Background
Trematode infections occur worldwide. Trematodes, also called flukes, cause various clinical infections in humans. The parasites are so named because of their conspicuous suckers, the organs of attachment (trematos means "pierced with holes"). All the flukes that cause infections in humans belong to the group of digenetic trematodes. Important features exhibited by adult digenetic trematodes are summarized below (see Features of digenic trematodes).
Trematode infections such as schistosomiasis have emerged as important tropical infections. An estimated 200 million people in the tropical belts of the world may have schistosomal infection. This makes Schistosoma infection the second most prevalent tropical infectious disease in areas such as sub-Saharan Africa after malaria. [1]
Depending on the habitat in the infected host, flukes can be classified as blood flukes, liver flukes, lung flukes, or intestinal flukes (see Classification of trematodes according to their habitat). The flukes that cause most human infections are Schistosoma species (blood fluke), Paragonimus westermani (lung fluke), and Clonorchis sinensis (liver fluke). Other less-important flukes include the liver flukes Fasciola hepatica and Opisthorchis viverrini and the intestinal flukes Fasciolopsis buski, Heterophyes heterophyes, and Metagonimus yokogawai.
Features of digenic trematodes
Digenic trematodes are unsegmented, leaf-shaped worms that are flattened dorsoventrally. They bear 2 suckers, one surrounding the mouth (oral sucker) and another on the ventral surface of the body (ventral sucker). These serve as the organs of attachment. The sexes of the parasites are not separate (monoecious). An exception is schistosomes, which are diecious (unisexual).
The alimentary canal is incomplete, and no anus is present. The excretory system is bilaterally symmetrical. It consists of flame cells and collecting tubes. These flame cells provide the basis for the identification of the species.
The reproductive system consists of male and female reproductive organs and is complete in each fluke. The flukes are oviparous. They lay operculated eggs. An exception is schistosome eggs, which are not operculated.
All have complicated life cycles, with alternating asexual and sexual developments in different hosts. [2]
Classification of trematodes according to their habitat
Blood flukes include Schistosoma haematobium, Schistosoma mansoni, Schistosoma japonicum, Schistosoma mekongi, and Schistosoma intercalatum (clade B – mammalian freshwater schistosomes).
Liver flukes include F hepatica, Fasciola gigantica, C sinensis, Opisthorchis felineus, O viverrini, Dicrocoelium dendriticum,Dicrocoelium hospes, and Metorchis conjunctus.
Pancreatic flukes include Eurytrema pancreaticum, Eurytrema coelomaticum, and Eurytrema ovis.
Lung flukes include Pwestermani, Paragonimusheterotremus, Paragonimus kellicoti, Paragonimusmexicana, Paragonimus skrjabin, Paragonimus miyazakii, Paragonimus compactus, and Paragonimushueit’ungensis.
Intestinal flukes include F buski, M yokogawai, Echinostoma ilocanum, Watsonius watsoni, H heterophyes, and Gastrodiscoides hominis.
Eye flukes include Philophthalmus lacrimosus, Philophthalmus palpebrarum, and Philophthalmus gralli (Philophthalmus lucipetus).
Other flukes include Alaria americana and Clinostomum complanatum.
Pathophysiology
The life cycle of trematodes is completed in 2 different classes of hosts: definitive (ie, humans, domestic animals, wild animals) and intermediate (ie, freshwater snails). See the figures below.
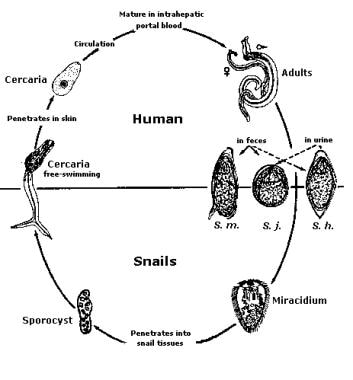 Adult worms in humans reside in the veins in various locations: Schistosoma mansoni in the inferior mesenteric veins, Schistosoma japonicum in the superior mesenteric veins, and Schistosoma haematobium in the vesical veins (these locations are not absolute). The females (size 7-20 mm; males slightly smaller) deposit eggs in the small venules of the portal and perivesical systems. The eggs are moved progressively toward the lumen of the intestine (S mansoni and S japonicum) and of the bladder and ureters (S haematobium), and they are eliminated with feces or urine, respectively. Under optimal conditions, the eggs hatch and release miracidia, which swim and penetrate specific snail intermediate hosts. The stages in the snail include 2 generations of sporocysts and the production of cercariae. Upon release from the snail, the infective cercariae swim, penetrate the skin of the human host, and migrate through several tissues and stages to their residence in the veins. Human contact with water is thus necessary for infection by schistosomes. Various animals serve as reservoirs for S japonicum and Schistosoma mekongi. Image courtesy of the US Centers for Disease Control and Prevention.
