Practice Essentials
Raynaud phenomenon manifests as recurrent vasospasm of the fingers and toes and usually occurs in response to stress or cold exposure. [1] The phenomenon is named for Maurice Raynaud, who, as a medical student, defined the first case in 1862 as "episodic, symmetric, acral vasospasm characterized by pallor, cyanosis, suffusion, and a sense of fullness or tautness, which may be painful." [2] See the image below.
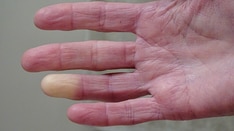
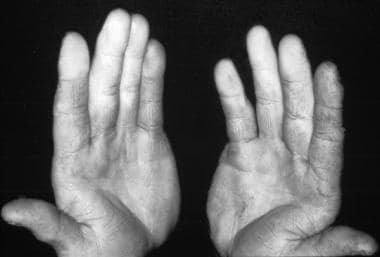 Photo of a patient with Raynaud phenomenon that resulted from working with a jackhammer. Courtesy of the CDC.
Photo of a patient with Raynaud phenomenon that resulted from working with a jackhammer. Courtesy of the CDC.
See Cutaneous Clues to Accurately Diagnosing Rheumatologic Disease, a Critical Images slideshow, to help recognize cutaneous manifestations of rheumatologic diseases.
Secondary Raynaud phenomenon should be distinguished from primary Raynaud phenomenon (Raynaud disease). They are distinct disorders that share a similar name. Raynaud disease is characterized by the occurrence of the vasospasm alone, with no association with another illness. Secondary Raynaud phenomenon is a designation usually used in the context of vasospasm associated with a variety of rheumatologic and non-rheumatologic diseases, environmental exposures, and/or medications. [3]
Diagnostic criteria for primary Raynaud phenomenon include the following [1] :
-
Attacks triggered by exposure to cold and/or stress
-
Symmetric bilateral involvement
-
Absence of necrosis
-
Absence of a detectable underlying cause
-
Normal capillaroscopy findings
-
Normal laboratory findings for inflammation
-
Absence of antinuclear factors
Young women who have had Raynaud phenomenon alone for more than 2 years and have not developed any additional manifestations are at low risk for developing an autoimmune disease. The same should not be said for older patients and male patients with Raynaud phenomenon, as vasospastic symptoms may predate systemic disease by as many as 20 years. In some studies, 46%-81% of affected patients have secondary Raynaud phenomenon.
Although Raynaud phenomenon has been described with various autoimmune diseases, the most common association is with progressive systemic sclerosis (90% in individuals with scleroderma) and mixed connective-tissue disease (85% prevalence). Raynaud phenomenon has also been described with such diverse diseases as systemic lupus erythematosus and other disorders not classified as autoimmune, including frostbite, vibration injury, polyvinyl chloride exposure, and cryoglobulinemia.
For primary Raynaud phenomenon, the first line of therapy consists of lifestyle measures. If these prove inadequate, the patient may benefit from pharmacologic treatment. Therapy for secondary Raynaud phenomenon must be tailored to the underlying disorder. Patients with secondary Raynaud phenomenon are more likely to require pharmacologic therapy. A variety of drugs are used off-label for treatment; the most commonly used drug is nifedipine. See Treatment and Medication.
For discussion of Raynaud phenomenon in children, see Pediatric Raynaud Phenomenon.
Pathophysiology
In individuals with Raynaud phenomenon, one or more body parts experience intense vasospasm with associated color change and subsequent hyperemia. Patients often describe 3 phases of change: initial white (vasoconstriction), followed by blue (cyanosis), and then red (rapid blood reflow). The affected body parts are usually those most susceptible to cold injury. A clear line of demarcation exists between the ischemic and unaffected areas (see the image below).
These effects are reversible, and they must be distinguished from irreversible causes of ischemia such as vasculitis or thrombosis. Rarely, tissue necrosis occurs distal to the affected vessel, usually in the periphery of the vasculature. It most commonly affects the fingers but may affect the toes, nose, and ears. Occasionally, even the tongue is involved.
