Practice Essentials
Renovascular hypertension (RVHT) reflects the causal relation between anatomically evident arterial occlusive disease and elevated blood pressure. The coexistence of renal arterial vascular (ie, renovascular) disease and hypertension roughly defines this type of nonessential hypertension. [1] More specific diagnoses are made retrospectively when hypertension improves after intravascular intervention. [2]
At present, no sufficiently accurate, noninvasive, radiologic, or serologic screening test is available that, if negative, completely excludes the presence of renal artery stenosis (RAS). Current guidelines of the American College of Cardiology (ACC) and the American Heart Association (AHA) advocate screening for RAS only when a corrective procedure will be considered if renovascular disease is detected. [3]
When the history is highly suggestive and no risk of radiocontrast-mediated kidney injury is present, renal arteriography is the appropriate test. When a moderate suspicion of renovascular disease exists, computed tomography angiography (CTA), magnetic resonance angiography (MRA), or duplex ultrasonography should be considered for screening, the latter depending on availability and local experience.
Antihypertensive drug therapy is indicated. Optimal blood pressure control plays an essential role in the therapeutic management of RVHT; however, aggressive control of other risk factors for atherosclerosis also is crucial. Cessation of smoking is important for its positive impact on the cardiovascular risk profile in patients with hypertension. Similarly, antidyslipidemic therapy for those patients with hyperlipidemia likely provides benefit in atherosclerotic RVHT.
The invasive and surgical options for treatment of RVHT include percutaneous transluminal renal angioplasty (PTRA), surgical revascularization, and nephrectomy. Intravascular stents may be placed during angioplasty, although research has called the clinical benefit of this into question. (See Treatment.)
Patient education regarding hypertension should include information about the clinical features associated with RVHT (see Presentation) and about the importance of good blood pressure control. For patient education information, see What Is Renal Hypertension?.
Background
Since the seminal experimental work by Goldblatt and colleagues in 1934, [4] RVHT has increasingly been recognized as an important cause of clinically atypical hypertension and chronic kidney disease, the latter by virtue of renal ischemia. RVHT is the clinical consequence of activation of the renin-angiotensin-aldosterone system (RAAS).
Renal artery occlusion creates ischemia, which triggers the release of renin and a secondary elevation in blood pressure. Hyperreninemia promotes conversion of angiotensin I to angiotensin II, causing severe vasoconstriction and aldosterone release. The ensuing cascade of events varies, depending on the presence of a functioning contralateral kidney.
When two kidneys are present, aldosterone-mediated sodium and water retention is handled properly by the nonstenotic kidney, precluding volume from contributing to the angiotensin II–mediated hypertension. By contrast, a solitary ischemic kidney has little or no capacity for sodium and water excretion; allowing volume to play an additive role in the hypertension.
Pathophysiology
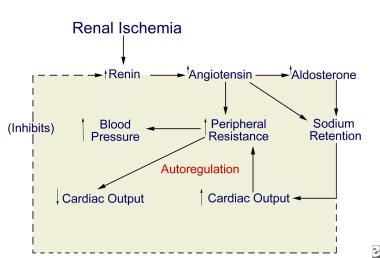
The chief pathophysiologic mechanism underlying RVHT involves activation of both limbs of the RAAS and depends on the presence or absence of a contralateral kidney (see the image below).
Unilateral renal ischemia initiates hypersecretion of renin, which accelerates conversion of angiotensin I to angiotensin II and enhances adrenal release of aldosterone. The result is profound angiotensin-mediated vasoconstriction and aldosterone-induced sodium and water retention.
In the two-kidney one-clip model, where the clinical correlate is unilateral renal artery disease, sodium and water handling via pressure diuresis of the contralateral kidney may be sufficient to prevent a volume component to the hypertension. In the setting of a solitary kidney (experimentally, the one-kidney one-clip model), sodium and water handling is compromised, sodium and water retention ensues, and volume-mediated hypertension occurs.
