Practice Essentials
Sertoli-cell-only (SCO) syndrome, also called germ cell aplasia or Del Castillo syndrome, describes a condition of the testes in which only Sertoli cells line the seminiferous tubules. [1] Sertoli cells help to make up the blood-testis barrier and are responsible assisting with sperm production. These cells respond to follicle-stimulating hormone (FSH) released by the hypothalamus, which helps to promote spermatogenesis. Typically, men with SCO syndrome present between age 20-40 years for evaluation of infertility and are found to be azoospermic, a term describing the absence of sperm in the ejaculate.
The physical examination findings are often unremarkable, and the diagnosis is made on the basis of testicular biopsy findings. While investigation to identify a cause of SCO syndrome is ongoing, the etiology and mechanism of this process are currently unknown. No known effective treatment exists, but these men may be able to reproduce with assisted reproductive technology.
See the image below.
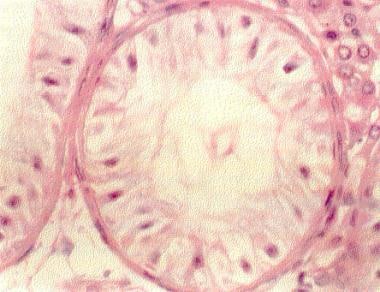 This hematoxylin and eosin section of a testis biopsy (400X) demonstrates an individual tubule lined only with Sertoli cells (Sertoli-cell-only [SCO] syndrome). The Sertoli cells line the seminiferous tubule.
This hematoxylin and eosin section of a testis biopsy (400X) demonstrates an individual tubule lined only with Sertoli cells (Sertoli-cell-only [SCO] syndrome). The Sertoli cells line the seminiferous tubule.
Pathophysiology
Sertoli cells have in general have several functions. They provide support to the developing spermatogonia and secrete a number of substances that aid in fetal development. For example, Sertoli cells secrete anti-müllerian hormone (AMH), which helps to ensure regression of müllerian ducts as a fetus develops into a male. They also secrete inhibin and activin, which help to regulate FSH secretion by the hypothalamus. [2] Activin has a positive feedback on the hypothalamus, causing increased levels of FSH necessary for sperm production. Inhibin has a negative feedback on the hypothalamus and helps to maintain testicular homeostasis. See the image below.
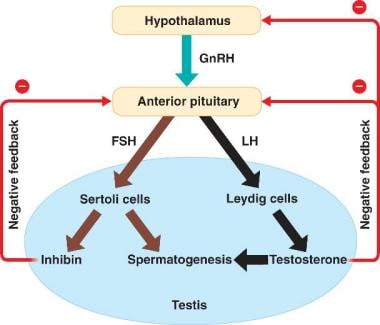 Interaction between the hypothalamus and the testes. Courtesy of Wikispaces at https://malereprobio12.wikispaces.com/.
Interaction between the hypothalamus and the testes. Courtesy of Wikispaces at https://malereprobio12.wikispaces.com/.
Involvement of other organ systems is rare, but is secondary to the underlying condition causing SCO syndrome. As an example, Klinefelter syndrome is characterized by SCO and Leydig cell hyperplasia.
A study investigating the PRPS2 protein found that PRPS2 expression was significantly greater in patients with SCO syndrome than in those with normal spermatogenesis. In SCO syndrome mouse model, PRPS2 overexpression significantly inhibited cell apoptosis and promoted cell cycle transition in TM4 Sertoli cells. [3]
Ovol1 and Ovol2, a family of zinc finger transcription factors, are expressed in spermatocytes at the pachytene stage and are suggested to be critical regulators of pachytene progression in male germ cells. Taniguchi and colleagues reported that while hOvol1 and hOvol2 were detected by reverse transcription-polymerase chain reaction (RT-PCR) in the testes of patients capable of spermatogenesis, they were not detected in those with Sertoli cell-only syndrome. The researchers concluded that further investigation of Ovol1 and Ovol2 functions in human spermatogenesis might lead to a novel therapy. [4]
Yao and colleagues found 174 microRNAs (miRNAs) were differentially expressed in human Sertoli cells in men with SCO syndrome compared with men with obstructive azoospermia, suggesting that these miRNAs may be associated with the pathogenesis of SCO syndrome. [5]
Etiology
Most cases of SCO syndrome are idiopathic. A congenital absence of germ cells due to failure of migration of gonocytes is theoretically possible.
