflammatory cells.
The eggs are found embedded in the lamina propria and muscularis propria of the bladder wall. Many of the eggs are destroyed by host reaction and become calcified, resulting in a lesion commonly known as a sandy patch, which appears as a granular, yellow-tan surface lesion.
In normal epithelial cells, S haematobium total antigen reportedly induces increased proliferation, migration, and invasion and decreases apoptosis. [30] Keratinous squamous metaplasia has been associated with the increased risk of developing SCC, with approximately one half of the cases arising subsequent to the metaplasia. [31, 32]
The majority of schistosomiasis-related cases of SCC will arise in the setting of chronic cystitis. [33] Chronic irritation secondary to lithiasis, [6, 7] urinary retention, and indwelling catheters has also been linked to the development of SCC. [7]
Other squamous cell carcinoma risk factors
Having bladder diverticula may increase an individual’s chance of developing SCC. [34] Rarely, bacillus Calmette-Guerin (BCG) treatment for CIS has been reported to lead to development of SCC. [35] Development of bladder cancer at a younger age has been associated with bladder exstrophy. [36, 37, 38, 39] SCC has also been described in urachal remnants. [40, 41, 42, 43, 44]
Coffee consumption does not increase the risk of developing bladder cancer. Early studies of rodents and a minority of human studies suggested a weak connection between artificial sweeteners (eg, saccharin, cyclamate) and bladder cancer; however, most recent studies show no significant correlation.
Epidemiology
Occurrence in the United States
The American Cancer Society estimates that 82,290 new cases of bladder cancer will be diagnosed in the United States in 2023 and that 16,710 people will die of the disease. [45] The incidence of bladder cancer increases with age, with the median age at diagnosis being 73 years; bladder cancer is rarely diagnosed before age 40 years. [46]
Bladder cancer is about 4 times more common in men than in women. [45] The male predominance in bladder cancer in the United States reflects the prevalence of transitional cell carcinoma (TCC). With small cell carcinoma—in contrast to TCC—the male-to-female incidence ratio is 1:2.
Bladder cancer is the fourth most common cancer in men in the United States, after prostate, lung, and colorectal cancer, but it is not among the top 10 cancers in women. Accordingly, more men than women are expected to die of bladder cancer in 2023, with 12,160 deaths in men versus 4550 in women. [45] Nevertheless, women generally have a worse prognosis than men.
The incidence of bladder cancer is twice as high in White men as in Black men in the United States. However, Blacks have a worse prognosis than Whites. [46, 47]
From 2000 to 2019, incidence and death rates for bladder cancer decreased in most racial and ethnic groups in both men and women in the US. On average, incidence rates decreased by 1.88% annually in men and 1.34% in women; death rates decreased by 2.16% in men and 2.44% in women. However, incidence rates showed a steady increase in American Indian and Alaska Native men and women, and death rates stabilized in Asian American and Pacific Islander men and Hispanic women. [48]
Limited data indicate that small cell carcinoma of the urinary bladder probably has the same epidemiologic characteristics as urothelial carcinoma. Patients are more likely to be male and older than 50 years. [49, 50]
International occurrence
Worldwide, bladder cancer is diagnosed in approximately 275,000 people each year, and about 108,000 die of this disease. In industrialized countries, 90% of bladder cancers are TCC. In developing countries—particularly in the Middle East and Africa—the majority of bladder cancers are SCCs, and most of these cancers are secondary to Schistosoma haematobium infection. Urothelial carcinoma is reported to be the most common urologic cancer in China.
In Africa, the highest incidence of SCC has been seen in schistosomal-endemic areas, notably Sudan and Egypt, where SCC ranges from two thirds to three quarters of all malignant tumors of the bladder. In recent years, a few studies from Egypt have shown a reversal of this trend due to the better control of schistosomiasis in the region, whereas in other parts of Africa the association is unchanged. [10, 51, 52] Increased smoking incidence is believed to have contributed to the shift in Egypt toward TCC, which has a stronger smoking association.
Prognosis
Untreated bladder cancer produces significant morbidity, including the following:
-
Hematuria
-
Dysuria
-
Irritative urinary symptoms
-
Urinary retention
-
Urinary incontinence
-
Ureteral obstruction
-
Pelvic pain
The recurrence rate for superficial TCC of the bladder is high. As many as 80% of patients have at least one recurrence.
The most significant prognostic factors for bladder cancer are grade, depth of invasion, and the presence of CIS. In patients undergoing radical cystectomy for muscle-invasive bladder cancer, the presence of nodal involvement is the most important prognostic factor. To date, there is no convincing evidence of genetic factors affecting outcome. [53]
Non–muscle-invasive bladder cancer has a good prognosis, with 5-year survival rates of 82-100%. The 5-year survival rate decreases with increasing stage, as follows:
-
Ta, T1, CIS – 82-100%
-
T2 – 63-83%
-
T3a – 67-71%
-
T3b – 17-57%
-
T4 – 0-22%
Prognosis for patients with metastatic urothelial cancer is poor, with only 5-10% of patients living 2 years after diagnosis.