In unilateral RAS, renin production is increased in the ischemic kidney but suppressed in the unaffected nonstenotic kidney, which lacks the same ischemic stimulus. Consequently, when two kidneys are present with a unilateral stenosis (two-kidney one-clip model), hyperreninemia persists and blood pressure remains elevated because of an angiotensin II–induced vasoconstrictive effect. Renin production decreases in the contralateral kidney, a pressure diuresis ensues, and hypertension is maintained by high levels of angiotensin II.
A solitary kidney rendered ischemic by RAS is unable to achieve the pressure diuresis required to handle the aldosterone-induced sodium and water retention. The resultant volume expansion contributes to the elevation in blood pressure and also suppresses the production of renin by the stenotic kidney.
The sympathetic nervous system does not appear to play a role in perpetuating elevated blood pressure in the two-kidney one-clip model of RVHT. Evidence for a role in the one-kidney one-clip model of RVHT has been presented but is not clear or definitive.
Stages in development of renovascular hypertension
The evolution of RVHT [ref61], [ref62] has been described as having the following three stages or phases:
- Renin-angiotensin–dependent phase
- Salt-retention phase
- Systemic renin-angiotensin–independent phase
In the first phase, the immediate rise in blood pressure is a direct consequence of hyperreninemia. Over days to weeks, blood pressure remains elevated, but the course and presence of hyperreninemia vary with the presence and function of the contralateral kidney. The mechanism by which hypertension is produced in patients with renovascular disease thus changes over time and varies with the state of sodium balance.
When the contralateral kidney is functional, volume expansion is avoided and renin levels remain high. The two kidneys are in opposition; the stenotic kidney avidly retains sodium and produces excess renin in response to renal ischemia, while the nonstenotic kidney excretes sodium and water to maintain euvolemia and renin production decreases. The end result is systemic hypertension that is mediated by both renin and angiotensin.
In the second phase, in the setting of an ischemic solitary kidney, sodium and water retention, together with the vasopressor effects of angiotensin II, act to maintain renal perfusion pressure. The stimulus to produce renin is stifled, and renin levels fall. Hypertension becomes less dependent on angiotensin II and predominantly results from volume expansion. Thus, perfusion pressure is restored at the expense of systemic hypertension and volume overload.
If blood flow is restored during these first two phases and renal perfusion is reinstated, blood pressure soon returns to a normal level. If renal hypoperfusion persists and the third phase is reached, restoration of renal blood flow may not normalize blood pressure, presumably because of secondary irreversible vascular or renal parenchymal disease.
In the third phase, hypertension often is unremitting, persisting well after the removal of the stenosis. Recalcitrant hypertension in this setting likely represents the presence of ischemic nephropathy in either or both kidneys; patients in whom stenoses were not hemodynamically significant initially also may have persistent hypertension.
RAAS and control of intrarenal hemodynamics
Angiotensin II exerts a vasoconstrictive effect on both afferent and efferent arterioles, but because the efferent arteriole has a smaller basal diameter, the increase in efferent resistance exceeds the increase in afferent resistance. Afferent vasoconstriction is further minimized by angiotensin II–mediated release of vasodilatory prostaglandins and nitric oxide. In addition, angiotensin II can constrict the glomerular mesangium, thereby reducing the surface area available for filtration.
The net effect of angiotensin II on filtration invokes the opposing factors of reduced renal blood flow and mesangial surface area (causing a decrease in filtration) and the increase in glomerular capillary pressure (which tends to increase filtration). The end result depends on the clinical setting in which it occurs.
In the healthy kidney, a fall in systemic blood pressure activates the RAAS, which triggers a decrease in renal blood flow secondary to increased renal vascular (afferent) resistance. The preferential increase in efferent resistance mediated by angiotensin II results in increased glomerular capillary hydraulic pressure, which maintains the glomerular filtration rate (GFR).
In the ischemic kidney with reduced afferent blood flow, intraglomerular pressure and glomerular filtration are maintained by and depend upon angiotensin II–mediated efferent vasoconstriction. In this setting, removal of the efferent vasoconstrictive effect by angiotensin blockade, as achieved by angiotensin-converting enzyme (ACE) inhibitors, results in a decrease in intraglomerular pressure and GFR.
