Practice Essentials
Renal transitional cell carcinoma (TCC), or renal urothelial carcinoma (UC), is a malignant tumor arising from the transitional (urothelial) epithelial cells that line the urinary tract from the renal calyces to the ureteral orifice (see the image below). UC is the most common tumor of the renal pelvis. Upper urinary tract TCC is estimated to occur in 5% of all urothelial cancers and in fewer than 10% of renal tumors. Evidence indicates that the frequency of upper urinary tract malignancies is increasing. [1]
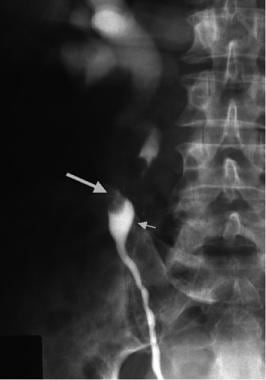 Right retrograde pyelogram demonstrates large filling defect in midureter due to transitional cell carcinoma (large arrow). Note characteristic appearance of radiographic contrast material just distal to obstruction (small arrow), which gives rise to so-called goblet sign. Contrast is also visible beyond partially obstructed segment of ureter in renal pelvis and collecting system.
Right retrograde pyelogram demonstrates large filling defect in midureter due to transitional cell carcinoma (large arrow). Note characteristic appearance of radiographic contrast material just distal to obstruction (small arrow), which gives rise to so-called goblet sign. Contrast is also visible beyond partially obstructed segment of ureter in renal pelvis and collecting system.
See Renal Cell Carcinoma: Recognition and Follow-up, a Critical Images slideshow, to help evaluate renal masses and determine when and what type of follow-up is necessary.
Surgical intervention is the main form of radical treatment for localized disease. Medical therapy usually is administered as an adjuvant to surgical therapy or to patients in whom surgical treatment is contraindicated (eg, because of poor general condition or the presence of advanced disease). The role of radiation therapy in the management of upper urinary tract TCC is not well defined.
For patient education resources, see Kidney Cancer: Renal Pelvis and Ureter.
Pathophysiology
TCC accounts for more than 90% of renal pelvic tumors; other cancer types seen include squamous cell carcinoma (SCC) and adenocarcinoma. The predominant histologic pattern of UC is a papillary tumor with stratified, nonkeratinizing epithelium supported on a thin fibrovascular core.
TCC of the upper urinary tract is histologically identical to urinary bladder cancer. These 2 malignancies share the same risk factors and can occur as a part of “field cancerization,” which results from exposure of urothelium to carcinogens excreted or activated in the urine. Hence, upper urinary tract urothelial tumors may be multifocal, and in 2-10% of cases, they are bilateral as well.
Patients with upper urinary tract urothelial tumors are at risk for the development of bladder tumors, which have an estimated incidence of 20-48%. Bladder cancer usually appears within 5 years. Patients with primary bladder cancer develop upper urinary tract UC in 2-4% of cases. The frequency of upper urinary tract UC may reach 21% in patients with bladder carcinoma in situ (CIS) and in those with certain occupational exposures.
Etiology
The exact cause of upper urinary tract TCC is not known; however, several risk factors have been identified.
Workers in the chemical, petrochemical, aniline dye, and plastics industries, as well as those exposed to coal, coke, tar, and asphalt, are at increased risk for renal pelvis and ureteral tumors.
Cigarette smoking appears to be the most significant acquired risk factor for upper urinary tract UC. It is suggested that 70% of upper urinary tract urothelial tumors in men and 40% of those in women can be attributed to smoking.
Two chronic tubulointerstitial disorders, Balkan endemic nephropathy and Chinese herbs nephropathy, are also risk factors for upper urinary tract urothelial tumors. Both have been linked to exposure to aristolochic acid. [2] Balkan endemic nephropathy is confined to regions located along the Danube River and its tributaries. It appears to result from dietary exposure to aristolochic acid in the seeds of Aristolochia clematitis (European birthwort), a native plant that may comingle with local wheat. [3] Chinese herbs nephropathy likely results from aristolochic acid found in certain herbal remedies used in traditional Chinese medicine. [2]
Analgesic abuse is a risk factor; a combination of phenacetin use and papillary necrosis results in a 20-fold increase in risk for renal urothelial tumors. Phenacetin was removed from the US market in 1983, and phenacetin-related upper urinary tract UC has become vanishingly rare. [4]
Chronic bacterial infection with urinary calculus and obstruction may predispose to the development of urothelial cancer. SCC is the most common entity in these cases. Schistosomiasis also may predispose to SCC.
The chemotherapy drugs cyclophosphamide and ifosfamide are implicated in the development of urothelial cancers in the upper and lower urinary tract, particularly after drug-induced hemorrhagic cystitis.
Epidemiology
United States statistics
According to American Cancer Society, an estimated 79,000 kidney and renal pelvis cancers and 81,180 bladder cancers will be diagnosed in the United States in 2022, with 13,920 deaths from the former and 17,100 deaths from the latter. Primary malignancies of the ureters and other urinary organs are much less common; it is estimated that 4010 such cancers will be diagnosed and 970 patients will die of this disease in 2022. [5] Deaths from urothelial malignancies have been decreasing since 1995.
