Practice Essentials
Renal cell carcinoma (see the image below) is the most common type of kidney cancer in adults. It accounts for approximately 85% of neoplasms arising from the kidney. [1]
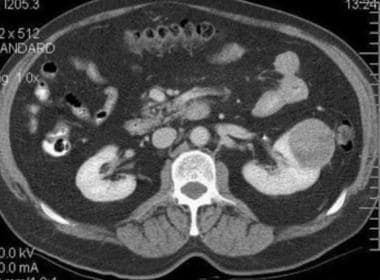 Typical renal cell carcinoma. CT scan obtained before contrast enhancement has an attenuation measurement of 33.9 HU.
Typical renal cell carcinoma. CT scan obtained before contrast enhancement has an attenuation measurement of 33.9 HU.
See Renal Cell Carcinoma: Recognition and Follow-up, a Critical Images slideshow, to help evaluate renal masses and determine when and what type of follow-up is necessary.
Signs and symptoms
Renal cell carcinoma may remain clinically occult for most of its course. Only 10% of patients present with the classic triad of flank pain, hematuria, and flank mass.
Other signs and symptoms include the following:
-
Weight loss
-
Fever
-
Hypertension
-
Hypercalcemia
-
Night sweats
-
Malaise
-
A varicocele, usually left sided, due to obstruction of the testicular vein
See Presentation for more detail.
Diagnosis
Lab studies used for evaluation of suspected renal cell carcinoma include the following:
-
Urinalysis (UA)
-
Complete blood cell (CBC) count with differential
-
Electrolytes
-
Kidney function tests
-
Liver function tests (LFTs; aspartate aminotransferase [AST] and alanine aminotransferase [ALT])
-
Serum calcium
Imaging studies used to evaluate and stage renal masses may include the following:
-
Excretory urography
-
CT scanning
-
PET scanning
-
Ultrasonography
-
Arteriography
-
Venography
-
MRI
See Workup for more detail.
Management
The principal treatment options for renal cell cancer are as follows:
-
Surgery
-
Molecular-targeted therapy
-
Immunotherapy
-
Radiation therapy
-
Thermal Ablation
-
Active surveillance
Surgical resection remains the only known curative treatment for localized renal cell carcinoma, and it is also used improve outcome or for palliation in metastatic disease. Targeted therapy and immunomodulatory agents are considered standard of care in patients with metastatic disease. Chemotherapy is used only occasionally, in certain tumor types. Experimental treatment approaches include vaccines and nonmyeloablative allogeneic peripheral blood stem cell transplantation.
See Treatment and Medication for more detail.
Background
Renal cell carcinoma (RCC) accounts for approximately 3% of adult malignancies and 90-95% of neoplasms arising from the kidney. This disease is characterized by a lack of early warning signs, diverse clinical manifestations (see Presentation), and resistance to radiation and chemotherapy. [2, 3, 4]
Increasingly, renal cell cancers are diagnosed at an earlier stage, and nephron-sparing surgery, thermal ablation, and surveillance are gaining acceptance as a treatment of choice for smaller tumors. Radical nephrectomy is the standard for larger and central tumors. (See Treatment.)
In recent years, multiple agents have been developed for systemic treatment of metastatic disease. Although the optimal treatment strategy continues to evolve, three agents that target angiogenesis (sunitinib, bevacizumab/interferon, and pazopanib) and a mammalian target of rapamycin (mTOR)–targeted therapy (temsirolimus) are approved as front-line agents. For selected patients, cabozantinib or combination therapy with nivolumab plus ipilimumab are also used as first-line treatments. Finally, high-dose interleukin-2 (IL-2) and axitinib can be used in selected patients.
A number of agents are available to be used in second and subsequent lines of therapy, these include anti-angiogenic therapy (if not already used in first-line treatment), nivolumab, cabozantinib, and mTOR inhibitors. Recommendations regarding how to sequence approved agents during subsequent therapy are evolving; more work is needed. Clinical trials are currently exploring future directions, including combinations of approved agents and the optimal sequencing of these agents.