Adult worms in humans reside in the veins in various locations: Schistosoma mansoni in the inferior mesenteric veins, Schistosoma japonicum in the superior mesenteric veins, and Schistosoma haematobium in the vesical veins (these locations are not absolute). The females (size 7-20 mm; males slightly smaller) deposit eggs in the small venules of the portal and perivesical systems. The eggs are moved progressively toward the lumen of the intestine (S mansoni and S japonicum) and of the bladder and ureters (S haematobium), and they are eliminated with feces or urine, respectively. Under optimal conditions, the eggs hatch and release miracidia, which swim and penetrate specific snail intermediate hosts. The stages in the snail include 2 generations of sporocysts and the production of cercariae. Upon release from the snail, the infective cercariae swim, penetrate the skin of the human host, and migrate through several tissues and stages to their residence in the veins. Human contact with water is thus necessary for infection by schistosomes. Various animals serve as reservoirs for S japonicum and Schistosoma mekongi. Image courtesy of the US Centers for Disease Control and Prevention.
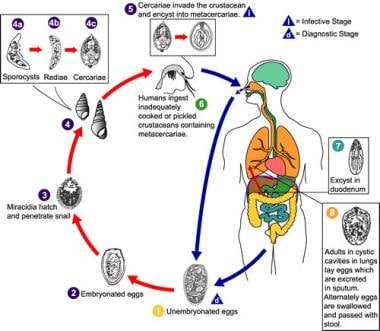 Eggs are excreted unembryonated in the sputum, or, alternately, they are swallowed and passed with stool (1). In the external environment, the eggs become embryonated (2), and miracidia hatch and seek the first intermediate host, a snail, and penetrate its soft tissues (3). Miracidia go through several developmental stages inside the snail (4): sporocysts (4a), rediae (4b), with the latter giving rise to many cercariae (4c), which emerge from the snail. The cercariae invade the second intermediate host, a crustacean such as a crab or crayfish, in which they encyst and become metacercariae. This is the infective stage for the mammalian host (5). Human infection with Paragonimus westermani occurs by eating inadequately cooked or pickled crab or crayfish that harbor metacercariae of the parasite (6). The metacercariae excyst in the duodenum (7), penetrate through the intestinal wall into the peritoneal cavity, and then through the abdominal wall and diaphragm into the lungs, where they become encapsulated and develop into adults (8) (7.5-12 mm X 4-6 mm). The worms can also reach other organs and tissues, such as the brain and striated muscles, respectively. However, when this occurs, completion of the life cycle is not achieved because the eggs laid cannot exit these sites. Time from infection to oviposition is 65-90 days. Infections may persist for 20 years in humans. Animals such as pigs, dogs, and a variety of feline species can also harbor P westermani. Image courtesy of the US Centers for Disease Control and Prevention.
Eggs are excreted unembryonated in the sputum, or, alternately, they are swallowed and passed with stool (1). In the external environment, the eggs become embryonated (2), and miracidia hatch and seek the first intermediate host, a snail, and penetrate its soft tissues (3). Miracidia go through several developmental stages inside the snail (4): sporocysts (4a), rediae (4b), with the latter giving rise to many cercariae (4c), which emerge from the snail. The cercariae invade the second intermediate host, a crustacean such as a crab or crayfish, in which they encyst and become metacercariae. This is the infective stage for the mammalian host (5). Human infection with Paragonimus westermani occurs by eating inadequately cooked or pickled crab or crayfish that harbor metacercariae of the parasite (6). The metacercariae excyst in the duodenum (7), penetrate through the intestinal wall into the peritoneal cavity, and then through the abdominal wall and diaphragm into the lungs, where they become encapsulated and develop into adults (8) (7.5-12 mm X 4-6 mm). The worms can also reach other organs and tissues, such as the brain and striated muscles, respectively. However, when this occurs, completion of the life cycle is not achieved because the eggs laid cannot exit these sites. Time from infection to oviposition is 65-90 days. Infections may persist for 20 years in humans. Animals such as pigs, dogs, and a variety of feline species can also harbor P westermani. Image courtesy of the US Centers for Disease Control and Prevention.
Snails that act as intermediate hosts for trematodes of medical importance are listed in Table 1. The list of these hosts for different trematodes and the source of infections are summarized in Table 2.