Thus, in patients with renovascular disease, particularly those with bilateral RAS or those with a stenotic renal artery to a single kidney, ACE inhibitors may cause a deterioration of renal function and azotemia. The propensity for angiotensin receptor blockers (ARBs) to affect GFR adversely is based on similar pathophysiology. It should be kept in mind that an acute decline in renal function in this setting is reversible once the ACE inhibitor (or the ARB) is discontinued. [2]
Manifestations
In adults, renovascular disease tends to appear at different times and affects the sexes differently. Atherosclerotic disease affects mainly the proximal third of the main renal artery and is most common among older men. Fibromuscular dysplasia (FMD) involves the distal two thirds and branches of the renal arteries and is most common among younger women. Midaortic syndrome is considered a variant of FMD. Neurofibromatosis may be seen.
Fibromuscular dysplasia
FMD involves fibrous or muscular hypertrophy of the vessel tunica media with fibrous intimal hyperplasia; accordingly, it is sometimes referred to as fibromuscular hyperplasia. Often, poststenotic dilatation is also present. The process may range from mild occlusion to complete occlusion of the vessel. FMD may be multifocal or unifocal. Multifocal FMD, which is the more common form in adults, has the radiologic appearance of a so-called “string of beads”, while unifocal FMD appears as a circumferential or tubular stenosis. [2] Louis et al reported that in children, unifocal FMD is more common than multifocal FMD, and the stenosis is often tubular. [5]
The most common site of stenosis is the orifice of the renal artery at its origin in the aortic wall (see the images below). The next most common location is within the main renal artery, and the segmental arteries are the least common site of stenosis. Total occlusion most often occurs at the orifice of the renal artery.
 Aortogram of 4-year-old child with renovascular hypertension caused by stenosis of left renal artery. Note that left kidney has 2 renal arteries and that artery to superior pole has stenosis.
Aortogram of 4-year-old child with renovascular hypertension caused by stenosis of left renal artery. Note that left kidney has 2 renal arteries and that artery to superior pole has stenosis.
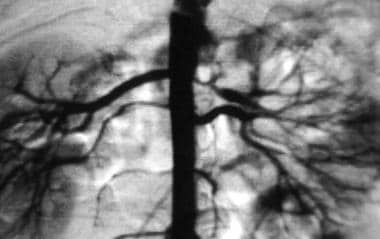 Close-up view of aortogram of 4-year-old child. Stenotic lesion begins at ostium of left superior renal artery. This lesion was caused by fibromuscular dysplasia and did not respond well to balloon angioplasty.
Close-up view of aortogram of 4-year-old child. Stenotic lesion begins at ostium of left superior renal artery. This lesion was caused by fibromuscular dysplasia and did not respond well to balloon angioplasty.
The inciting event of FMD is unknown. Some have suggested an autoimmune origin. In 1995, Stanley proposed that the lesion forms as a developmental disease in the muscular layer, which is followed by intimal hyperplasia from the abnormal flow through the constricted lumen. [6]
Midaortic syndrome
In midaortic syndrome, vascular involvement extends beyond the renal artery. Aortic narrowing is present, often extending from the aortic hiatus to just above the inferior mesenteric artery (IMA). One or both of the renal arteries are usually involved, and the celiac artery and the superior mesenteric artery (SMA) may be narrowed. Midaortic syndrome may result in total renal artery occlusion, with perfusion dependent on collateral circulation. Extensive collateralization from the IMA and a Riolan arcade may exist. Renal artery stenosis is usually bilateral. [7, 8]
Neurofibromatosis
Hypertension in patients with neurofibromatosis is often essential, but some patients also present with RVHT (see the image below). These patients have a pattern of RAS similar to that observed in FMD. However, involvement of the intrarenal arteries and arterioles may also exist. Neurofibromatosis usually involves the renal arteries of both kidneys. [9]
Etiology
The term renovascular hypertension (RVHT) implies that the cause of the elevated blood pressure is decreased arterial inflow to the kidneys. Overall, approximately 90% of RVHT cases are caused by atherosclerotic disease, 9% are caused by fibromuscular dysplasia (FMD), and miscellaneous causes make up the remainder. [2, 10] Other clinical entities that may be associated with RVHT include the following:
-
Cholesterol embolic disease
-
Acute arterial thrombosis or embolism
-
Aortic or renal artery dissection
-
Renal arterial trauma
-
Arterial aneurysm
-
Arteriovenous malformation of the renal artery
-
Diaphragmatic crus compression
Acquired RVHT may also be a consequence of:
-
Subisthmic coarctation
-
Vasculitis
-
Vascular trauma
-
Renal artery thrombosis
-
Tumors
-
Midaortic syndrome
-
Anastomotic stenosis (eg, after transplantation)
Epidemiology
United States statistics
RVHT is a common type of potentially correctable secondary hypertension. Although it accounts for less than 1% of mild hypertension, the prevalence may be as high as 38% in patients with severe hypertension and general atherosclerotic vascular or peripheral vascular disease. [11]
The incidence of hypertension in children is reported to be 1-5%, and in adolescents may be as high as 10%. In children, unlike adults, 70-80% of hypertension may be secondary hypertension, which is often correctable. RVHT is second only to coarctation of the aorta as a surgically correctable cause of hypertension in children. [6]
International statistics
The prevalence of RVHT internationally is not clear, but it likely accounts for the sole etiology in a relatively small percentage of unselected patients with hypertension. Significant geographic differences in the overall prevalence of RVHT have not been reported, though the etiology does appear to vary geographically.
In the western hemisphere, FMD is the most common cause of pediatric RVHT. Reports from Asia and South Africa identify Takayasu arteritis affecting the renal artery as the most common cause of RVHT in children. [12] One pediatric study in south Asia found that 87% of the patients presenting with RVHT had arteritis. [13]
Other demographics
The onset of RVHT tends to occur in patients younger than 30 years or older than 50 years. Systemic hypertension is less common in children than in adults, but the incidence of hypertension in children is approximately 1-5%. The presence of hypertension in younger children is usually indicative of an underlying disease process (secondary hypertension). In children, approximately 5-25% of secondary hypertension is attributed to renovascular disease. [14]
In children, the prevalence of renovascular disease as the cause of hypertension is inversely related to age with younger children more likely to have hypertension that is due to renovascular disease. In children younger than 5 years, the incidence of potentially surgically correctable hypertension is close to 80%. This incidence drops to 40-45% in children aged 6-10 years. In children aged 11-20 years, a 20% incidence of surgically correctable hypertension is observed. [14]
RVHT is most common in younger women and older adults. [11] Younger women develop RVHT most commonly from FMD affecting the distal two thirds and branches of the renal arteries. Older men develop RVHT most often from atherosclerotic disease affecting mainly the proximal third of the main renal artery. In children, multiple studies have failed to demonstrate any clear sex difference with regard to the prevalence of RVHT.
Overall, RVHT seems to be less common in the black population than in the white population. [15] Blood pressure has been shown to be higher in black children than in white children, but the difference has not been deemed clinically significant. When adjusted for height, much of this difference is eliminated. RVHT is less common among older black children than among adolescent whites, but the prevalence is actually higher in young black children. [16]
Prognosis
The prognosis of patients with RVHT is difficult to ascertain and varies with the extent of the occlusive phenomena, the sensitivity of the individual to antihypertensive therapy, and the efficacy of surgical repair or angioplasty. In patients with hypertension, the presence of atherosclerotic renal artery disease is a strong predictor of increased mortality relative to the general population. RVHT in the setting of renal dysfunction is associated with the greatest mortality.
Although the actual mortality of untreated RVHT is not clear, the prognosis is clearly poor, and the severity of the hypertension places considerable amount of strain on target organs and can lead to death. Fortunately, renovascular disease may be correctable with surgical treatment or invasive intervention.