Go to Clear Cell Renal Cell Carcinoma and Sarcomatoid and Rhabdoid Renal Cell Carcinoma for complete information on these topics.
Pathophysiology
The tissue of origin for renal cell carcinoma (RCC) is the proximal renal tubular epithelium. Renal cancer occurs in a sporadic (nonhereditary) and a hereditary form, and both forms are associated with structural alterations of the short arm of chromosome 3 (3p). Genetic studies of the families at high risk for developing renal cancer led to the cloning of genes whose alteration results in tumor formation. These genes are either tumor suppressors (VHL, TSC) or oncogenes (MET).
Multiple hereditary syndromes associated with renal cell carcinoma are recognized, as follows:
-
von Hippel-Lindau (VHL) syndrome
-
Hereditary papillary renal carcinoma (HPRC)
-
Familial renal oncocytoma (FRO) associated with Birt-Hogg-Dube syndrome (BHDS)
-
Succinate dehydrogenase (SDH)–deficient RCC
-
Hereditary renal carcinoma (HRC)
von Hippel-Lindau syndrome
von Hippel-Lindau syndrome, or von Hippel-Lindau disease, is an autosomal dominant syndrome that confers predisposition to a variety of neoplasms, including the following:
-
Renal cell carcinoma with clear cell histologic features
-
Pancreatic cysts and islet cell tumors
-
Retinal angiomas
-
Central nervous system (CNS) hemangioblastomas
-
Endolymphatic sac tumors
-
Epididymal cystadenomas
Renal cell carcinoma develops in nearly 40% of patients with von Hippel-Lindau disease and is a major cause of death in these patients. Deletions of chromosome 3p occur commonly in renal cell carcinoma associated with von Hippel-Lindau disease. Chromosome 3p contains several of the genes associated with kidney cancer, including BAP-1 and PBRM-1, among others.
The VHL gene is mutated in a high percentage of tumors and cell lines from patients with sporadic (nonhereditary) clear cell renal carcinoma. Several kindreds with familial clear cell carcinoma have a constitutional balanced translocation between 3p and either chromosome 6 or chromosome 8.
Mutations of the VHL gene result in the accumulation of hypoxia-inducible factors (HIFs) that stimulate angiogenesis through vascular endothelial growth factor (VEGF) and its receptor (VEGFR). VEGF and VEGFR are important new therapeutic targets.
Hereditary papillary renal carcinoma
Hereditary papillary renal carcinoma is an inherited disorder with an autosomal dominant inheritance pattern; affected individuals develop bilateral, multifocal papillary renal carcinoma. Germline mutations in the tyrosine kinase domain of the MET gene have been identified.
Familial renal oncocytoma and Birt-Hogg-Dube syndrome
Individuals affected with familial renal oncocytoma can develop bilateral, multifocal oncocytoma or oncocytic neoplasms in the kidney. Birt-Hogg-Dube syndrome is a hereditary cutaneous syndrome. Patients with Birt-Hogg-Dube syndrome have a dominantly inherited predisposition to develop benign tumors of the hair follicle (ie, fibrofolliculomas), predominantly on the face, neck, and upper trunk, and these individuals are at risk of developing renal tumors, colonic polyps or tumors, and pulmonary cysts.
Hereditary renal carcinoma
Affected individuals with this inherited medical condition have an increased tendency to develop oncocytomas, benign kidney tumors that have a low malignant potential.
Renal carcinoma patients diagnosed at 45 years or younger and those with family history of kidney cancer should receive genetic counseling.
Etiology
A number of environmental and genetic factors have been studied as possible causes for renal cell carcinoma (RCC). Cigarette smoking doubles the risk of renal cell carcinoma and contributes to as many as one third of all cases. The risk appears to increase with the amount of cigarette smoking in a dose-dependent fashion. Smoking has also been associated with advanced disease at presentation.
Obesity is a risk factor, particularly in women. Increasing body weight has a linear relationship with increasing risk. Hypertension is a possible risk factor.