The risk of progression, defined as an increased tumor grade or stage, depends primarily on the tumor grade, as follows:
-
Grade I – 2-4%
-
Grade II – 5-7%
-
Grade III – 33-64%
Prognosis in carcinoma in situ
CIS in association with T1 papillary tumor carries a poorer prognosis. It has a recurrence rate of 63-92% and a rate of progression to muscle invasion of 50-75% despite intravesical BCG. Diffuse CIS is an especially ominous finding; in one study, 78% of cases progressed to muscle-invasive disease. [54]
Prognosis in squamous cell carcinoma
Tumor stage, lymph node involvement, and tumor grade have been shown to be of independent prognostic value in SCC. [55, 56] However, pathologic stage is the most important prognostic factor. In one relatively large series of 154 cases, the overall 5-year survival rate was 56% for pT1 and 68% for pT2 tumors. However, the 5-year survival rate for pT3 and pT4 tumors was only 19%. [53]
Several studies have demonstrated grading to be a significant morphologic parameter in SCC. [53] In one series, 5-year survival rates for grade 1, 2, and 3 SCC was 62%, 52%, and 35%, respectively. [53] In the same study of patients undergoing cystectomy, the investigators suggested that a higher number of newly formed blood vessels predicts unfavorable disease outcome.
In SCC, the survival rate appears to be better with radical surgery than with radiation therapy and/or chemotherapy. In locally advanced tumors, however, neoadjuvant radiation improves the outcome. [57] Sex and age have not been prognostically significant in SCC. [53]
Prognosis in small cell carcinoma
Patients with small cell carcinoma of the bladder usually have disease in an advanced stage at diagnosis, and they have a poor prognosis. [58, 59, 60] Overall median survival is only 1.7 years. The 5-year survival rates for stage II, III, and IV disease are 64%, 15%, and 11%, respectively. [61]
Recurrent bladder cancer
Bladder cancer has the highest recurrence rate of any malignancy (ie, 70% within 5 y). Although most patients with bladder cancer can be treated initially with organ-sparing therapy, most experience either recurrence or progression. The underlying genetic changes that result in a bladder tumor occur in the entire urothelium, making the whole lining of the urinary system susceptible to tumor recurrence.
Risk factors for recurrence and progression include the following [62, 63] :
-
Female sex
-
Larger tumor size
-
Multifocality
-
Larger number of tumors
-
High tumor grade
-
Advanced stage
-
Presence of CIS
The time interval to recurrence is also significant. Patients with tumor recurrences within 2 years, and especially with recurrences within 3-6 months, have an aggressive tumor and an increased risk of disease progression.
-
In an ileal conduit, a small segment of ileum is taken out of continuity with the gastrointestinal tract but is maintained on its mesentery. Ureters are anastomosed to one end of this ileal segment, and the other end is brought out as a stoma to the abdominal wall.
-
In an Indiana pouch, a urinary reservoir is created from detubularized right colon and an efferent limb of terminal ileum. Terminal ileum is plicated and brought to the abdominal wall. The continence mechanism is the ileocecal valve.
-
In an orthotopic neobladder, a segment of ileum is used to construct a neobladder, which is connected to the urethra. Orthotopic neobladder most closely restores the natural storage and voiding function of the native bladder.
-
Bladder cancer. The classic appearance of carcinoma in situ is as a flat, velvety patch. However, using special staining techniques such as 5-aminolevulinic acid, it has been shown that significant areas of carcinoma in situ are easily overlooked by conventional cystoscopy. Courtesy of Abbott and Vysis Inc.
-
Bladder cancer. Papillary bladder tumors such as this one are typically of low stage and grade (Ta-G1). Courtesy of Abbott and Vysis Inc.
-
Bladder cancer. Sessile lesions as shown usually invade muscle, although occasionally a tumor is detected at the T1-G3 stage prior to muscle invasion. Courtesy of Abbott and Vysis Inc.
-
Bladder cancer. Photograph in which fluorescence in situ hybridization centromere staining identifies aneuploidy of chromosome 3. Multiple instances of overexpression of the chromosome (note the multiple red dots, which identify centromeres of this chromosome) prove aneuploidy.
-
Bladder cancer. Cross-section through the bladder, uterus, and vagina with squamous cell carcinoma of the bladder infiltrating through the bladder wall into the vaginal wall.
-
Bladder cancer. High power, Pap stain showing high grade urothelial carcinoma on a bladder wash cytology.
-
Bladder cancer, Intermediate power, H&E stain of urothelial carcinoma in situ. The superficial cells shed into the urine and correlate with those seen in cytologic bladder washing or urine cytology.
-
Bladder cancer. High power, H&E stain of high grade urothelial carcinoma. This tumor is now invasive into the muscularis propria (smooth muscle seen in center of image).
-
Bladder cancer. Histopathology of bladder shows eggs of Schistosoma haematobium surrounded by intense infiltrates of eosinophils and other inflammatory cells.
-
Bladder cancer. (A) When infused into the bladder, the optical imaging agent hexaminolevulinate (Cysview) accumulates preferentially in malignant cells. (B) On blue-light cystoscopy, the collection of hexaminolevulinate within tumors is visible as bright red spots. Courtesy of Gary David Steinberg, MD, FACS.