Despite several years of research, the full understanding of the pathophysiology of Raynaud phenomenon remains to be elucidated. [4]
Primary Raynaud phenomenon is related to functional alterations alone. In contrast, secondary Raynaud phenomenon also reflects structural microvascular abnormalities. Herrick (2005) reviewed the pathogenesis of Raynaud phenomenon and describes the mechanisms under 3 categories: vascular, neural, and intravascular abnormalities. [5]
Vascular abnormalities
A deficiency of vasodilatory mediators, including nitric oxide, has been implicated in the pathogenesis of Raynaud phenomenon. [6] In addition, endothelin-1, a potent vasoconstrictor found in the endothelium, has been found to be circulating in high levels in patients with secondary Raynaud phenomenon. [6] Release of endothelin-1 is triggered by vasoactive stimuli, including angiotensin, vasopressin, and transforming growth factor-beta (TGF-beta) [7] Conflicting results regarding the levels of endothelin-1 in patients with primary Raynaud phenomenon are noted.
Angiotensin has vasoconstrictive and profibrotic effects. [8] A study by Kawaguchi et al revealed higher levels of angiotensin II in patients with diffuse cutaneous systemic sclerosis. [9]
In patients with systemic sclerosis, structural abnormalities related to fibrotic proliferation of the vasculature leading to reduced blood flow to the digits have been found. This differs from primary Raynaud disease. [10]
Neural abnormalities
Edwards et al proposed that primary Raynaud's disease involves abnormal function of brain stem areas that integrate the cardiovascular components of the response to acute stress. These researchers found that initially, both patients with Raynaud phenomenon and healthy individuals responded to sound stimuli with vasodilation in forearm muscles and vasoconstriction in the skin of the digit. Over 5 days, however, healthy individuals showed habituation to the stimuli, while patients with primary Raynaud phenomenon did not. [11]
Impaired vasodilation may be involved in Raynaud phenomenon. An important neuropeptide, calcitonin gene-related peptide, is a potent vasodilator secreted by nerves that supply blood vessels. [12] A diminished number of calcitonin gene–related peptide-releasing neurons has been found in skin biopsy samples of patients with primary Raynaud and systemic sclerosis. [13]
Enhanced vasoconstriction in Raynaud phenomenon may involve overactivity of α2C -adrenoreceptors; these adrenoreceptors have been found to enable cold-induced vasoconstriction of the blood vessels. [14] Two studies by Furspan et al showed that the enhanced contractile response to α2 -adrenergic agonists and cooling in patients with primary Raynaud phenomenon may be linked to increased protein tyrosine kinase activity. [15] These data suggest that protein tyrosine kinase inhibitors may be beneficial in the treatment of Raynaud phenomenon.
In patients with Raynaud phenomenon secondary to systemic sclerosis, increased levels of neuropeptide Y have been found. Neuropeptide Y is a potent vasoconstrictor.
