Practice Essentials
Tularemia is an acute, febrile, granulomatous, infectious zoonosis caused by Francisella tularensis, an aerobic, gram-negative, pleomorphic bacillus. [1] F tularensis is one of the most infectious bacterial species known. [1]
Signs and symptoms
The following are common findings in the various clinical forms of tularemia [1] :
-
Abrupt onset of fever and chills - These symptoms typically last for several days, remit for a brief interval, and then recur
-
Pulse-temperature disassociation [2]
-
Headache
-
Anorexia
-
Malaise and fatigue or prostration
-
Myalgias
-
Cough
-
Vomiting
-
Pharyngitis
-
Abdominal pain
-
Secondary pneumonitis - May occur in 45-83% of patients with the typhoidal form of tularemia
As many as 20% of patients with tularemia have a rash, which may begin as blotchy, macular, or maculopapular and progress to pustular. Erythema nodosum and erythema multiforme are rare.
More specific signs and symptoms are as follows [1] :
-
Ulceroglandular tularemia - Includes painful regional lymphadenopathy and an ulcerated skin lesion
-
Glandular tularemia - Tender lymphadenopathy without evidence of local cutaneous lesions
-
Oculoglandular tularemia - Unilateral conjunctivitis, corneal ulceration, lymphadenopathy, photophobia, lacrimation, lid edema, vision loss (rare)
-
Oropharyngeal tularemia - Stomatitis and exudative pharyngitis or tonsillitis; abdominal pain, nausea, and vomiting; cervical lymphadenopathy; diarrhea; gastrointestinal bleeding
-
Intestinal tularemia - Abdominal pain, vomiting, and diarrhea
-
Pneumonic tularemia - Dry cough, dyspnea, and pleuritic-type chest pain
-
Typhoidal tularemia - Fever, chills, myalgias, malaise, and weight loss
Diagnosis
Serology
The diagnosis of tularemia usually is based on serology results. Tests vary from antibody detection (using latex agglutination or enzyme-linked immunosorbent assay [ELISA] testing) to the examination of a range of polymerase chain reaction (PCR) assay products. [1, 3, 4, 5]
An agglutination titer greater than 1:160 is considered presumptively positive, and treatment may be started if this result is obtained. A second titer, demonstrating a 4-fold increase after 2 weeks, confirms the diagnosis.
Indirect fluorescent antibody testing
Indirect fluorescent antibody testing of suppurative material is rapid and specific. Microscopic examination of tissue and smear specimens is possible using fluorescently labeled antibodies at reference laboratories, possibly providing rapid confirmation of disease.
Histologic studies
Early tularemic lesions may demonstrate areas of focal necrosis surrounded by neutrophils and macrophages. Later, the necrotic areas become surrounded by epithelioid cells and lymphocytes. Caseating granulomata with or without multinucleated giant cells develops in some lesions.
Bacterial culturing
Although F tularensis has been cultured from sputum, pleural fluid, wounds, blood, lymph node biopsy samples, and gastric washings, the yield is extremely low and culturing poses a danger to laboratory personnel.
Imaging
-
Chest radiography - To evaluate for pneumonia; this is indicated in any patient in whom the diagnosis of tularemia is suspected
-
Ultrasonography - To examine lymph nodes for findings suggestive of infection; however, these findings lack specificity
Management
Medical care in tularemia is directed primarily toward antibiotic eradication of F tularensis, with streptomycin being the drug of choice (DOC) for this treatment. Research increasingly supports the use of fluoroquinolones to treat the disease, but clinical experience and in vitro data regarding their efficacy are limited. [6]
Symptomatic and supportive care is applied for accompanying conditions (eg, osteomyelitis, pericarditis, peritonitis) in patients with tularemia, as clinically indicated.
Vaccination
No tularemia vaccine is currently available. A vaccine based on a live strain of the bacterium previously was available but no longer is produced because of concerns about unknown attenuation, safety, and production.