A genetic basis for SCO syndrome is under intense investigation. [6] Massive deletions in the azoospermia factor (AZF) region of the Y chromosome, specifically in AZFb/b+c, have been found in men with SCO syndrome. Five deletions arose from nonallelic homologous recombination between palindromes P5 and P1 and 2 between P4 and P1. In addition, two deletions were found at novel proximal breakpoints in the interval region between P4 and P3. [7]
Y-chromosome microdeletions are also occasionally identified as a cause of SCO syndrome. [8, 9] As research continues, more genetic and chromosomal abnormalities associated with SCO may be found.
Expression of Fas, FasL, and active caspase-3 has been detected in Sertoli cells and hyperplastic interstitial cells. This may be associated with apoptotic elimination or altered maturation of Fas-expressing germ cells through the activation of caspase-3. [10]
Exposure to chemicals and toxins may cause SCO; however, direct cause-and-effect relationships in humans have been difficult to document.
Klinefelter syndrome, 47 XXY, results in a characteristic biopsy appearance of SCO and Leydig cell hyperplasia. [11]
SCO secondary to a high-grade varicocele has been reported. [12]
Attempting to distinguish between primary (congenital) and secondary (acquired) SCO syndrome is of no prognostic significance.
Epidemiology
The prevalence of SCO syndrome in the overall population is extremely low. Approximately 10% of US couples are affected by infertility. Of these couples, approximately 30% have a pure male factor as the underlying cause, and another 20% have a combined male and female factor. Although precise figures are difficult to obtain, less than 5%-10% of these infertile men have SCO syndrome. [13]
SCO syndrome has no known racial predilection; however, SCO is more common in white men. In most series, most couples who present for evaluation of male infertility are white. The most common age at presentation is 20-40 years and represents most men who are trying to initiate a pregnancy.
Prognosis
Sertoli-cell-only (SCO) syndrome remains a stable condition with no appreciable improvement in azoospermia. A few patients may be able to initiate pregnancy with sperm obtained by testicular biopsy with testicular sperm extraction (TESE). [13]
A number of models have been developed to predict successful sperm retrieval with TESE, although efficacy of these models is moderate. In general, higher patient age, higher values for serum testosterone, and lower values for serum FSH and LH were predictive for successful sperm retrieval. Idiopathic nonobstructive azoospermia and the presence of an AZFc deletion were predictive for unsuccessful sperm retrieval. [14]
Individuals with SCO syndrome may be at increased risk for testicular nodules and cancer. Initial reports show a 26% risk of nodules and a 10.5% risk of malignancy in testicles of men with pure SCO syndrome. [15] Further studies would be helpful to support this initial report.
Patient Education
Couples faced with a diagnosis of SCO syndrome should be given the options of adoption, use of donor sperm with intrauterine insemination and testicular sperm extraction with in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI). See Infertility Treatments.
Although sperm may be successfully extracted from small pockets of spermatogenesis in up to 20%-40% of men with a diagnosis of SCO syndrome, the use of these sperm for IVF/ICSI is successful in only a small percentage of patients and thus should not be offered as a standard of care. A fewAdoption is an effective option; however, couples must understand that adoption can be a lengthy and costly process. For intrauterine insemination, donor sperm may be obtained locally or nationally through sperm banks.
-
This hematoxylin and eosin section of a testis biopsy (400X) demonstrates an individual tubule lined only with Sertoli cells (Sertoli-cell-only [SCO] syndrome). The Sertoli cells line the seminiferous tubule.
-
Interaction between the hypothalamus and the testes. Courtesy of Wikispaces at https://malereprobio12.wikispaces.com/.