Intravascular abnormalities
Raynaud phenomenon has been associated with the following intravascular abnormalities:
-
In primary Raynaud and systemic sclerosis, increased platelet activation and aggregation has been demonstrated [16]
-
An increased production of platelet thromboxane A 2, a potent vasoconstrictor, has been found in patients with Raynaud phenomenon
-
In patients with systemic sclerosis, an impaired fibrolytic system has been reported, probably contributing to vascular obstruction [17]
-
Oxidative stress by reactive oxygen species has also been implicated in the pathogenesis of Raynaud phenomenon
Etiology
The cause of primary Raynaud phenomenon remains unknown. Ascherman et al propose an autoimmune etiology, with cytokeratin 10 (K10) as a candidate autoantigen; their study in mice showed that anti-K10 antibodies can mediate ischemia similar to that seen in Raynaud phenomenon. [18] A study from northern Sweden found that cold injury may precede the onset of Raynaud’s phenomenon, suggesting this as a possible causal factor. [19]
Possible causes for secondary Raynaud can be divided into several broad categories, including the following:
-
Occupational
-
Hematologic
-
Collagen-vascular (autoimmune)
-
Miscellaneous disorders such as Fabry disease, [23] pheochromocytoma, neoplasms, acromegaly, carpal tunnel syndrome, and myxedema
Rarely, secondary Raynaud phenomenon may be a paraneoplastic syndrome. Cases have been reported in patients with hematologic, lung, breast, uterine, and ovarian cancers, principally adenocarcinomas. [24]
A study using data from the population-based LifeLines Cohort Study from the Netherlands found an association between low body weight and prior involuntary weight loss and risk of Raynaud phenomenon in both men and women. The odds ratio (OR) for association of low body weight and Raynaud phenomenon was 5.55 in men (95% confidence interval [CI] 2.82-10.93) and 3.14 (CI 2.40-4.10) in women; the OR for association with involuntary weight loss was 1.32 (CI 1.17-1.48) in men and 1.31 (CI 1.20-1.44) in women. Low-fat diet was also associated with Reynaud phenomenon in women (OR 1.27, CI 1.15-1.44). [25]
Epidemiology
A 7-year study of Raynaud phenomenon in whites in the United States showed baseline prevalence rates of 11% in women and 8% in men and yearly incidence rates of 2.2% in women and 1.5% in men. [26] Internationally, the prevalence of primary Raynaud phenomenon varies among different populations, from 4.9%-20.1% in women to 3.8%-13.5% in men. As in the United States, the prevalence of secondary Raynaud phenomenon depends on the underlying disorder.
Racial, sexual, and age-related demographics
Primary Raynaud phenomenon has no racial predilection. Secondary Raynaud phenomenon approximates the racial prevalence of the underlying disease, if any.
Primary Raynaud phenomenon occurs more frequently in women than in men. The prevalence by sex varies in different populations, ranging from 4.9%-20.1% in women to 3.8%-13.5% in men.
Primary Raynaud phenomenon usually occurs in the second or third decade of life. Secondary Raynaud phenomenon begins in accordance with the underlying disorder.
Prognosis
The prognosis for patients with primary Raynaud phenomenon is usually very good, with no mortality and little morbidity. A study from northern Sweden reported annual remission rates of 4.4% in women and 5.5% in men. [19]
In very rare cases, ischemia of the affected body part can result in necrosis. In addition, a study in 830 participants of the Charleston Heart Study cohort identified a potentially significant relationship between Raynaud phenomenon and all-cause mortality, especially in elderly subjects. Furthermore, in whites the presence of Raynaud phenomenon (when broadly defined as including both blanching and cyanotic color changes) was associated with a 1.6-fold higher risk of death related to cardiovascular disease. [27]
In a study by Mueller et al of 2958 consecutive patients with incipient Raynaud phenomenon without previously known connective tissue disease, survival during a median follow-up period of 9.3 years was poorer than in a demographically matched standard population (log-rank test P < 0.0001). Mortality was higher in men than in women (P< 0.0001). In women, but not in men, the presence of abnormal nailfold capillaries, antinuclear antibodies, and anti-Scl-70 antibodies were associated with all-cause mortality. [28]
The prognosis for patients with secondary Raynaud phenomenon is related to the underlying disease. The prognosis for the involved digit or digits in these patients is related to the severity of the ischemia and the effectiveness of maneuvers to restore blood flow.
Patient Education
Patients with Raynaud phenomenon should avoid situations that precipitate their attacks, and they should insulate their hands from the cold. Smoking should be prohibited.
If ulcerations develop, patients need to keep them sterile and aggressively treat any infections that may develop. All of this should be done under the supervision of a physician; consultation with a wound care specialist may be useful.
For patient education information, see Raynaud Phenomenon.
-
A 9-year-old with Raynaud phenomenon. Notice the discoloration of the fingers.
-
Photo of a patient with Raynaud phenomenon that resulted from working with a jackhammer. Courtesy of the CDC.
-
Raynaud phenomenon showing demarcation of color difference.