Prevention
-
Avoid tick-infested areas
-
Wear trousers and long-sleeved shirts to avoid tick bites
-
Use tick repellants
-
Frequently inspect the body and clothing for evidence of ticks
-
Avoid exposure to dead or wild mammals and wear gloves if such exposure is necessary; hands should be thoroughly washed afterwards
Background
Tularemia is an acute, febrile, granulomatous, infectious zoonosis caused by Francisella tularensis, an aerobic, gram-negative, pleomorphic bacillus. F tularensis is one of the most infectious bacterial species known, as it can cause illness in humans with exposure to as few as 10-50 organisms. Four major subspecies, or biovars, exist; they differ in virulence and geographic range, with F tularensis biovar tularensis, found primarily in North America, being the most virulent. (See Pathophysiology and Etiology.)
Worldwide, more than 100 species of animals, including mammals, birds, amphibians, and arthropods, host F tularensis. The bacillus, which causes acute infectious illness in humans, also may be found in mud and water. (See Etiology.)
Although tularemia was quite common in the United States before World War II, incidence of the disease began a steady decline in the 1950s, falling to fewer than 0.15 cases per 100,000 population by 1965. [7] As a result, tularemia was removed from the reportable disease list in 1995, although outbreaks and sporadic cases have continued to occur worldwide. The disease again was put on the reportable list in 2000, owing to its potential as a bioweapon. [8, 9] (See Epidemiology.)
Although most cases of tularemia in the United States have occurred in the south-central states of Arkansas, Kansas, Missouri, Nebraska, and Oklahoma, the incidence of cases occurring north of these states has been increasing. [10, 11, 12]
Types of tularemia
Some authorities classify tularemia into 2 groups, which include the far more common ulceroglandular form (in which local or regional symptoms and signs predominate) and the more lethal typhoidal form (in which systemic symptoms dominate the clinical picture). More commonly, however, tularemia is divided into the following 7 forms [13, 1] :
-
Ulceroglandular tularemia: Cutaneous ulcer with regional lymphadenopathy
-
Glandular tularemia: Regional lymphadenopathy with no ulcer
-
Oculoglandular tularemia: Conjunctivitis with preauricular lymphadenopathy
-
Oropharyngeal tularemia: Stomatitis, pharyngitis, or tonsillitis with cervical lymphadenopathy
-
Intestinal tularemia: Abdominal pain, vomiting, and diarrhea
-
Pneumonic tularemia: Primary pleuropulmonary disease
-
Typhoidal tularemia: Febrile illness without early localizing signs and symptoms
Immunocompromised patients are more likely to present with pneumonic or typhoidal illness. [13]
Each form reflects the mode of transmission. The organism gains access to the host by means of inoculation into skin or mucous membrane or through inhalation or ingestion.
Biovars of F tularensis
F tularensis is an aerobic, gram-negative, pleomorphic coccobacillus. It is capable of growing within several different cell types, including macrophages, endothelial cells, and hepatocytes. [14, 15]
Four subspecies of F tularensis, all of which have been associated with human disease, have been described, but only the F tularensis biovar tularensis (Jellison type A) and F tularensis biovar holarctica (Jellison type B) are common causes. The two forms are serologically identical, differing primarily in their geographic distribution, fermentation reactions, and virulence.
F tularensis biovar tularensis predominantly is found in North America (although cases have been described in Europe) and is an extremely virulent organism. As few as 10-50 organisms may result in disease if inhaled or injected intradermally, although oral ingestion would require as many as 108 organisms for disease to occur.
F tularensis biovar holarctica (also known as F tularensis biovar palearctica) primarily is found in Europe [16] and Asia but also has been identified in cases of tularemia in North America. It is from this less-virulent subspecies that the live virus vaccine is derived. F tularensis biovars novicida and mediasiatica are of low virulence. A Japanese F tularensis biovar holarctica variant, japonica, more recently has been characterized.
Traditionally, Francisella subspecies have been characterized on the basis of biochemical reactions, growth characteristics, and virulence properties. However, biochemical methods for differentiating between the subspecies are imprecise; newer molecular typing methodology has advanced classification of these organisms.