Table 1. Vectors and Geographical Areas Associated With Certain Trematode Types (Open Table in a new window)
Vector |
Geographical Area |
Type of Trematode |
Biomphalaria glabrata |
Brazil |
S mansoni |
Bulinus globosa |
Nigeria |
S haematobium |
Bulinus truncate |
Iran |
S haematobium |
Oncomelania hupensis nosophora |
Japan |
S japonicum |
Thiara granifera |
China |
P westermani; M yokogawai |
Semisulcospira libertine |
China |
P westermani; M yokogawai |
Polypylis hemisphaerula |
China |
F buski |
Parafossarulus manchouricus |
China |
C sinensis |
Bithynia leachi |
Germany |
O felineus |
Pirenella conica |
Egypt |
H heterophyes |
Lymnaea truncatula |
England |
F hepatica |
Table 2. List of Definitive and Intermediate Hosts and Sources of Infection of Major Trematodes (Open Table in a new window)
Trematode |
Definitive Host |
Intermediate Host 1st 2nd |
Source of Infection |
|
S haematobium |
Humans |
Freshwater snails (genus Bulinus) |
Absent |
Contact with water contaminated by cercariae |
S mansoni |
Humans, occasionally baboons and rodents |
Freshwater snails (genus Biomphalaria) |
Absent |
Penetration of skin by cercariae |
S japonicum |
Humans, dogs, pigs, cattle, mice, mustelids, and monkeys |
Amphibian snails (Oncomelania species) |
Absent |
Penetration of skin by cercariae |
S mekongi |
Humans and dogs |
Aquatic snails (Tricula aperta) |
Absent |
Penetration of skin by cercariae |
F hepatica |
Sheep, goats, cattle, and other herbivorous animals |
Amphibian snails (family Lymnaeidae) |
Aquatic vegetations and watercress |
Ingestion of aquatic plants and watercress infected with metacercariae |
C sinensis |
Humans, dogs, pigs, cats, rats, and several species of wild animals |
Freshwater snails (family Bulinidae) |
Freshwater fish (family Cyprinidae) |
Eating raw or partially cooked freshwater fish or dried, salted, or pickled fish infected with encysted metacercariae |
O felineus |
Humans and other fish-eating mammals |
Aquatic snails |
Freshwater fish |
Eating fish infected with metacercariae |
P westermani |
Humans, wolves, foxes, tigers, leopards, lions, cats, dogs, and monkeys |
Freshwater snails (family Pleuroceridae and Thiaridae) |
Freshwater crab or crayfish |
Ingestion of freshwater crabs or crayfish infected with metacercariae |
F buski |
Pigs and humans |
Planorbid snails of the genera Segmentina, Hippeutis, and Polypylis |
Freshwater plants such as water caltrops, water chestnut, water bamboo, water hyacinth, and lotus |
Ingestion of freshwater aquatic plants that harbor metacercariae |
Blood flukes (Schistosoma species)
Schistosomiasis, or bilharzia, is a tropical parasitic disease caused by blood-dwelling fluke worms of the genus Schistosoma, from the Greek for skhistos (split) and soma (body).Originally thought to be a single organism with a split body, the parasite was eventually recognized as having male and female forms. The main schistosomes that infect human beings include S haematobium (transmitted by Bulinus snails and causing urinary schistosomiasis in Africa and the Arabian peninsula), S mansoni (transmitted by Biomphalaria snails and causing intestinal and hepatic schistosomiasis in Africa, the Arabian peninsula, and South America), and S japonicum (transmitted by the amphibious snail Oncomelania and causing intestinal and hepatosplenic schistosomiasis in China, the Philippines, and Indonesia).
S intercalatum and S mekongi are only of local importance. S japonicum is a zoonotic parasite that infects a wide range of animals, including cattle, dogs, pigs, and rodents. S mansoni also infects rodents and primates, but human beings are the main host. A dozen other schistosome species are animal parasites, some of which occasionally infect humans.
Unlike other trematodes, schistosomes have separate sexes, but males and females are found together. The male is short and stout and holds the relatively long female worm in its gynecophoric canal, a groovelike structure. With S haematobium, both male and female live together in the veins that drain the urinary bladder, pelvis, and ureter, whereas S japonicum and S mansoni live in the inferior and superior mesenteric veins, respectively. Hence, these flukes are known as blood flukes. These species are distinguished from the other schistosomal species based on the morphology of their eggs and their adult and cercarial forms. Shaematobium eggs have a terminal spine, whereas Smansoni and Sjaponicum eggs have lateral spines and central spines, respectively.
Humans are infected by free-swimming, fork-tailed cercaria in fresh water by penetration of the skin. The cercaria loses its tail and outer layer of glycocalyces, transforms into a schistosomula (a larval form), and travels through venous circulation to the heart, lungs, and portal circulation. Larvae mature and develop into adult worms in approximately 3 weeks and reach the vessels that drain the urinary bladder (Shaematobium) or the mesentery (Sjaponicum, Smansoni). At these venous sites, they live and lay eggs for the duration of the host’s life.