A retrospective review of a cohort that included 30 severely hypertensive children with renovascular disease found an overall 18% incidence of hypertensive retinopathy. Most of the children had severe disease (retinal hemorrhages, exudates, and optic disc edema) and in some cases permanent visual reduction. [17]
PTRA yields normal blood pressures in some patients and others experience a decrease in blood pressures. Unfortunately, a high rate of recurrence of hypertension and vascular stenosis appears to be observed in patients treated with PTRA. [18] Some patients may experience resolution of their hypertension after nephrectomy.
Revascularization using PTRA with or without stenting in combination with medical therapy has been investigated in randomized trials of patients with unilateral atherosclerotic renal artery stenosis. A meta-analysis of these trials found no benefit from PTRA on mortality or end-stage renal disease as major cardiovascular events. [19]
Patients who have a high likelihood of benefit from revascularization with PTRA with stenting and medical therapy versus medical therapy alone are those that have unilateral renal artery stenosis, bilateral renal artery stenosis, or stenosis of a solitary kidney and meet one or more of the following criteria [13] :
- Recurrent congestive heart failure or sudden unexplained pulmonary edema
- Unstable angina
- Accelerated, resistant, or malignant hypertension
- Hypertension with unexplained unilateral small kidney and intolerance to medication
Successful surgical intervention is expected to offer patients a normal lifespan without complications. Children who undergo surgical revascularization appear to do well for at least 16 years postoperatively. They are able to participate in active sports and similar vigorous activities without problems. Further long-term follow-up is needed to determine the durability of these reconstructions and the actual life potential of these children.
-
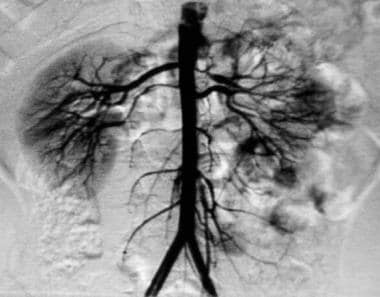
Magnetic resonance angiography (MRA) showing renal artery stenosis. Courtesy of Patricia Stoltzfus, MD, Chief of Interventional Radiology, West Virginia University.
-
Proposed pathogenesis of renovascular hypertension.
-
Angiogram showing bilateral renal artery stenosis. Courtesy of Department of Radiology, Henry Ford Hospital.
-
After percutaneous transluminal angioplasty (right renal artery). Courtesy of Department of Radiology, Henry Ford Hospital.
-
After percutaneous transluminal angioplasty and stent placement (left renal artery). Courtesy of Department of Radiology, Henry Ford Hospital.
-
Close-up of the Palmaz stent. Courtesy of Department of Radiology, Henry Ford Hospital.
-
Aortogram of 4-year-old child with renovascular hypertension caused by stenosis of left renal artery. Note that left kidney has 2 renal arteries and that artery to superior pole has stenosis.
-
Close-up view of aortogram of 4-year-old child. Stenotic lesion begins at ostium of left superior renal artery. This lesion was caused by fibromuscular dysplasia and did not respond well to balloon angioplasty.
-
Operative photograph of 4-year-old child. Patient underwent aortorenal bypass with reinforced saphenous vein graft. Inferior pole renal artery was preserved.
-
Aortogram of 8-year-old child with neurofibromatosis and renovascular hypertension caused by right renal artery stenosis.
-
Operative photograph of 8-year-old child. Aortorenal bypass was performed with Dacron-reinforced saphenous vein graft. Aorta is completely exposed, and graft is visible inferior to native renal artery.
-
Although nephrectomy is rarely indicated in treatment of renovascular hypertension in children, it can be safely performed with modern pediatric surgical laparoscopy technique. This 3-month-old child with renal dysplasia and refractory hypertension underwent laparoscopic nephrectomy. Photograph illustrates patient positioning and placement of small trocars at time of nephrectomy. Dysplastic kidney was easily removed through slightly enlarged umbilical incision.
-
3-month-old child immediately after laparoscopic nephrectomy. This patient was discharged from hospital 2 days after surgery. This approach eliminates need for large incisions and facilitates recovery from surgery, minimizing pain and length of hospital stay.