In addition to F tularensis, the Francisella genus contains other species that may be human pathogens (F philomiragia, F opportunistica, and F hispanensis), fish pathogens (F salimarina, F halioticida, F orientalis, and F noatunensis), or environmental organisms. [17, 18, 19]
Bioweapon potential
The species is considered a category A biowarfare agent due to its high infectivity, relative ease of growth and stability in liquid formulation, ease of dissemination, and ability to cause substantial illness and death. [20, 21]
The World Health Organization (WHO) conducted modeling studies in 1970 on the possible use of F tularensis as an aerosolized bioweapon. The WHO estimated that an aerosol dispersal of 50 kg of virulent F tularensis over a metropolitan area with 5 million inhabitants would result in 250,000 incapacitating casualties, including 19,000 fatalities. This dispersal also would result in relapses occurring for many months after the initial exposure and the potential to establish enzootic reservoirs of tularemia in wild animals, leading to possible subsequent outbreaks.
A subsequent modeling study found that mitigation strategies will have to depend on the size of the release, the stockpile level of antibiotics, and the speed of antibiotic distribution. As previously mentioned, due to concerns regarding its potential use as a biowarfare agent, tularemia, which had been taken off the list of reportable diseases in 1995, was reinstated on the list in 2000. [22, 23]
Patient education
Direct patient education at limiting tick and deer fly exposure. If the patient hunts, stress the importance of learning contact precautions and good hygiene for handling rabbits and other wild animals. [1]
For patient education information, see Ticks.
Pathophysiology
The cell wall of F tularensis possesses high levels of fatty acids, and wild strains have an electron-transparent, lipid-rich capsule. Loss of this capsule may result in loss of serum resistance and virulence; however, the capsule exhibits no innate immunogenicity or toxicity.
A subcutaneous inoculum of 10 organisms is sufficient to induce disease, whereas an inhalational exposure of 25 organisms may cause a severely debilitating or fatal disease. Over the first 3-5 days after cutaneous exposure, the organism multiplies locally and a papule forms.
During the next 2-4 days, the site ulcerates. Organisms spread from the entry site to regional lymph nodes and may disseminate lympho-hematogenously to involve multiple organs. Pulmonary findings may be primary after direct inhalation of aerosolized bacteria or may be present in up to half of all tularemia cases from hematogenous spread (secondary pneumonia). Patients are most likely bacteremic at this time, although this usually is not detected.
Immune response
Infection produces an acute inflammatory response initially involving local macrophages, neutrophils, and fibrin. T lymphocytes, epithelioid cells, and giant cells then migrate into local necrotic tissue. [24] As the area of necrosis expands, thrombosis of adjacent arteries and veins may occur. Granulomas develop, which may caseate and be mistaken for tuberculosis, and necrotic foci may coalesce to form abscesses. These changes occur in infected sites and have been demonstrated on autopsy in lymph nodes, the liver, the spleen, bone marrow, and the lungs. [25] F tularensis may remain viable for prolonged periods. They may remain viable in the tissues, where they cause infection.
During the second to third week of Francisella infection, humoral immunity develops against the bacterium's carbohydrate antigens. Agglutinating immunoglobulin M (IgM), IgG, and IgA antibodies are seen at this time. [15, 26] Opsonizing IgG and IgM antibodies also are produced; these act in conjunction with complement (C3).
B-cell ̶ deficient mice have been shown to have impaired clearance of organisms after primary infection with the less virulent vaccine strain of Francisella. [27] In addition, alpha/beta T-cell ̶ dependent immunity, involving either CD4+ or CD8+ T cells and directed against protein antigens, has been demonstrated to be necessary for effective eradication of Francisella. [14, 15]
It also has been found that in humans, tularemia antigens, whether introduced by natural exposure or vaccination, elicit a vigorous and long-lasting response by memory T cells (TM cells). Some of these TM cells have lytic potential, and they demonstrate the ability to enter intestinal mucosal and nonmucosal lung sites. [28]
Preformed molecules on the surface of Francisella trigger rearrangements of the host cell cytoskeleton. Macrophage complement receptors interact with complement factor C3 fixed by molecules on the bacterial cell surface, and bacteria are internalized by looping phagocytosis.