The eggs penetrate the vascular endothelium, enter the bladder or gut lumen, and are excreted in urine (Shaematobium) or stool (Sjaponicum, Smansoni). If these excreted eggs gain access to fresh water, the miracidium emerges from the egg and swims freely until it finds an appropriate snail. In the snail host, after 2 generations of asexual multiplication (sporocysts), the forked-tailed cercariae emerge in water to infect other susceptible human hosts. A single miracidium can multiply in the snail to produce nearly 100,000 cercariae.
Table 3. Comparative Features of Major Human Schistosoma Species (Open Table in a new window)
|
S haematobium |
S mansoni |
S japonicum |
Adult |
|
|
|
Body surface of male |
Finely tuberculate |
Grossly tuberculate |
Nontuberculate (smooth) |
Testes |
4-6, in a cluster |
6-9, in a cluster |
7, in a linear series |
Position of ovary |
Posterior to middle of body |
Anterior to middle of body |
Posterior to middle of body |
Number of eggs in uterus |
20-30 |
1-4 |
50-300 |
Egg |
|
|
|
Size and shape |
110-170 μm long 40-70 μm wide Terminal spine |
114-175 μm long 45-68 μm wide Lateral spine |
70-100 μm long 50-65 μm wide Central spine |
Cercaria |
|
|
|
Cephalic glands |
2 pairs, oxyphilic |
2 pairs, basophilic |
4 pairs, oxyphilic |
Lung flukes
The genus Paragonimus contains more than 30 species that have been reported to cause infections in animals and humans. Among these, approximately 10 species have been reported to cause infection in humans, of which Pwestermani is the most important. Pwestermani, also known as the Oriental lung fluke, is the most widespread species in Africa, South America, and parts of Asia. Among other species of Paragonimus that have been reported to cause human disease from around the world is Paragonimus heterotremus, which has been reported from northeastern parts of the Indian subcontinent. [3]
P westermani is a thick, fleshy, reddish brown, egg-shaped worm (7.5-12 mm in length, 4-6 mm in breadth, and 3.5-5 mm in thickness). It inhabits parenchyma of the lung close to bronchioles in humans, foxes, wolves, and various feline hosts (eg, lions, leopards, tigers, cats).
The infection is typically transmitted via ingestion of metacercariae contained in raw freshwater crabs or crayfish. Additionally, consumption of the raw meat of paratenic hosts (eg, omnivorous mammals) may also contribute to human infection. Freshwater snails and crabs are first and second intermediate hosts of Paragonimus species, respectively. In the duodenum, the cyst wall is dissolved, and the metacercariae are released. The metacercariae migrate by penetrating through the intestinal wall, peritoneal cavity, and, finally, through the abdominal wall and diaphragm into the lungs. There, the immature worms finally settle close to the bronchi, grow, and develop to become sexually mature hermaphrodite worms.
Adult worms begin to lay the eggs, which are unembryonated and are passed out in the sputum. However, if they are swallowed, they are excreted in the feces. The eggs develop further in the water. In each egg, a ciliated miracidium develops during a period of 2-3 weeks. The miracidium escapes from the egg and penetrates a suitable species of snail (first intermediate host), in which it goes through a generation of sporocysts and 2 generations of rediae to form the cercariae. The cercariae come out of the snail, invade a freshwater crustacean (crayfish or crab), and encyst to form metacercariae. When ingested, these cause the infection, and the cycle is repeated. Note the image below.
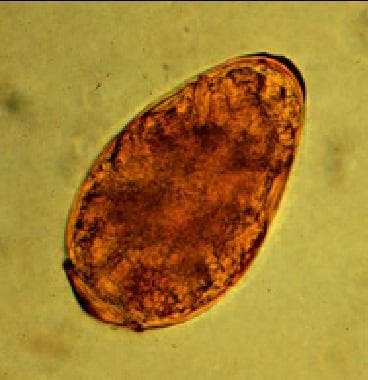 The average egg size is 85 μm by 53 μm (range, 68-118 μm X 39-67 μm). They are yellow-brown, ovoidal or elongate, have a thick shell, and are often asymmetrical with one end slightly flattened. At the large end, the operculum is clearly visible. The opposite (abopercular) end is thickened. The eggs of P westermani are excreted unembryonated. Image courtesy of the US Centers for Disease Control and Prevention.