International statistics
Worldwide statistics vary and are inaccurate, in that renal pelvis tumors are not reported separately. The highest incidence is found in Balkan countries (eg, Bosnia, Bulgaria, Croatia, Romania, and Serbia), where UCs account for 40% of all renal cancers and are bilateral in 10% of cases.
Age-, sex-, and race-related demographics
Renal pelvis tumors rarely occur before age 40 years. The peak incidence is in the 60- to 70-year age group. Men are affected approximately 2 times as frequently as women are. The incidence is slightly higher in African Americans than in other races; reported rates are similar among white Americans, Hispanics, and Native Americans. Renal pelvic tumors are less common in Asian Americans.
Prognosis
Renal UC is uniformly fatal unless it is treated. In a multicenter study of 1363 patients with upper urinary tract urothelial carcinoma who were treated with radical nephroureterectomy, the 5-year cancer-specific survival probability was approximately 73%. [6]
Tumor stage is the most important prognostic factor for upper urinary tract UC. Survival correlates closely with tumor stage. The TNM staging system developed by the International Union Against cancer (UICC) for upper urinary tract carcinomas is the most comprehensive (see Staging).
Tumor grade is another predictor of prognosis (see Histologic Findings). Tumor grade usually follows tumor stage, and patients with high-grade carcinomas have more advanced (ie, high-stage) disease. Stage and grade correlate in as many as 83% of cases, though stage remains a more accurate predictor of prognosis.
Patients with stage T3 renal tumors have a better prognosis than those with ureteral tumors. A retrospective study by Park et al found that in patients with stage pT3 disease, 5-year cancer-specific survival rates were 77.5% for renal pelvic tumors invading the renal parenchyma versus 49.7% for tumors invading peripelvic or periureteral fat; 5-year recurrence-free survival rates for the 2 tumor types were 75.6% and 32.0%, respectively. [7] The authors suggested that the thickness of the renal parenchyma may protect against local tumor spread.
The 5-year survival rate after radical surgery depends on the disease stage, as follows:
-
Stages Tis, Ta, or T1 – 91%
-
Stage T2 – 43%
-
Stages T3, T4, N1, or N2 – 23%
-
Stages N3 or M1 – 0%
TCC may develop in the contralateral kidney after radical nephroureterectomy. In a European multicenter dataset of patients who underwent nephroureterectomy for nonmetastatic TCC, a history of bladder TCC preceding the upper urinary tract TCC was the only predictor of TCC recurrence in the contralateral upper tract. [8] The 5-year probabilities of freedom from TCC in the contralateral upper tract TCC were as follows:
-
96.6% for patients with de novo upper-tract disease
-
91.1% for those having prior non–muscle-invasive bladder TCC
-
55.3% for those with prior muscle-invasive bladder TCC
The 5-year survival rate in selected patients after conservative surgery is reported to be 70-90%.
Recurrences in the remaining urothelium after conservative treatment are relatively frequent because of the multifocal nature of TCCs. Ipsilateral recurrence rates may reach 25-50%. Most low-grade recurrences can be treated with repeat conservative excision. The 5-year survival rates in these patients with low-grade, low-stage disease can approach 100%.
The prognosis is poor for patients with advanced SCC.
Vecchi et al have developed and validated two nomograms for predicting overall survival in patients with metastatic UC. The nomograms are intended to be used before and after completion of first-line platinum-based chemotherapy for metastatic UC. [9]
Adverse prognostic factors in patients receiving salvage systemic therapy for advanced UC include the following [10] :
-
Eastern Cooperative Oncology Group (ECOG) performance status > 0
-
Liver metastasis
-
Hemoglobin level < 10 g/dL
-
< 3 months since prior chemotherapy
-
Albumin level below the lower limit of normal [11]
-
CT scan with contrast, vascular phase. Mass can be seen in left renal pelvis (black arrows). Patient underwent nephroureterectomy. Tumor was high-grade urothelial carcinoma invading subepithelial tissue (stage T1) and measuring 7.5 × 3.2 × 3 cm.
-
CT scan, delayed phase. Enhancing mass can be visualized in left renal pelvis (white arrows).
-
Retrograde pyelography. Filling defect can be seen in left renal pelvis and lower calyx (black arrows). Patient underwent left nephroureterectomy. Tumor was low-grade urothelial carcinoma measuring 2.5 × 2 × 1 cm.
-
Right retrograde pyelogram demonstrates large filling defect in midureter due to transitional cell carcinoma (large arrow). Note characteristic appearance of radiographic contrast material just distal to obstruction (small arrow), which gives rise to so-called goblet sign. Contrast is also visible beyond partially obstructed segment of ureter in renal pelvis and collecting system.
-
Pathology specimen shows urothelial tumor of renal pelvis (white arrows).