Immediately after phagocytosis, the bacteria are housed in a nonacidified phagosome. Bacteria may degrade the phagosomal membrane and escape into the cytoplasm, where they actively multiply. This can lead to cell death and liberation of bacteria. In rodent macrophages, bacterial survival has been shown to be associated with failure of phagosome-lysosome fusion, phagosome acidification, and utilization of host iron. [29]
It also has been found that a lack of CD14 on human dendritic cells in the lung, or the existence of just minimal amounts of it, appears to contribute to F tularensis evasion of the host’s immune response. [30]
Interferon gamma (IFN-gamma) and tumor necrosis factor-alpha (TNF-alpha) activate macrophages to kill Francisella through the production of reactive nitrogen products such as nitric oxide (NO), [31] whereas neutrophils and mononuclear cells have been demonstrated to accumulate at infected liver foci and lyse Francisella -containing hepatocytes, releasing organisms from their relatively protected, sequestered environment. [32]
The ability of F tularensis to impair phagocyte function and survive in infected cells is central to its virulence. This intracellular life cycle has been shown to be related to the tightly regulated expression of a series of genes. [33, 34]
In the unique environment of the lung, oxygen-dependent neutrophil killing of wild virulent strains appears to be only partially effective. [35] Shortly after inhalation, Francisella are found inside cells that typically act as cytokine-producing first responders to infection, including airway macrophages and alveolar epithelial cells. Mouse experiments demonstrate lack of production of pro-inflammatory cytokines, including interleukin 12p40 (IL-12p40), TNF, IL-6, and IL-1 alpha. The exact mediators responsible for immunosuppression remain unclear; however, immunomodulatory factors, such as transforming growth factor-beta (TGF-beta) and prostaglandin E2 (PGE2), may be actively involved.
Approximately 48-72 hours postinfection, a number of cytokines and chemokines, such as IFN-gamma and TNF, are upregulated, and levels of proinflammatory mediators, such as RANTES (regulated on activation, normal T cell expressed and secreted), IL-6, and IL-1 beta, are detected. Unfortunately, the lung may contain more than 108 colony-forming units (CFU) of F tularensis, and this upregulation may be too late to prevent death. [14, 36] This late "cytokine storm," similar to that demonstrated in other instances of severe bacterial sepsis, actually may prove harmful to the host, causing capillary leakage, tissue injury, and lethal organ failure. [37, 38]
The incubation period for tularemia depends on the size of the inoculum, but ranges from 1-21 days (average 2-6 days). Individuals with tularemia may be asymptomatic or acutely septic with rapid death. Six clinical forms of tularemia have been identified. Each form is influenced by factors related to the host, organism, route of transmission, and host entry site.
Etiology
As previously mentioned, four subspecies of F tularensis have been described. Although all have been associated with human disease, however, only the F tularensis biovar tularensis (Jellison type A) and F tularensis biovar holarctica (Jellison type B) are common causes. The two forms are serologically identical, differing primarily in their geographic distribution, fermentation reactions, and virulence.
F tularensis biovar tularensis generally is found in North American rabbits and ticks and causes severe disease in humans. An inoculum of 10 organisms subcutaneously is sufficient to induce disease, and an inhalational exposure of only 25 organisms may cause disease.
F tularensis biovar holarctica is found primarily in Asian and European rodents and results in a milder form of disease in humans.
Methods of transmission include inhalation, ingestion, contact with an infected animal, and vector-associated exposure.
Transmission
Human-to-human spread of tularemia is not thought to occur. Transmission of disease to humans most often results from an insect bite or contact with contaminated animals or animal products. [39, 40] Thorough cooking of meat before consumption is believed to lessen the likelihood of transmission.
Transmission also has been described via ingestion of or contact with contaminated water, exposure to contaminated mud, animal bites, [41, 42] and exposure to aerosolized water droplets or dust from contaminated soil or grains.