Occupational exposure to certain chemicals, such as trichloroethylene, has been linked to increased risk for renal cell carcinoma. [5] In addition, the risk of renal cell carcinoma increases with duration of exposure to benzene, benzidine, cadmium, herbicides, and vinyl chloride. [6]
A prospective evaluation by Cho et al concluded that longer duration of use of nonaspirin nonsteroidal anti-inflammatory drugs (NSAIDs) may increase the risk for renal cell cancer. [7] Phenacetin-containing analgesics taken in large amounts may be associated with increased incidence of renal cell carcinoma; this agent is no longer approved for use in the United States.
Patients undergoing long-term dialysis have an increased incidence of acquired cystic disease of the kidney, which predisposes to renal cell cancer. In kidney transplant recipients, acquired renal cystic disease also predisposes to renal cell cancer. Chronic hepatitis C infection [8] and, according to a meta-analysis of pooled data, kidney stones in males [9] are associated with higher incidences of kidney cancer.
Genetic disorders associated with renal cell carcinoma include von Hippel-Lindau syndrome, hereditary papillary renal carcinoma, Birt-Hogg-Dube syndrome, and hereditary renal carcinoma. The genetic disease tuberous sclerosis appears to be associated with renal cell carcinoma, although the exact nature of the association is unclear.
Epidemiology
The incidence of kidney cancer in the United States has been rising since the 1990s, reaching 15.4 cases per 100,000 population in 2019. [10] From 2010 to 2019, the incidence rate increased by about 1% per year. Most of the increase has been in localized-stage cancers, and the increase appears to have been due in part to increased incidental detection of asymptomatic tumors because of the wider use of medical imaging. [5]
Currently, cancers of the kidney and renal pelvis are the sixth most common cancer in US men, accounting for 5% of cases, and the ninth most common in US women, accounting for 3% of cases. The American Cancer Society estimates that in 2023 there will be 81,800 cases (52,360 in males and 29,440 in females) of malignant tumors of the kidney and renal pelvis diagnosed, with 13,780 deaths (8790 in males and 4990 in females). [5] Renal cell carcinoma is expected to account for 80% of this incidence and mortality.
In most of Europe, the incidence of kidney cancer has decreased or stabilized over the past decade, perhaps in part because of reduced tobacco smoking in men. Mortality from kidney cancer has also declined in most of Europe, principally in Scandinavia and other western European countries. In men, the mortality rate per 100,000 population fell from 4.8 in 1990-1994 to 4.1 in 2000-2004; in women, the rate fell from 2.1 to 1.8. [11]
Renal cell carcinoma is more common in people of Northern European ancestry (Scandinavians) and North Americans than in those of Asian or African descent. In the United States, the incidence is slightly higher in Blacks than in Whites: 26.2 versus 24.3 per 100,000 population in men, and 13.2 versus 11.9 per 100,000 population in women. The highest incidence rates in the US are in American Indian/Alaska Native men, at 36.2 cases per 100,000 population. [10]
The median age at diagnosis from 2015 to 2019 was 65 years. In familial clusters, however, the disease has been reported in younger people. [10]
Prognosis
In the United States, death rates from kidney and renal pelvis cancer decreased by about 2% per year from 2013 to 2020. [5] These cancers currently constitute the twelfth leading cause of cancer death. [10] The 5-year survival rates initially reported by Robson in 1969 were 66% for stage I renal carcinoma, 64% for stage II, 42% for stage III, and only 11% for stage IV. [12, 13] Except for stage I, these survival statistics have remained essentially unchanged for several decades. Surveillance, Epidemiology, and End Results (SEER) data from 2012–2018 showed 5-year relative survival for localized kidney and renal pelvic cancer as 93.0% for localized disease, 72.3% for regional disease, and 15.3% for distant disease. [10]
Prognostic systems for metastatic renal cell carcinoma
Two similar prognostic systems are commonly used to calculate risk in patients with metastatic renal cell carcinoma (RCC). The Memorial Sloan-Kettering Cancer Center (MSKCC)/Motzer score includes the following criteria [14] :
-