The average egg size is 85 μm by 53 μm (range, 68-118 μm X 39-67 μm). They are yellow-brown, ovoidal or elongate, have a thick shell, and are often asymmetrical with one end slightly flattened. At the large end, the operculum is clearly visible. The opposite (abopercular) end is thickened. The eggs of P westermani are excreted unembryonated. Image courtesy of the US Centers for Disease Control and Prevention.
Liver flukes (C sinensis and F hepatica )
C sinensis
C sinensis is a widespread parasite found in Southeast Asia that infects the biliary passage in humans. The fluke is oblong, flat, transparent, and relatively small (10-25 mm long by 3-5 mm wide). It has a pointed anterior and rounded posterior end. Humans are infected by eating raw or partially cooked freshwater fish or dried, salted, or pickled fish infected with the metacercariae. In the duodenum, the cyst is digested and an immature larva released. The larva enters the biliary duct, where it develops and matures into an adult worm. The adult worm feeds on the mucosal secretions and begins to lay fully embryonated operculated eggs, which are excreted in the feces.
Upon reaching fresh water and upon ingestion by a suitable species of operculate snails (first intermediate host), the eggs hatch to produce a miracidium. Inside the snail, the miracidia multiply asexually through a single generation of sporocysts and generations of rediae to fork-tailed cercariae.
See the image below.
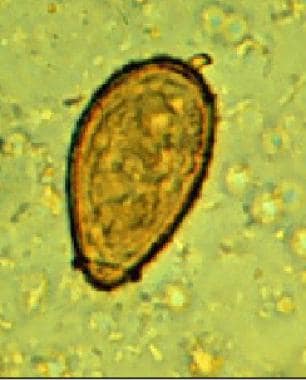 These are small operculated eggs. Size is 27-35 μm X 11-20 μm. The operculum, at the smaller end of the egg, is convex and rests on a visible "shoulder." At the opposite (larger, abopercular) end, a small knob or hooklike protrusion is often visible (as here). The miracidium is visible inside the egg. Image courtesy of the US Centers for Disease Control and Prevention.
These are small operculated eggs. Size is 27-35 μm X 11-20 μm. The operculum, at the smaller end of the egg, is convex and rests on a visible "shoulder." At the opposite (larger, abopercular) end, a small knob or hooklike protrusion is often visible (as here). The miracidium is visible inside the egg. Image courtesy of the US Centers for Disease Control and Prevention.
The cercariae escape from the snail to the water and penetrate under scales of freshwater cyprinid fish (second intermediate host). In the fish, the cercariae lose their tails and encyst in the scale or muscle of the fish to the metacercariae, which are infectious to humans. When ingested, the infected fish cause infection in humans.
F hepatica
Fascioliasis, a zoonotic disease caused by infection with Fhepatica (a digenetic trematode), is a major disease of livestock that is associated with important economic losses due to mortality; liver condemnation; reduced production of meat, milk, and wool; and expenditures for anthelmintics. The disease has a cosmopolitan distribution, with cases reported from Scandinavia to New Zealand and southern Argentina to Mexico.
Fhepatica, also known as the sheep liver fluke, is a large liver fluke. This fluke primarily causes zoonotic disease in sheep and other domestic animals. Humans are infected by eating watercress and other aquatic plants contaminated by the metacercariae, which enter the duodenum and excyst. They then penetrate the intestinal wall, peritoneal cavity, and liver capsule (Glisson capsule) to reach the bile duct of the liver, where they develop and mature into adult worms.
The adult worms begin to lay the unembryonated eggs, which are excreted in the stool. They develop further in the fresh water. A miracidium hatches out of the egg and invades the appropriate snail host. Inside the snail host, the larva multiplies asexually through a single generation of sporocysts and 2 generations of rediae to finally develop into cercariae. Upon exiting the snail, the cercariae encyst on aquatic plants to form metacercariae. When humans and sheep eat these plants, they become infected, repeating the life cycle.
D dendriticum, D hospes
Dicrocoeliasis is a parasitic disease caused by the small liver flukes D dendriticum and Dhospes. The disease represents a worldwide and widespread problem in grazing livestock, and it is sometimes (although rarely) found in humans. However, because of its unusual method of transmission by ingestion of infected ants, human dicrocoeliasis remains a relatively rare occurrence in humans. Cases of human dicrocoeliasis have been reported throughout Eastern Europe, Western Europe, Africa, Australia, India, and Saudi Arabia.
Pancreatic flukes (E pancreaticum , E coelomaticum , E ovis )
These flukes have a thick body and are 8-16 mm long and 6 mm wide. They parasitize the pancreatic ducts and occasionally the bile ducts of sheep, pigs, and cattle in Brazil and Asia. Three species, Epancreaticum, Ecoelomaticum, and E ovis, are recognized.