Francisella survives for prolonged periods of time in frozen water, mud, and animal carcasses. [9, 43] The persistence of Francisella in water may be related to enhanced survival of the organism in the presence of amoeba. [44]
Carnivores, such as domestic cats, may transiently harbor Francisella in their mouths or on their claws after killing or feeding on infected prey, regardless of whether they have actually become infected. Cases have developed in laboratory workers, who should be notified in advance to safely handle specimens when tularemia is suspected.
Animal hosts
F tularensis is capable of infecting hundreds of different invertebrate, aquatic, and terrestrial vertebrate species, including lagomorphs, rodents, ticks, mosquitos, and flies. In any geographic region, usually no more than a dozen mammals are important to its ecology. However, the overall ecology of F tularensis remains poorly characterized, particularly transmission cycles and specific differences between the four subspecies. [45, 46]
Lagomorphs, including Sylvilagus and Lepus species, historically have been recognized as common sources of transmission (hence the common names wild hare disease and rabbit skinners' disease for tularemia). In North America, squirrels, muskrat, beavers, and voles have also been identified as natural reservoirs of F tularensis. In the former Soviet Union, in addition to hares, animal hosts include hamsters, voles, water rats, and mice. [41]
Tularemia has been recovered from more than 54 species of arthropods. [47] Blood-feeding flies and arthropods are the most important vectors for tularemia in the United States. Biting flies, such as deer flies (Chrysops discalis), are the predominant vectors in the far western states, whereas ticks, primarily Amblyomma americanum, Dermacentor andersoni, and D variabilis, are important vectors from the Rocky Mountains eastward. In Northern Europe and the former Soviet Union, mosquitos serve as the most important insect vector.
Ticks are a particularly important reservoir and vector because at least 13 different species have been found to be infected with F tularensis. In addition, vertical transmission of the bacterium transovarially has been demonstrated. F tularensis may be present in tick feces or saliva and can be inoculated directly or indirectly into the bite wound. Tick colonization by the organism may be enhanced, as intraerythrocytic F tularensis are protected from the acidic pH in the tick gut. [48]
Specific etiologies
Ulceroglandular form
This form occurs in 70-80% of cases. The organism enters through a scratch, abrasion, or tick or insect bite and spreads via the proximal lymphatic system. Within the ulceroglandular form, more differentiation exists. A subcutaneous inoculum of as few as 10 organisms can cause disease.
Glandular form
This form of the disease is rare. No ulcer is present, and the organism is presumed to have gained access to the lymphatic system and/or bloodstream through clinically unapparent abrasions.
Oculoglandular form
In this form of tularemia, which makes up 1% of cases, the organism enters through the conjunctiva from either a splash of infected blood or rubbing the eyes after contact with infectious materials (eg, blood from a rabbit carcass).
Oropharyngeal form
This form of the disease, which also is rare, occurs after ingestion of undercooked rabbit meat containing the organism or consumption of contaminated water.
Pneumonic form
This uncommon form of tularemia occurs when the organism is inhaled via aerosols of water or dust from soil, grains, or pelts or arises secondary to hematogenous spread of the bacterium. It is observed in laboratory workers and occasionally occurs naturally. Pneumonia also occurs in 10-15% of patients with ulceroglandular tularemia and in 50% of those with typhoidal tularemia. The mortality rate can reach 60% if untreated.
Typhoidal (or septicemic) form
This form, which occurs in 10-15% of cases, is more severe than the others and often includes pneumonia. Ingestion may be the mode of transmission; in most cases, however, the portal of entry remains unknown.
Epidemiology
Tularemia is widely distributed; however, it largely is found in the northern hemisphere from 30-71° north latitude. The disease most commonly is described in the western and south-central regions of the United States and in continental Europe and Asia.
Occurrence in the United States
A few hundred cases of tularemia are reported annually in the United States. Most reported cases occur in Arkansas, Tennessee, Texas, Oklahoma, Kansas, Utah, and Missouri.
In the past, tularemia infections reportedly occurred more frequently during the cold-weather months (eg, rabbit-associated disease); however, the infections now are being reported more frequently during warm-weather months (eg, tick-associated disease).