Time from diagnosis to systemic treatment less than 1 year
-
Hemoglobin concentration below the lower limit of normal (LLN)
-
Serum calcium concentration > 10 mg/dL (2.5 mmol/L)
-
Lactate dehydrogenase (LDH) level more than 1.5 times the upper limit of normal (ULN)
-
Performance status ( Karnofsky) less than 80%
The International Metastatic RCC Database Consortium (IMDC) risk model, validated and further developed by Heng et al, includes the following prognostic criteria [15] :
-
Time from diagnosis to systemic treatment less than 1 year
-
Karnofsky performance status less than 80%
-
Hemoglobin concentration below LLN
-
Serum calcium (corrected for hypoalbuminemia) above ULN
-
Platelet count above the ULN
-
Neutrophil count above the ULN
Both systems categorize patients into the following 3 risk groups:
-
Favorable risk (no risk factors) - Median survival 20 months (MSKCC/Motzer); 2-year overall survival (2y OS) 75% (IMDC)
-
Intermediate risk (1 or 2 risk factors) - Median survival 10 months; 2y OS 53%
-
Poor/high risk group (3 or more risk factors) - Median survival 4 months; 2y OS 7%
In addition, Albiges and colleagues identified obesity as a favorable prognostic factor in patients with metastatic renal cell carcinoma treated with targeted therapy. In a study of 1975 patients from the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC), median overall survival was 25.6 months in patients with a body mass index (BMI) of 25 kg/m2 or higher, compared with 17.1 months in patients with a BMI of less than 25 kg/m2. The adjusted hazard ratio (HR) for obesity was 0.84. [16]
Review of an external validation cohort of 4657 patients treated for kidney cancer in clinical trials from 2003 to 2013 also demonstrated longer overall survival in obese patients, with median overall survivals of 23.4 months versus 14.5 months for those with low BMI. [16]
The longer survival may relate to the fatty acid synthase (FASN) pathway. FASN acts as a metabolic oncogene and its overexpression has been associated with poor prognosis in renal cell carcinoma and other types of cancers. FASN was downregulated in overweight and obese patients in this study. [16]
Patient Education
Patients with a family history of genetic syndromes associated with increased risk for renal cell carcinoma should be educated about these syndromes, and genetic counseling should be offered to the patients and family members. For example, renal cell carcinoma develops in nearly 40% of patients with von Hippel-Lindau (VHL) disease and is a major cause of death in patients with that disorder.
Patients at high risk should be made aware of the early signs and symptoms of the disease, and the need for early intervention for possible cure should be stressed. For patients with early-stage disease who have undergone treatment, education about possible relapse should be provided.
For patient education information, see Renal Cell Cancer.
-
Gross image of a bivalved kidney showing renal cell carcinoma in the upper half. The periphery of the carcinoma is yellow (due to high lipid content) with a central gelatinous area of necrosis.
-
H and E, high power of a clear cell renal cell carcinoma. The tumor cells have abundant pale "clear" cytoplasm.
-
H and E, low power of a papillary renal cell carcinoma. There are "finger-like" projections of fibrovascular stroma lined by malignant tumor cells that lack the abundant clear cytoplasm seen in a clear cell carcinoma.
-
H and E, high power of a chromophobe RCC composed of cells with clear, reticular cytoplasm and some with eosinophilic cytoplasm. The cell borders are often more distinct in this carcinoma than others and the nuclei are often smaller and darker.
-
H and E, high power of a collecting duct carcinoma composed of tubules lined by malignant cells in a background stroma that is fibrotic.
-
Typical renal cell carcinoma. CT scan obtained before contrast enhancement has an attenuation measurement of 33.9 HU.
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Treatment
- Guidelines
- Guidelines Summary
- Diagnosis and Staging
- Management of Stage I Renal Mass
- Management of Clinical Stage II and III Renal Masses
- Primary Treatment of Stage IV Kidney Cancer
- Management of Advanced and Metastatic Disease
- Molecular-Targeted Therapy in Metastatic Renal Cell Carcinoma
- Long-Term Monitoring for Clinically Localized Renal Neoplasms
- Show All
- Medication
- Questions & Answers
- Media Gallery
- References