The first intermediate hosts are terrestrial snails (Bradybaena species), and the cercariae encyst in grasshoppers (Conocephalus species), which are the second intermediate host. After a suitable animal hosts ingests a grasshopper with cercariae, the immature flukes are released and migrate to the pancreatic duct, where they mature and produce eggs within approximately 11-14 weeks.
There are no obvious clinical signs of infection with these parasites. Dicrocoelium -like eggs can be demonstrated in feces. Light infections cause proliferative inflammation of the pancreatic duct, which may become enlarged and occluded. In heavy infections, fibrotic, necrotic, and degenerative lesions develop. Losses are reported due to condemned pancreas, but the pathogenesis suggests an additional loss of production.
Intestinal flukes (F buski, H heterophyes, M yokogawai , G hominis )
F buski is the most common intestinal nematode that causes infections in humans. The trematodes Hheterophyes, Myokogawai, and G hominis are less-common causes of human infection.
F buski, known as the giant intestinal fluke, is found in the duodenum and jejunum of pigs and humans and is the largest intestinal fluke to parasitize humans. Humans are infected by eating freshwater aquatic plants such as water caltrops, water chestnuts, and water bamboo, which can harbor the metacercariae. In the intestine, the metacercariae excyst, attach to the duodenum or jejunum, develop, and grow into adult worms. They lay unembryonated eggs, which are excreted in the feces.
In water, inside the egg, a ciliated miracidium develops, comes out, and penetrates a suitable snail host. Inside the snail, after several stages of asexual multiplication, large numbers of cercariae are produced. The latter emerge from the snail and encyst on the surface of aquatic plants to metacercariae. Ingestion of these plants causes infection in humans, and the cycle is repeated.
Epidemiology
Frequency
United States
Infection with blood flukes, lung flukes, liver flukes, and intestinal flukes in the United States is extremely rare. The condition is observed in travelers and emigrants from endemic areas. The liver fluke M conjunctus is found to be endemic in North America (Canadian population).
International
Trematode infections in general are becoming more prevalent. Schistosomiasis affects about 200 million people worldwide, and more than 650 million people live in endemic areas. Worldwide, more than 250 million people in 74 countries are infected. Currently, 601 million individuals are at risk for Csinensis infection, 293.8 million for infection with Paragonimus species, 91.1 million for infection with Fasciola species, and 79.8 million for infection with Opisthorchis species.
The geographic distribution of schistosomiasis depends on the presence of the freshwater snails that act as the intermediate hosts. Human infection is caused by skin penetration by the schistosomal cercariae upon contact with the contaminated water sources. Persons susceptible to infection include farmers working in irrigated fields, anglers working in culture ponds and rivers, and persons who wash utensils or clothes along banks of canals or rivers.
Residents who live near freshwater bodies have a risk of infection that is 2.15 times that of persons who live farther from water. Exponential growth of aqua culture may be the most important risk factor for the emergence of foodborne trematodiasis.
Foodborne trematodiasis, which is caused by liver flukes (Csinensis,Fasciola species, Opisthorchis species), lung flukes (Paragonimus species), and intestinal flukes (Echinostoma species, Fbuski, heterophyids), is an emerging public health problem in Southeast Asia and the West Pacific region.