Approximately 200 cases of tularemia are reported annually in the United States, although many cases probably are undiagnosed, misdiagnosed, or unreported. The disease has been reported in all states except Hawaii.
Transmission of tularemia previously was most frequent from June through August and in December. The summer peak was thought to be due to insect bites, whereas the winter peak was attributed to hunting-associated cases. Peak reporting now occurs in late spring and early summer. [7] From 1990-2000, 56% of cases reported in the United States were from Arkansas, Missouri, Oklahoma, and South Dakota, with high numbers of cases also reported in Massachusetts, Kansas, and Montana. [29] High incidence rates among indigenous Alaskans and Native Americans also have been reported. [7, 49]
Occupations and avocations associated with an increased risk for tularemia include the following:
-
Laboratory work
-
Veterinary practice
-
Farming
-
Landscaping
-
Working with sheep
-
Hunting and trapping
-
Meat handling
International occurrence
Tularemia occurs throughout the Northern Hemisphere, except for in the United Kingdom. Cases have been reported in the United States, the former Soviet Union, Japan, Canada, Mexico, and Europe. [39, 46, 49] Tularemia has not been reported in Africa and South America.
Sex- and age-related demographics
Although both sexes are equally susceptible to tularemia, males are affected more frequently than females. This primarily results from increased exposure to specific activities (eg, hunting and skinning animals) and increased occupational vulnerability among males.
People of all ages are susceptible to the tularemia; however, young to middle-aged people are more likely to participate in activities that predispose them to exposure. [7]
Prognosis
Untreated tularemia has a mortality rate of 5-15%; if treated, the disease carries a mortality rate of 1-3%. The mortality rate is 2-3 times higher in patients with typhoidal tularemia than in those with other forms.
Other factors associated with increased mortality include elevated creatine kinase levels, renal failure, and other serious comorbidities, as well as late diagnosis. The mortality rate also depends on the subspecies involved; F tularensis biovar tularensis is significantly more virulent than the others and is responsible for almost all reported deaths.
Complications
Complications of tularemia include the following:
-
Pneumonia
-
Lung abscess
-
Respiratory failure, including possible acute respiratory distress syndrome (ARDS)
-
Rhabdomyolysis
-
Renal failure with possible hemodialysis
-
Hemoptysis
-
Meningitis
-
Endocarditis
-
Suppurative lymphadenitis
-
Pericarditis
-
Peritonitis
-
Appendicitis
-
Perisplenitis
-
Osteomyelitis
-
Guillain-Barré syndrome
-
Hepatitis
-
Sepsis
-
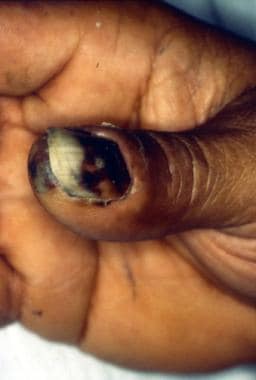
Eschar on thumb and under thumbnail at the site of a rabbit bite in a patient with tularemia.
-
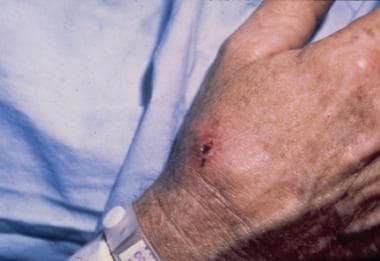
Axillary bubo in a patient with tularemia.
-
Ulceroglandular type of tularemia on the face. Courtesy of Dr Hon Pak.
-
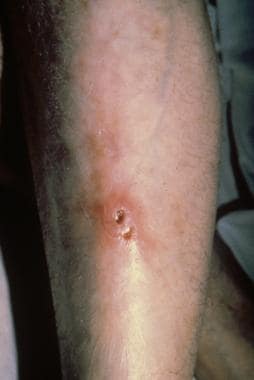
Ulceroglandular tularemia on an extremity. Courtesy of Dr Hon Pak.
-
Ulceroglandular type of tularemia on the hand. Courtesy of Dr Hon Pak.