The liver fluke M conjunctus has been reported in Russia. The life cycle of this parasite resembles that of Opisthorchis species. Aquatic snails serve as the first intermediate host, white sucker (Catostomus commersoni) serve as the second intermediate host, and humans are incidental hosts. Unlike Clonorchis and Opisthorchis, M conjunctus is not linked to the causation of cholangiocarcinoma. [4] Clonorchiasis is believed to be the third most prevalent worm parasite disease in the world. It is endemic to Japan, China, Taiwan, and South Asia and is currently infecting an estimated 35 million humans, of which 15 million are from China. [5]
The different species of Schistosoma have different geographic distributions. Urinary schistosomiasis caused by Shaematobium is found in 54 countries in Africa and the eastern Mediterranean. Intestinal schistosomiasis is caused by Sjaponicum and is limited to 4 countries in the Far East (ie, China, Thailand, Indonesia, Philippines). Smansoni is found in 52 countries in Africa and Latin America. Smekongi is found along the banks of the Mekong River area in Southeast Asia. It has been estimated that after malaria, schistosomiasis is the most common tropical infectious disease, with 207 million estimated cases. [6, 7] Schistosoma gimvicum and Schistosoma incognitum are rare species of schistosomes reported from India that are known to cause urinary and intestinal schistosomiasis, respectively. [8]
Schistosomal coinfection has been found to affect the malaria transmission in areas in Africa, where both these infections are common. Higher incidence of parasitemia and density of gametocytes has been seen in patients with coinfections. [1]
Cercarial dermatitis is commonly caused by various mammalian schistosomes (belonging to clade B) and rarely by avian schistosomes belonging to clade A (marine avian schistosomes - Austrobilharzia and Ornithobilharzia) and clade D (freshwater avian schistosomes - Trichobilharzia, Gigantobilharzia, Dendritobilharzia, among others). In India and Nepal, clade B mammalian freshwater schistosomes such as like Schistosoma turkestanicum, Schistosoma nasale, Schistosoma indicum, and Schistosoma spindale have been implicated in outbreaks of cercarial dermatitis. The snail Indoplanorbis exustus, seen abundantly in Asia (in contrast to Bulinus and Biomphalaria in Africa), acts as the intermediate host, and domesticated farm cattle act as definitive hosts. [9]
Liver fluke infection is endemic in China, Japan, Korea, Taiwan, and Vietnam (Csinensis); Thailand and Laos (Oviverrini); and the Russian Federation and Eastern Europe (Ofelineus). People who habitually eat raw or partially cooked fish or dried, salted, or pickled fish are more susceptible to infection by Clonorchis species. C sinensis the most common liver fluke in East Asia. Its importance lies in the fact that it has been shown to be responsible for cholangiocarcinoma in humans. In 2009, it was given the status of a group 1 biological carcinogen. [10]
Human fascioliasis occurs worldwide in temperate regions. F hepatica is found on every continent except Antarctica, with the estimated number of infected people over 2 million. The prevalence is highest in areas of extensive sheep and cattle farming and where dietary practices include the consumption of raw aquatic vegetables. In many locations (eg, Portugal, the Nile delta, northern Iran, parts of China, the Andean highlands of Ecuador, Bolivia, and Peru), high infection rates have made fascioliasis a serious public health concern. Outbreaks of Fgigantica infection have been reported from tropical areas of Southeast Asia, Africa, and Hawaii. Human fascioliasis has been reported with increasing frequency from countries such as Turkey in the past few years. [11]
Nearly 100 million people worldwide are infected with F buski. The infection is found most commonly in China, Taiwan, Thailand, Indonesia, Bangladesh, and India. Human infection occurs after ingestion of various parts (eg, fruits, pods, roots, stems) of infected water chestnut, lotus, and other aquatic plants, when they are bitten or peeled off with the teeth. Human infection with H heterophyes has been reported in Egypt's Nile delta.
Metagonimiasis is common in East Asia (mainly Japan, China, Taiwan, and the Republic of Korea). Echinostoma is endemic in Southeast Asia and the Far East (eg, China, Indonesia, Vietnam, Taiwan, Thailand). The life cycle of all the intestinal flukes is similar. [12]
Human lung fluke infection, most commonly with P westermani, is most common in China, Korea, Thailand, Philippines, and Laos. P skrjabini is more prevalent in China and P miyazakii is common in Japan. Isolated endemic foci have also been reported from the states of Manipur, Nagaland, and Arunachal Pradesh in India, where P heterotremus is the most common agent, followed by P westermani. Infection with a new subspecies of P miyazakii named P miyazakii manipurinus has been recently reported from India. P kellicotti is the agent responsible for pulmonary paragonimiasis in North America. [13]
A low prevalence has been reported from the African countries of Cameroon and Nigeria, where infections with Paragonimus africanus and Paragonimus uterobilateralis are reported. Rare reports of infections with other species of Paragonimus such as P compacta and P hueit’ungensis have been reported from a few countries, including India. [14]
Humans are infected by eating raw or partially cooked crab or crayfish or crabs soaked in wine as a food delicacy or by drinking juice from raw crabs or crayfish as a part of a food habit.
Systemic and intraocular infections in humans have been reported to be caused by A Americana, a 3-host trematode affecting the definitive host, dog. Humans acquire infection by consuming undercooked frogs (intermediate host) that contain the infective mesocercaria stage of the parasite. Humans and snakes act as paratenic hosts. Two intraocular (diffuse subacute neuroretinitis) and one disseminated infection by this parasite has been reported worldwide. [15]
The trematode Philophthalmus affects birds’ eyes (definitive host), and snails act as intermediate host. New definitive hosts (birds and, accidently, humans) acquire infections when encysted metacercaria are ingested or when they come into direct contact with the eye. Cases have been reported from Yugoslavia, Thailand, Sri Lanka, Japan, Mexico, and the United States. [16]
Clinostomum complanatum primarily affects birds (definitive host). Freshwater fish act as a second intermediate host, containing metacercaria, which are infective to birds. Humans acquire infection by ingesting undercooked freshwater fish, releasing the metacercaria into the stomach. The fluke migrates through the esophagus to the upper respiratory tract, causing pharyngitis or laryngitis. Most human Clinostomum infections have been reported in Japan, Korea, and Thailand. [17]
Race
No racial predisposition to trematode infections is apparent.
Sex
Most trematode infections have no sexual predisposition.
Age
Most trematode infections affect people of all ages equally. However, with intestinal trematode infections, children are affected more severely, as are children and adolescents with schistosomiasis.
Prognosis
Prognosis is excellent in patients with mild-to-moderate trematode infection, with early disease, and/or without severe complications.
Patients with heavier worm infection are less likely to improve, and the outcome in such infections may be serious and fatal.
Patient Education
Avoid high-risk food habits. Inform patients about the danger of eating raw or undercooked vegetables and fish and the importance of cleaning, washing, and adequately cooking vegetables and fish or raw liver. [2]
-
Adult worms in humans reside in the veins in various locations: Schistosoma mansoni in the inferior mesenteric veins, Schistosoma japonicum in the superior mesenteric veins, and Schistosoma haematobium in the vesical veins (these locations are not absolute). The females (size 7-20 mm; males slightly smaller) deposit eggs in the small venules of the portal and perivesical systems. The eggs are moved progressively toward the lumen of the intestine (S mansoni and S japonicum) and of the bladder and ureters (S haematobium), and they are eliminated with feces or urine, respectively. Under optimal conditions, the eggs hatch and release miracidia, which swim and penetrate specific snail intermediate hosts. The stages in the snail include 2 generations of sporocysts and the production of cercariae. Upon release from the snail, the infective cercariae swim, penetrate the skin of the human host, and migrate through several tissues and stages to their residence in the veins. Human contact with water is thus necessary for infection by schistosomes. Various animals serve as reservoirs for S japonicum and Schistosoma mekongi. Image courtesy of the US Centers for Disease Control and Prevention.
-
These are small operculated eggs. Size is 27-35 μm X 11-20 μm. The operculum, at the smaller end of the egg, is convex and rests on a visible "shoulder." At the opposite (larger, abopercular) end, a small knob or hooklike protrusion is often visible (as here). The miracidium is visible inside the egg. Image courtesy of the US Centers for Disease Control and Prevention.
-
Wet mounts with iodine. The eggs are ellipsoidal. They have a small, barely distinct operculum (upper end of the eggs in panel A). The operculum can be opened (egg in panel B), for example, when slight pressure is applied to the coverslip. The eggs have a thin shell that is slightly thicker at the abopercular end. They are passed unembryonated. Size range is 120-150 μm X 63-90 μm. Image courtesy of the US Centers for Disease Control and Prevention.
-
Adult flukes size range is 20-75 mm by 8-20 mm. Image courtesy of the US Centers for Disease Control and Prevention.
-
Eggs are excreted unembryonated in the sputum, or, alternately, they are swallowed and passed with stool (1). In the external environment, the eggs become embryonated (2), and miracidia hatch and seek the first intermediate host, a snail, and penetrate its soft tissues (3). Miracidia go through several developmental stages inside the snail (4): sporocysts (4a), rediae (4b), with the latter giving rise to many cercariae (4c), which emerge from the snail. The cercariae invade the second intermediate host, a crustacean such as a crab or crayfish, in which they encyst and become metacercariae. This is the infective stage for the mammalian host (5). Human infection with Paragonimus westermani occurs by eating inadequately cooked or pickled crab or crayfish that harbor metacercariae of the parasite (6). The metacercariae excyst in the duodenum (7), penetrate through the intestinal wall into the peritoneal cavity, and then through the abdominal wall and diaphragm into the lungs, where they become encapsulated and develop into adults (8) (7.5-12 mm X 4-6 mm). The worms can also reach other organs and tissues, such as the brain and striated muscles, respectively. However, when this occurs, completion of the life cycle is not achieved because the eggs laid cannot exit these sites. Time from infection to oviposition is 65-90 days. Infections may persist for 20 years in humans. Animals such as pigs, dogs, and a variety of feline species can also harbor P westermani. Image courtesy of the US Centers for Disease Control and Prevention.
-
The average egg size is 85 μm by 53 μm (range, 68-118 μm X 39-67 μm). They are yellow-brown, ovoidal or elongate, have a thick shell, and are often asymmetrical with one end slightly flattened. At the large end, the operculum is clearly visible. The opposite (abopercular) end is thickened. The eggs of P westermani are excreted unembryonated. Image courtesy of the US Centers for Disease Control and Prevention.







